Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
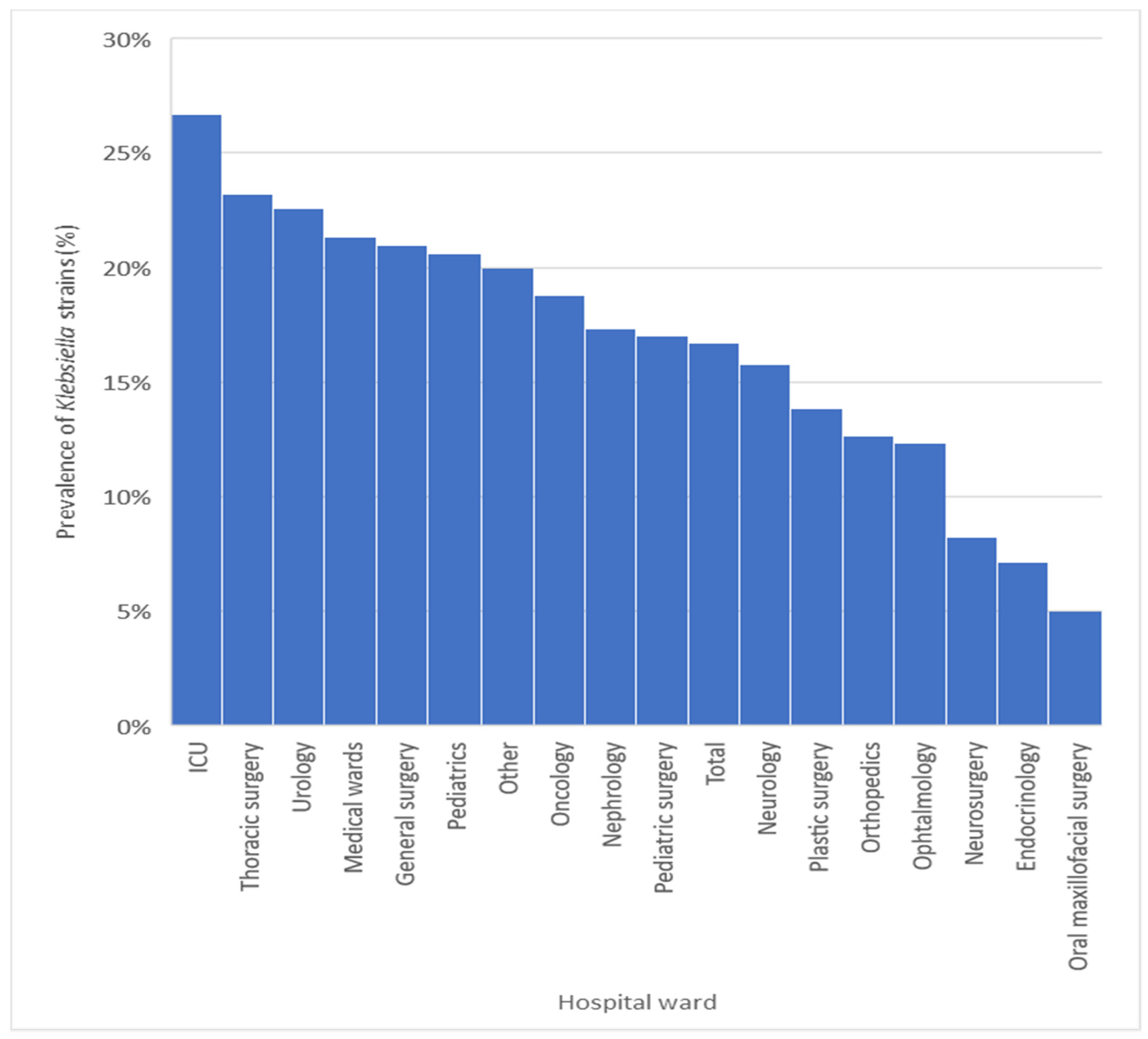
3.1. Prevalence of Klebsiella spp. Strains
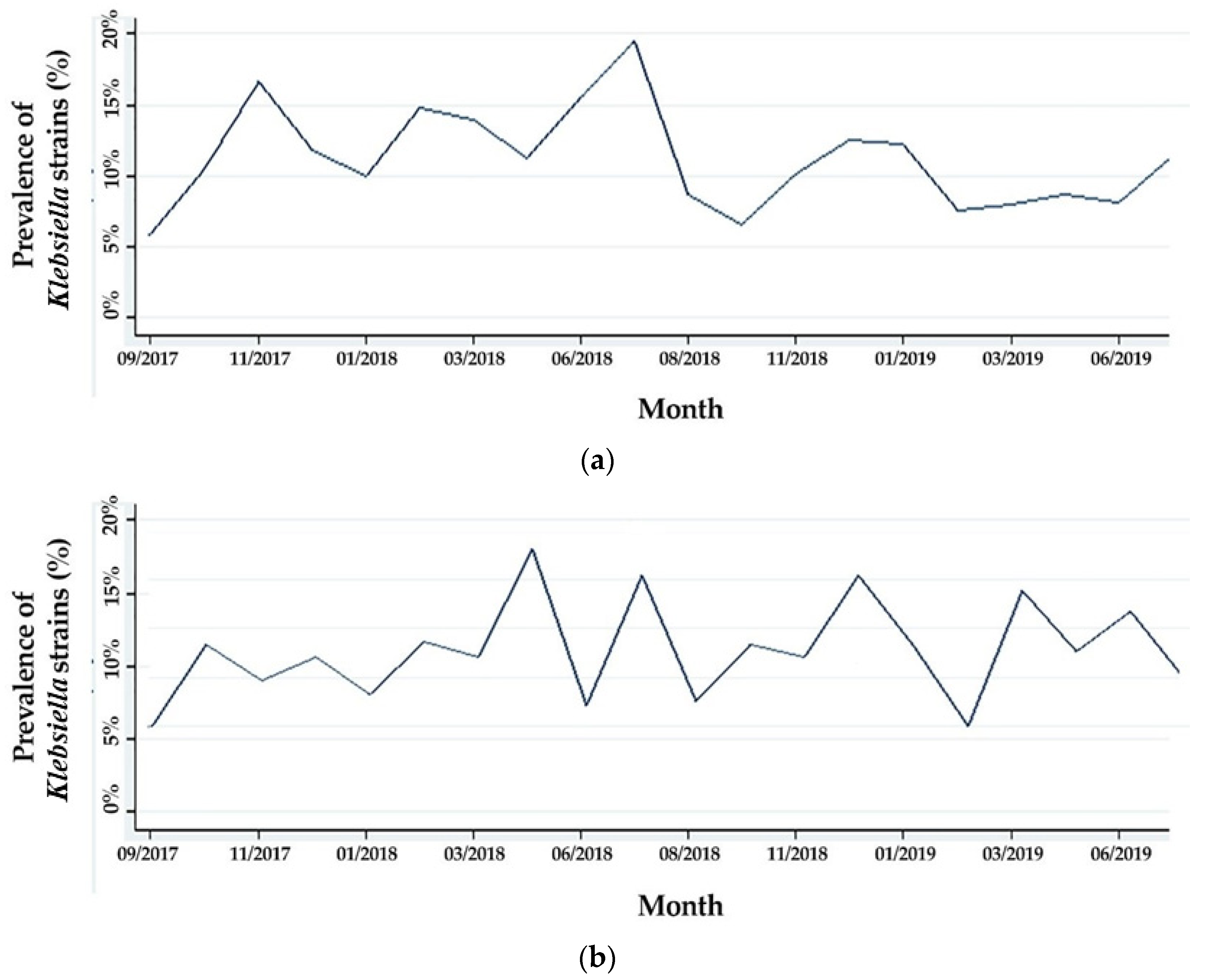
3.2. Evolution of the Prevalence of Klebsiella Strains
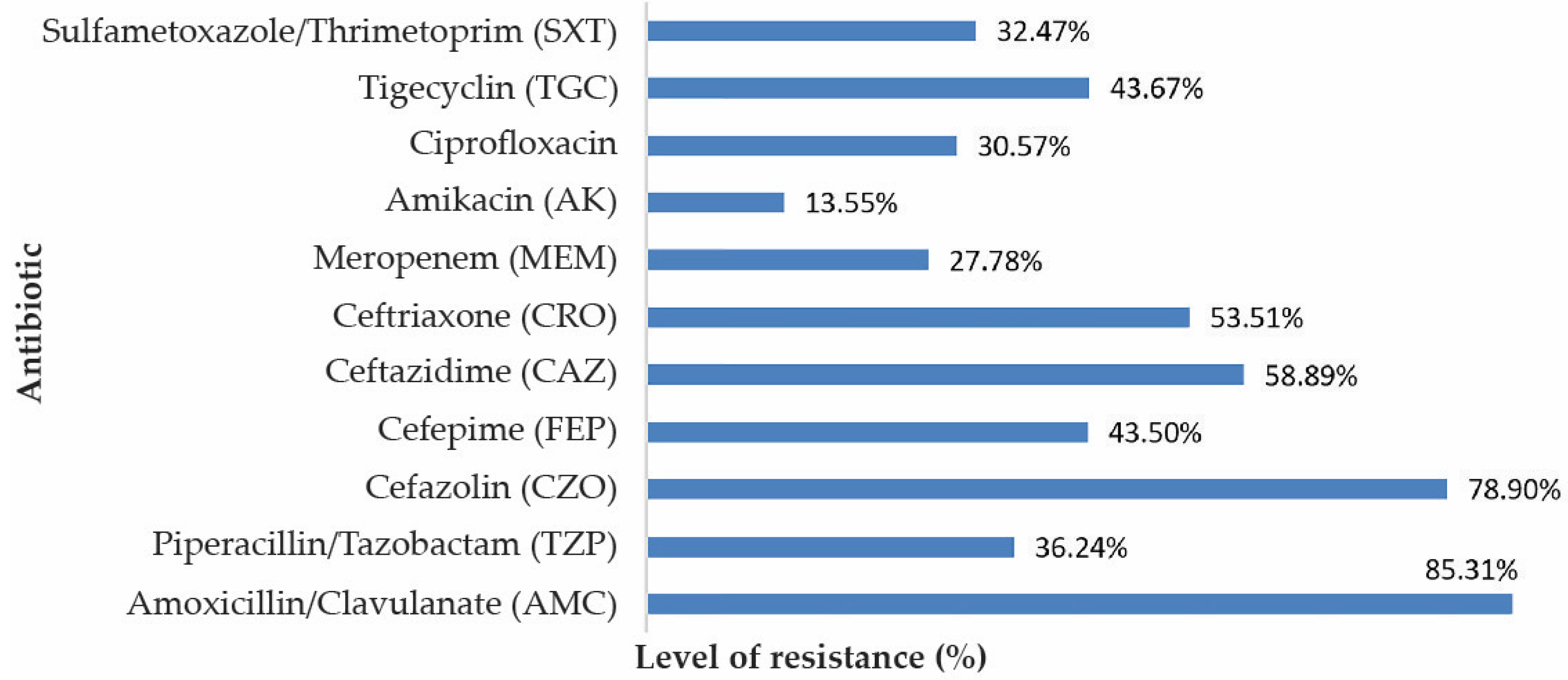
3.3. Resistance to Antibiotics of the Isolated Bacterial Species
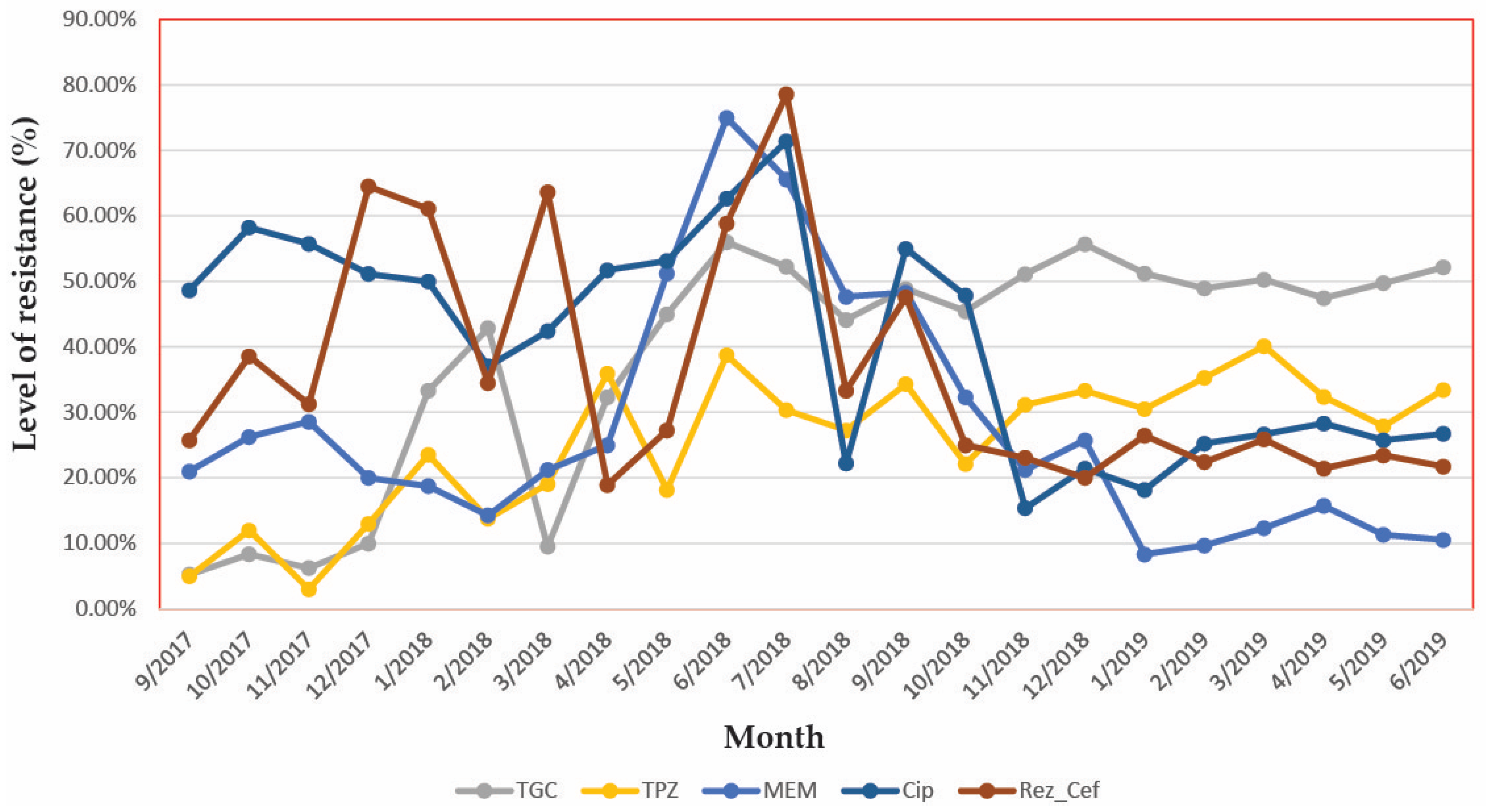
3.4. Analyse the Variation in Time of the Antibiotic Resistance
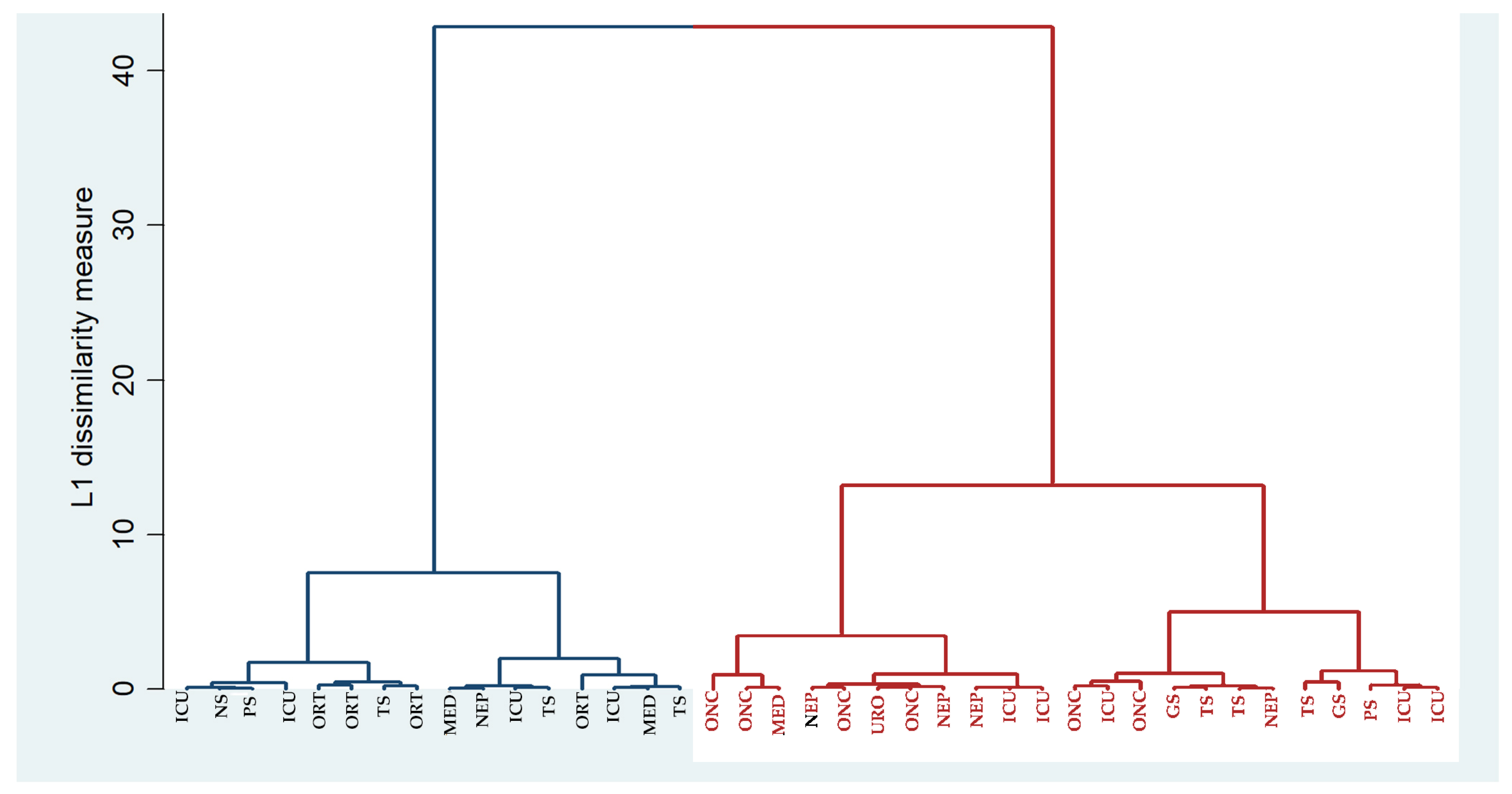
3.5. Analysis of Strains Relatedness Based on Clustering on MAR Index
3.6. Epidemiologic Tracing of the Antibiotic Resistance Phenotypes
4. Discussion
4.1. Prevalence of Klebsiella
4.2. Antibiotic Resistance of Klebsiella
4.3. The Link between the Antibiotic Resistance of Klebsiella and the Consumption of Antibiotics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Usha, P.T.A.; Jose, S.; Nisha, A.R. Antimicrobial Drug Resistance-A global concern. Vet. World 2010, 3, 138–139. [Google Scholar]
- Al-Tawfiq, J.A.; Tambyah, P.A. Healthcare associated infections (HAI) perspectives. J. Infect. Public Health 2014, 7, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; El Zowalaty, M.E.; Lundkvist, Å.; Järhult, J.D.; Khan Nayem, M.R.; Tanzin, A.Z.; Badsha, M.R.; Khan, S.A.; Ashour, H.M. Residual antimicrobial agents in food originating from animals. Trends Food Sci. Technol. 2021, 111, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Di Tella, D.; Tamburro, M.; Guerrizio, G.; Fanelli, I.; Sammarco, M.L.; Ripabelli, G. Molecular epidemiological insights into colistin-resistant and carbapenemases-producing clinical Klebsiella pneumoniae isolates. Infect. Drug Resist. 2019, 12, 3783–3795. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.J.; Moehring, R.W.; Sloane, R.; Schmader, K.E.; Weber, D.J.; Fowler, V.G.J.; Smathers, E.; Sexton, D.J. Bloodstream infections in community hospitals in the 21st century: A multicenter cohort study. PLoS ONE 2014, 9, e91713. [Google Scholar] [CrossRef] [Green Version]
- Zlatian, O.; Balasoiu, A.T.; Balasoiu, M.; Cristea, O.; Docea, A.O.; Mitrut, R.; Spandidos, D.A.; Tsatsakis, A.M.; Bancescu, G.; Calina, D. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp. Ther. Med. 2018, 16, 4499–4510. [Google Scholar] [CrossRef] [Green Version]
- Perl, T.M.; Cullen, J.J.; Wenzel, R.P.; Zimmerman, M.B.; Pfaller, M.A.; Sheppard, D.; Twombley, J.; French, P.P.; Herwaldt, L.A.; Team, M.A.T.R.O.S.A.S.; et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 2002, 346, 1871–1877. [Google Scholar] [CrossRef]
- Calina, D.; Staicus, C.; Rosu, L.; Rosu, A.F. Involvement of microbial flora in aetiology of surgical site infections. In Proceedings of the Clinical Pharmacy Congress, London, UK, 22–23 April 2016. [Google Scholar]
- Calina, D.; Roșu, L.; Roșu, A.F.; Ianoşi, G.; Ianoşi, S.; Zlatian, O.; Mitruț, R.; Docea, A.O.; Rogoveanu, O.; Mitruț, P.; et al. Etiological diagnosis and pharmacotherapeutic management of parapneumonic pleurisy. Farmacia 2016, 64, 946–952. [Google Scholar]
- Vading, M.; Nauclér, P.; Kalin, M.; Giske, C.G. Invasive infection caused by Klebsiella pneumoniae is a disease affecting patients with high comorbidity and associated with high long-term mortality. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.C.; Lee, J. The threat of carbapenem-resistant bacteria in the environment: Evidence of widespread contamination of reservoirs at a global scale. Environ. Pollut. 2019, 255, 113143. [Google Scholar] [CrossRef]
- Salehi, B.; Shivaprasad Shetty, M.; Anil Kumar, N.V.; Živković, J.; Calina, D.; Oana Docea, A.; Emamzadeh-Yazdi, S.; Sibel Kılıç, C.; Goloshvili, T.; Nicola, S.; et al. Veronica plants—Drifting from farm to traditional healing, food application, and phytopharmacology. Molecules 2019, 24, 2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungureanu, A.; Zlatian, O.; Mitroi, G.; Drocaş, A.; Ţîrcã, T.; Cǎlina, D.; Dehelean, C.; Docea, A.O.; Izotov, B.N.; Rakitskii, V.N.; et al. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol. Med. Rep. 2017, 16, 8771–8780. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [Green Version]
- Hendrik, T.C.; Voorintholt, A.F.; Vos, M.C. Clinical and molecular epidemiology of extended-spectrum beta-lactamase- Producing Klebsiella spp: A systematic review and meta-analyses. PLoS ONE 2015, 10, e140754. [Google Scholar] [CrossRef]
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic resistance in enterobacteriaceae: Mechanisms and clinical implications. BMJ 2016, 352. [Google Scholar] [CrossRef]
- Afunwa, R.A.; Odimegwu, D.C.; Iroha, R.I.; Esimone, C.O. Antimicrobial resistance status and prevalence rates of extended spectrum beta-lactamase producers isolated from a mixed human population. Bosn. J. Basic Med. Sci. 2011, 11, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Pournaras, S.; Protonotariou, E.; Voulgari, E.; Kristo, I.; Dimitroulia, E.; Vitti, D.; Tsalidou, M.; Maniatis, A.N.; Tsakris, A.; Sofianou, D. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 2009, 64, 348–352. [Google Scholar] [CrossRef]
- Galani, I.; Karaiskos, I.; Karantani, I.; Papoutsaki, V.; Maraki, S.; Papaioannou, V.; Kazila, P.; Tsorlini, H.; Charalampaki, N.; Toutouza, M.; et al. Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Eurosurveillance 2018, 23, 1700775. [Google Scholar] [CrossRef] [Green Version]
- Ripabelli, G.; Tamburro, M.; Guerrizio, G.; Fanelli, I.; Flocco, R.; Scutellà, M.; Sammarco, M.L. Tracking multidrug-resistant Klebsiella pneumoniae from an Italian Hospital: Molecular epidemiology and surveillance by PFGE, RAPD and PCR-Based resistance genes prevalence. Curr. Microbiol. 2018, 75, 977–987. [Google Scholar] [CrossRef]
- Phillips, I.K.S. Class I β-lactamases induction and derepression. Drugs 1989, 37, 402–407. [Google Scholar] [CrossRef]
- Davis, R.; Brown, P.D. Multiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J. Med. Microbiol. 2016, 65, 261–271. [Google Scholar] [CrossRef]
- Giacometti, A.; Siquini, F.M.; Cirioni, O.; Petroni, S.; Scalise, G. Imipenem and meropenem induced resistance to beta-lactam antibiotics in Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 315–318. [Google Scholar] [CrossRef]
- Rodloff, A.C.; Goldstein, E.J.C.; Torres, A. Two decades of imipenem therapy. J. Antimicrob. Chemother. 2006, 58, 916–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberoi, L.; Singh, N.; Sharma, P.; Aggarwal, A. ESBL, MBL and Ampc β Lactamases producing superbugs—Havoc in the Intensive Care Units of Punjab India. J. Clin. Diagn. Res. 2013, 7, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Feng, Y.S.; Sung, W.P.; Surampalli, R.Y. Quantification and analysis of airborne bacterial characteristics in a nursing care institution. J. Air Waste Manag. Assoc. 2011, 61, 732–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, P.W.; Sax, H.; Wolfensberger, A.; Clack, L.; Kuster, S.P. The preventable proportion of healthcare-associated infections 2005-2016: Systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 1277–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect. Control Hosp. Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef]
- Xu, J.; Shi, C.; Song, M.; Xu, X.; Yang, P.; Paoli, G.; Shi, X. Phenotypic and genotypic antimicrobial resistance traits of foodborne Staphylococcus aureus isolates from Shanghai. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, M.S.; Iriarte, A.; Reyes-Lamothe, R.; Sherratt, D.J.; Tolmasky, M.E. Small Klebsiella pneumoniae plasmids: Neglected contributors to antibiotic resistance. Front. Microbiol. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Schjørring, S.; Struve, C.; Krogfelt, K.A. Transfer of antimicrobial resistance plasmids from Klebsiella pneumoniae to Escherichia coli in the mouse intestine. J. Antimicrob. Chemother. 2008, 62, 1086–1093. [Google Scholar] [CrossRef] [Green Version]
- Călina, D.; Docea, A.O.; Rosu, L.; Zlatian, O.; Rosu, A.F.; Anghelina, F.; Rogoveanu, O.; Arsene, A.L.; Nicolae, A.C.; Drăgoi, C.M.; et al. Antimicrobial resistance development following surgical site infections. Mol. Med. Rep. 2017, 15, 681–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdal, J.E.; Bjørnholt, J.; Blomfeldt, A.; Smith-Erichsen, N.; Bukholm, G. Patterns and dynamics of airway colonisation in mechanically-ventilated patients. Clin. Microbiol. Infect. 2007, 13, 476–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzouvelekis, L.S.; Markogiannakis, A.; Piperaki, E.; Souli, M.; Daikos, G.L. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 2014, 20, 862–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef] [Green Version]
- Vatopoulos, A.C.; Kalapothaki, V.; Legakis, N.J. An electronic network for the surveillance of antimicrobial resistance in bacterial nosocomial isolates in Greece. Bull. World Health Organ. 1999, 77, 595–601. [Google Scholar]



| No. Samples | No. Strains | Percent | |
|---|---|---|---|
| Joint fluid | 8 | 0 | 0.00% |
| Bile | 5 | 0 | 0.00% |
| Blood | 130 | 21 | 16.15% |
| Catheter | 31 | 4 | 12.90% |
| Pleural fluid | 19 | 0 | 0.00% |
| Cerebrospinal fluid | 6 | 0 | 0.00% |
| Purulent secretion | 276 | 34 | 12.32% |
| Sputum | 128 | 35 | 27.34% |
| Urine | 1294 | 77 | 5.95% |
| Tracheal aspirate | 290 | 61 | 21.03% |
| Pharyngeal swab | 72 | 4 | 5.56% |
| Nasal swab | 38 | 5 | 13.16% |
| Puncture liquid | 120 | 3 | 2.50% |
| Conjunctival secretion | 32 | 4 | 12.50% |
| Ear discharges | 7 | 3 | 42.86% |
| total | 2456 | 251 | 10.22% |
| Staphylococcus aureus | E. coli | Klebsiella | NFR | |
|---|---|---|---|---|
| Staphylococcus aureus | 1 | |||
| E. coli | 0.3472 p = 0.1336 | 1 | ||
| Klebsiella | 0.4850 * p = 0.0302 | 0.2924 p = 0.2109 | 1 | |
| NFR | −0.1583 p = 0.5051 | 0.2769 p = 0.2372 | 0.1359 p = 0.5679 | 1 |
| Species | E. coli | Klebsiella | Ps. aeruginosa | NFR | Staphylococcus aureus |
|---|---|---|---|---|---|
| E. coli | r = 1.0000 | ||||
| Klebsiella | r = 0.5107 * p = 0.0214 | r = 1.0000 | |||
| Pseudomonas aeruginosa, | r = 0.3184 p = 0.1841 | r = 0.2755 p = 0.2536 | r = 1.0000 | ||
| NFR | r = 0.1906 p = 0.4209 | r = 0.1661 p = 0.4839 | r = 0.3599 p = 0.1302 | r = 1.0000 | |
| Staphylococcus aureus | r = 0.1972 p = 0.4046 | r = 0.3500 p = 0.1303 | r = −0.4089 * p = 0.0422 | r = 1.0000 |
| No. Patients | AK (g) | AMC (g) | CRO (g) | CZO (g) | FEP (g) | CIP (g) | CAZ (g) | SXT (g) | IPM (g) | MEM (g) | TPZ (g) | TGC (g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DDD/100 patient-days (WHO) | 1 | 3 | 2 | 3 | 1.8 | 0.5 | 0.24 | 1 | 2 | 2 | 14 | 0.1 | |
| ICU | 1646 | 1.01 | 0.9 | 12 | 3.5 | 1.27 | 0.16 | 0.11 | 0.35 | 1.83 | 6.5 | 23 | 0.04 |
| Medical wards | 1521 | 0.01 | 1.54 | 11.1 | 0.85 | 0 | 0.07 | 0.55 | 0 | 1.16 | 5.15 | 5.01 | 0.08 |
| Nephrology | 1003 | 0.08 | 0.7 | 16 | 1.49 | 1.25 | 0.03 | 0.08 | 0.36 | 2.83 | 7.4 | 12.29 | 0.02 |
| Neurology | 436 | 0.18 | 0.07 | 15 | 0 | 0.06 | 0.14 | 0.02 | 1.55 | 0 | 0.55 | 1.16 | 0 |
| Oncology | 804 | 0 | 0.95 | 9 | 0.44 | 0.29 | 0 | 0.04 | 0.07 | 0.32 | 2.47 | 0 | 0 |
| Cardiology | 219 | 0.09 | 12.9 | 16.17 | 0.13 | 0.3 | 0.49 | 0.4 | 0.25 | 2.45 | 2.54 | 1.24 | 0 |
| Pediatrics | 199 | 0.38 | 0.43 | 13.3 | 2.29 | 0 | 0.08 | 1.45 | 0 | 10.14 | 1.01 | 1.75 | 0 |
| Pediatric surgery | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neurosurgery | 1768 | 0.49 | 0.84 | 26 | 1.6 | 0.59 | 0.03 | 0.2 | 0 | 0 | 12.4 | 6.7 | 0 |
| Ophthalmology | 1696 | 0 | 0.15 | 15.8 | 0.04 | 0 | 0 | 0.11 | 0 | 0.05 | 0.21 | 0.77 | 0 |
| Orthopaedics | 2824 | 0 | 0.01 | 15.9 | 0.008 | 0.08 | 0.47 | 0 | 0 | 0 | 0.27 | 0 | 0 |
| Urology | 1927 | 0.98 | 0.06 | 18.8 | 0 | 0.03 | 0.02 | 0.58 | 0.06 | 3.63 | 1.17 | 0 | 0.003 |
| General surgery | 1329 | 0.6 | 0 | 17.6 | 6.32 | 0.009 | 0.12 | 0.12 | 1.7 | 1.44 | 3.61 | 1.49 | 0.003 |
| Thoracic surgery | 1131 | 0.52 | 0.12 | 19.3 | 0 | 2.28 | 0.26 | 0.55 | 0.08 | 0.25 | 4.22 | 9.75 | 0 |
| Plastic surgery | 34 | 0.39 | 0 | 15.5 | 0 | 0 | 1.3 | 0.33 | 0.07 | 0.35 | 0.6 | 1.86 | 0 |
| Oral maxillofacial surgery | 287 | 0 | 0 | 0 | 0 | 0 | 0 | 0.44 | 0 | 0 | 0 | 3.18 | 0 |
| Consumption of Antibiotics | Standardized Coefficient | Std. Err. | t | p | 95% Confidence Interval |
|---|---|---|---|---|---|
| Ciprofloxacin | −0.0021 | 0.0008975 | −2.34 | 0.101 | [−0.0049579, 0.0007546] |
| Tigecycline | 0.0118279 | 0.0032538 | 3.64 | 0.036 * | [0.001473, 0.0221829] |
| Piperacillin/ Tazobactam | −0.0027939 | 0.0008836 | −3.16 | 0.050 * | [−0.0056061, 0.0000182] |
| Meropenem | 0.003232 | 0.000955 | 3.38 | 0.043 * | [0.0001927, 0.0062714] |
| _constant | −0.3240754 | 0.1989285 | −1.63 | 0.202 | [−0.9571546, 0.3090037] |
| Outcome Used | R Sq. | Predictors in the Model | Coefficient | 95% Confidence Interval | p |
|---|---|---|---|---|---|
| Resistance to Meropenem | 0.627 | Consumption of Imipenem 1 month before | 0.0243 | [−0.0043, 0.0529] | 0.078 |
| Consumption of Meropenem 1 month before | −0.0016 | [−0.0057, 0.0025] | 0.341 | ||
| Resistance to Meropenem | 0.753 | Consumption of Imipenem 2 months before | 0.0075 | [0.0011, 0.0130] | 0.032 * |
| Consumption of Meropenem 2 months before | −0.0042 | [−0.0077, 0.0081] | 0.027 | ||
| Resistance to Ciprofloxacin | 0.753 | Consumption of Imipenem 2 months before | 0.0010 | [0.0007, 0.0014] | <0.001 * |
| Consumption of Meropenem 2 months before | −0.0002 | [−0.0001, 0.0010] | 0.094 | ||
| Consumption of Ciprofloxacin 2 months before | 0.0004 | [0.0001, 0.0007] | 0.0047 * | ||
| Resistance to Ciprofloxacin | 0.537 | Consumption of Imipenem 3 months before | 0.0064 | [0.0004, 0.0009] | <0.001 |
| Consumption of Meropenem 3 months before | 0.0001 | [−0.0002, 0.0004] | 0.571 | ||
| Consumption of Ciprofloxacin 3 months before | −0.0001 | [−0.0004, 0.0001] | 0.330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghenea, A.E.; Cioboată, R.; Drocaş, A.I.; Țieranu, E.N.; Vasile, C.M.; Moroşanu, A.; Țieranu, C.G.; Salan, A.-I.; Popescu, M.; Turculeanu, A.; et al. Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania. Antibiotics 2021, 10, 868. https://doi.org/10.3390/antibiotics10070868
Ghenea AE, Cioboată R, Drocaş AI, Țieranu EN, Vasile CM, Moroşanu A, Țieranu CG, Salan A-I, Popescu M, Turculeanu A, et al. Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania. Antibiotics. 2021; 10(7):868. https://doi.org/10.3390/antibiotics10070868
Chicago/Turabian StyleGhenea, Alice Elena, Ramona Cioboată, Andrei Ioan Drocaş, Eugen Nicolae Țieranu, Corina Maria Vasile, Aritina Moroşanu, Cristian George Țieranu, Alex-Ioan Salan, Mihaela Popescu, Adriana Turculeanu, and et al. 2021. "Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania" Antibiotics 10, no. 7: 868. https://doi.org/10.3390/antibiotics10070868
APA StyleGhenea, A. E., Cioboată, R., Drocaş, A. I., Țieranu, E. N., Vasile, C. M., Moroşanu, A., Țieranu, C. G., Salan, A. -I., Popescu, M., Turculeanu, A., Padureanu, V., Udriștoiu, A. -L., Calina, D., Cȃrţu, D., & Zlatian, O. M. (2021). Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania. Antibiotics, 10(7), 868. https://doi.org/10.3390/antibiotics10070868











