Biofilm Time-Kill Curves to Assess the Bactericidal Activity of Daptomycin Combinations against Biofilm-Producing Vancomycin-Resistant Enterococcus faecium and faecalis
Abstract
:1. Introduction
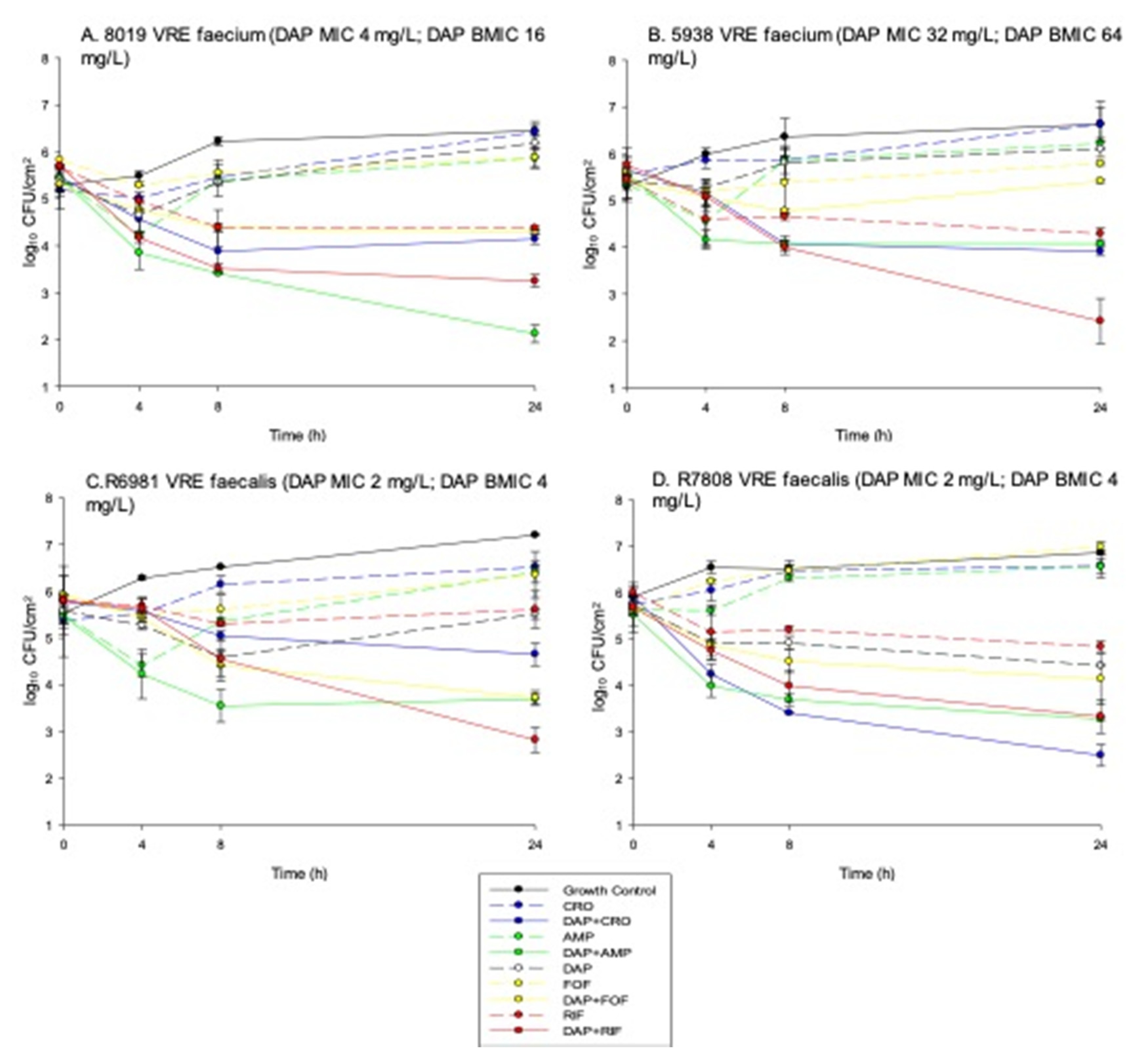
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antimicrobials
4.3. Media
4.4. Susceptibility Testing
4.5. Biofilm Time-Kill Evaluations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karklsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control. Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, A.; Wise, M.; Bell, M.; Cardo, D.; Edwards, J.; Fridkin, S.; Jernigan, J.; Kallen, A.; McDonald, L.C.; Patel, P.R.; et al. Vital Signs: Central Line-Associated Blood Stream Infections—United States, 2001, 2008, and 2009; MMWR Morbidity and Mortality Weekly Report 8; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2011; pp. 243–248. [Google Scholar]
- Parra-Ruiz, J.; Vidaillac, C.; Rose, W.E.; Rybak, M.J. Activities of high-dose daptomycin, vancomycin, and moxifloxacin alone or in combination with clarithromycin or rifampin in a novel in vitro model of Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2010, 10, 4329–4334. [Google Scholar] [CrossRef] [Green Version]
- Goeres, D.M.; Loetterle, L.R.; Hamilton, M.A.; Murga, R.; Kirby, D.W.; Donlan, R.M. Statistical assessment of a laboratory method for growing biofilms. Microbiology 2005, 3, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [Green Version]
- Stewart, P.S.; Davison, W.M.; Steenbergen, J.N. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 2009, 53, 3505–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sader, H.S.; Moet, G.J.; Farrell, D.J.; Jones, R.N. Antimicrobial susceptibility of daptomycin and comparator agents tested against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: Trend analysis of a 6-year period in US medical centers (2005–2010). Diagn. Microbiol. Infect. Dis. 2011, 70, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Stewart, P.S. Penetration of rifampin through Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2002, 46, 900–903. [Google Scholar] [CrossRef] [Green Version]
- Dunne, W.M., Jr.; Mason, E.O., Jr.; Kaplan, S.L. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 1993, 37, 2522–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, K.E.; Werth, B.J.; McRoberts, J.P.; Rybak, M.J. A novel approach utilizing biofilm time-kill curves to assess the bactericidal activity of ceftaroline combinations against biofilm-producing methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 2989–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, K.E.; King, S.T.; Stover, K.R.; Pogue, J.M. Therapeutic options for vancomycin-resistant enterococcal bacteremia. Expert Rev. Anti. Infect. Ther. 2015, 3, 363–377. [Google Scholar] [CrossRef]
- Barber, K.E.; Werth, B.J.; Rybak, M.J. The combination of ceftaroline plus daptomycin allows for therapeutic de-escalation and daptomycin sparing against MRSA. J. Antimicrob. Chemother. 2015, 70, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Berti, A.D.; Sakoulas, G.; Nizet, V.; Tewhey, R.; Rose, W.E. Beta-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 5005–5012. [Google Scholar] [CrossRef] [Green Version]
- Hall Snyder, A.D.; Werth, B.J.; Nonejuie, P.; McRoberts, J.P.; Pogliano, J.; Sakoulas, G.; Yim, J.; Singh, N.; Rybak, M.J. Fosfomycin Enhances the Activity of Daptomycin against Vancomycin-Resistant Enterococci in an In Vitro Pharmacokinetic-Pharmacodynamic Model. Antimicrob. Agents Chemother. 2016, 60, 5716–5723. [Google Scholar] [CrossRef] [Green Version]
- Sakoulas, G.; Bayer, A.S.; Pogliano, J.; Tsuji, B.T.; Yang, S.J.; Mishra, N.N.; Nizet, V.; Yeaman, M.R.; Moise, P.A. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2012, 56, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Hoffman, M.; Iznaola, O.; Goodwin, M.; Mohr, J. Combination therapy with ampicillin and daptomycin for treatment of Enterococcus faecalis endocarditis. Antimicrob. Agents Chemother. 2012, 56, 6064. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.R.; Barber, K.E.; Raut, A.; Rybak, M.J. β-Lactams enhance daptomycin activity against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium in In Vitro pharmacokinetic/pharmacodynamic models. Antimicrob. Agents Chemother. 2015, 59, 2842–2848. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.R.; Barber, K.E.; Raut, A.; Aboutaleb, M.; Sakoulas, G.; Rybak, M.J. β-Lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother. 2015, 70, 1738–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, K.E.; Smith, J.R.; Ireland, C.E.; Boles, B.R.; Rose, W.E.; Rybak, M.J. Evaluation of Ceftaroline Alone and in Combination against Biofilm-Producing Methicillin-Resistant Staphylococcus aureus with Reduced Susceptibility to Daptomycin and Vancomycin in an In Vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2015, 59, 4497–4503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, L.B.; Eliopoulos, C.T.; Yao, J.D.; Eliopoulos, G.M.; Moellering, R.C., Jr. In vivo activity of the combination of daptomycin and fosfomycin compared with daptomycin alone against a strain of Enterococcus faecalis with high-level gentamicin resistance in the rat endocarditis model. Diagn. Microbiol. Infect. Dis. 1992, 15, 173–176. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.; Morck, D.; Storey, D.; Read, R.; Buret, A.; Olson, B. The MBEC Assay System: Multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 2001, 337, 377–385. [Google Scholar] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| MIC (mg/L) | |||||||||
| Strain | DAP | AMP | CRO | FOF | RIF | DAP + AMP | DAP + CRO | DAP + FOF | DAP + RIF |
| E. faecalis | |||||||||
| R6981 | 2 | 512 | >1024 | 64 | 0.0156 | 0.125 | 0.125 | 0.5 | 0.5 |
| R7808 | 2 | >64 | >1024 | 64 | <0.0078 | 0.25 | 1 | 0.5 | 0.25 |
| R6797 | 1 | 2 | >64 | 64 | 2 | 0.25 | 1 | 0.0625 | 0.5 |
| R6798 | 1 | 1 | >64 | 64 | 2 | 0.25 | 0.5 | <0.016 | 1 |
| R6799 | 2 | 2 | >64 | 64 | 2 | 0.25 | 1 | 0.0625 | 0.5 |
| E. faecium | |||||||||
| 8019 | 4 | 4 | 32 | 64 | 0.5 | 0.125 | 0.0625 | 1 | 0.5 |
| 5938 | 32 | 16 | 64 | 64 | 0.0625 | 0.0625 | <0.031 | 8 | 8 |
| R1026 | 1 | 32 | >64 | >64 | <0.0156 | 0.5 | 1 | 0.125 | 0.25 |
| R1027 | 2 | 32 | >64 | 64 | 4 | 1 | 1 | 0.25 | 0.25 |
| R1028 | 4 | 32 | >64 | >64 | 32 | 1 | 1 | 0.5 | 0.015 |
| Biofilm MIC (mg/L) | |||||||||
| Strain | DAP | AMP | CRO | FOF | RIF | DAP + AMP | DAP + CRO | DAP + FOF | DAP + RIF |
| E. faecalis | |||||||||
| R6981 | 4 | >64 | >64 | >64 | 0.03125 | 0.25 | 0.5 | 0.25 | 0.25 |
| R7808 | 4 | >64 | >64 | >64 | 0.0156 | 0.25 | 1 | 0.5 | 0.5 |
| R6797 | 8 | >64 | >64 | >64 | 2 | 1 | 4 | 1 | 8 |
| R6798 | 8 | >64 | >64 | >64 | 2 | 2 | 4 | 2 | 8 |
| R6799 | 8 | >64 | >64 | >64 | 1 | 2 | 4 | 1 | 8 |
| E. faecium | |||||||||
| 8019 | 16 | >64 | >64 | >64 | 1 | 1 | 2 | 0.5 | 2 |
| 5938 | 64 | >64 | >64 | >64 | 0.25 | 4 | 8 | 8 | 16 |
| R1026 | 8 | 64 | >64 | 32 | 0.0156 | 1 | 1 | 1 | 0.25 |
| R1027 | 2 | 64 | >64 | 32 | <0.031 | 1 | 1 | 1 | 0.25 |
| R1028 | 8 | >64 | >64 | >64 | >64 | 1 | 1 | 1 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barber, K.E.; Shammout, Z.; Smith, J.R.; Kebriaei, R.; Morrisette, T.; Rybak, M.J. Biofilm Time-Kill Curves to Assess the Bactericidal Activity of Daptomycin Combinations against Biofilm-Producing Vancomycin-Resistant Enterococcus faecium and faecalis. Antibiotics 2021, 10, 897. https://doi.org/10.3390/antibiotics10080897
Barber KE, Shammout Z, Smith JR, Kebriaei R, Morrisette T, Rybak MJ. Biofilm Time-Kill Curves to Assess the Bactericidal Activity of Daptomycin Combinations against Biofilm-Producing Vancomycin-Resistant Enterococcus faecium and faecalis. Antibiotics. 2021; 10(8):897. https://doi.org/10.3390/antibiotics10080897
Chicago/Turabian StyleBarber, Katie E., Zade Shammout, Jordan R. Smith, Razieh Kebriaei, Taylor Morrisette, and Michael J. Rybak. 2021. "Biofilm Time-Kill Curves to Assess the Bactericidal Activity of Daptomycin Combinations against Biofilm-Producing Vancomycin-Resistant Enterococcus faecium and faecalis" Antibiotics 10, no. 8: 897. https://doi.org/10.3390/antibiotics10080897
APA StyleBarber, K. E., Shammout, Z., Smith, J. R., Kebriaei, R., Morrisette, T., & Rybak, M. J. (2021). Biofilm Time-Kill Curves to Assess the Bactericidal Activity of Daptomycin Combinations against Biofilm-Producing Vancomycin-Resistant Enterococcus faecium and faecalis. Antibiotics, 10(8), 897. https://doi.org/10.3390/antibiotics10080897






