Antimicrobial Activity of the Circular Bacteriocin AS-48 against Clinical Multidrug-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Collection and Identification
2.2. Antimicrobial-Resistance Profile of the Isolates
2.3. Antimicrobial Activity of AS-48 Alone and in Combination with Lysozyme
2.4. Effect of AS-48 on S. aureus Biofilms
2.4.1. Biofilms Formation by S. aureus Isolates
2.4.2. Effect of Bacteriocin AS-48 against S. aureus Biofilms
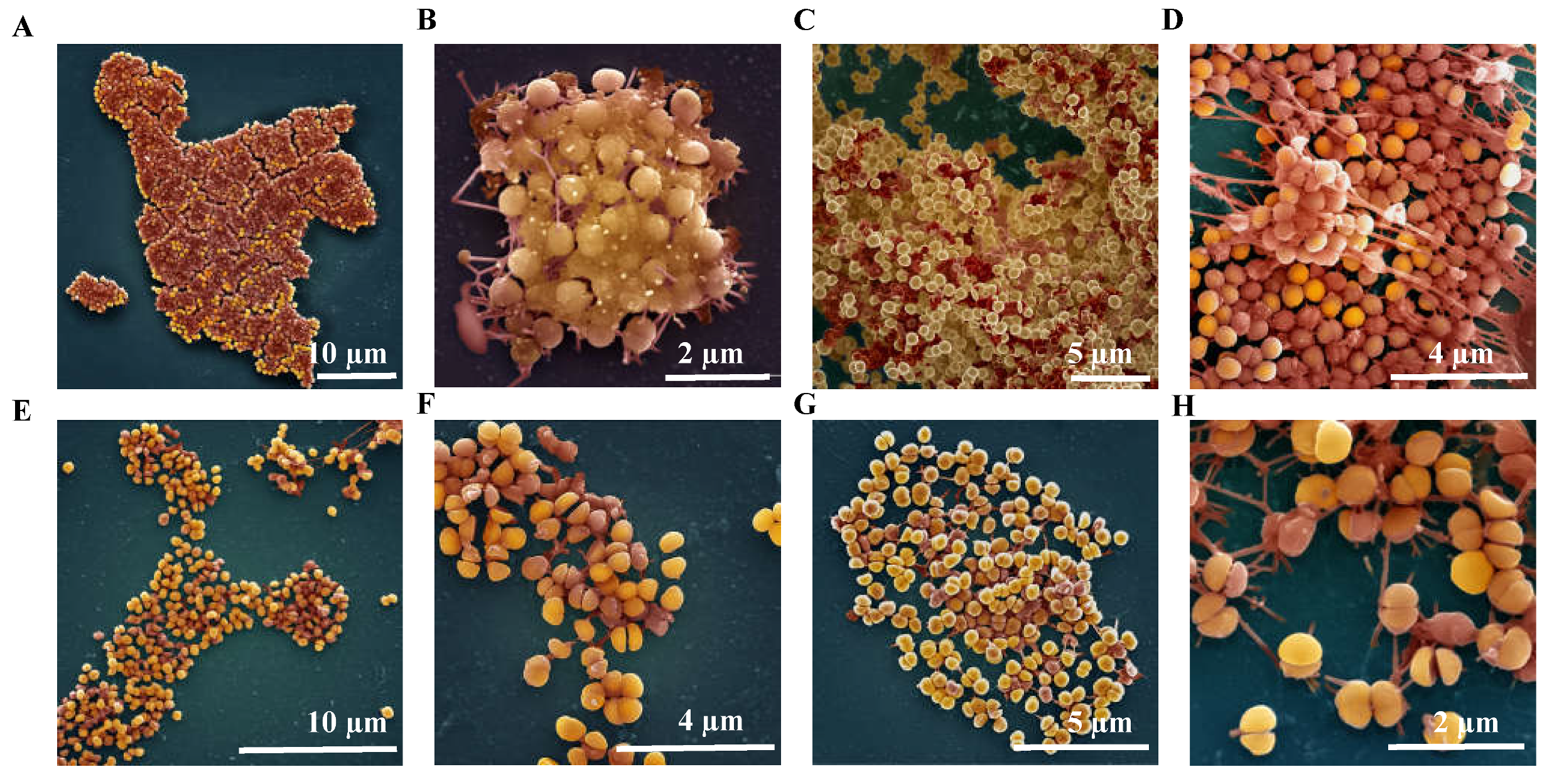
2.4.3. Ultrastructure of S. aureus Biofilms Treated with AS-48
3. Materials and Methods
3.1. Bacterial Isolation and Growth Conditions
3.2. Genotyping and Molecular Identification: RAPD-Analysis and 16S rDNA Gene Sequencing
3.3. Enterocin AS-48 Purification
3.4. Determination of the Minimal Inhibitory Concentration (MIC)
3.5. Antimicrobial Activity of AS-48 on S. aureus Biofilms
3.5.1. Biofilm Formation by the Staphylococci
3.5.2. Visualization under the Scanning Electron Microscope (SEM) of Effects Caused by AS-48 on S. aureus Biofilms
3.5.3. Studies of the Effects Caused by AS-48 on the Viability of S. aureus Cells Imbibed in Biofilms
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Ryder, V.J.; Chopra, I.; O’Neill, A.J. Increased Mutability of Staphylococci in Biofilms as a Consequence of Oxidative Stress. PLoS ONE 2012, 7, e47695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus Aureus Biofilms Promote Horizontal Transfer of Antibiotic Resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control Antimicrobial Resistance in the EU/EEA(EARS-Net)—Annual Epidemiological Report 2019. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data?f%5B0%5D=output_types%3A1256 (accessed on 18 June 2021).
- Sampedro, G.R.; Bubeck Wardenburg, J. Staphylococcus Aureus in the Intensive Care Unit: Are These Golden Grapes Ripe for a New Approach? J. Infect. Dis. 2017, 215, S64–S70. [Google Scholar]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-Resistant Staphylococcus Aureus (MRSA): Antibiotic-Resistance and the Biofilm Phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus Aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Brown, P.D.; Ngeno, C. Antimicrobial Resistance in Clinical Isolates of Staphylococcus Aureus from Hospital and Community Sources in Southern Jamaica. Int. J. Infect. Dis. 2007, 11, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H. Methicillin (Oxacillin)-Resistant Staphylococcus Aureus Strains Isolated from Major Food Animals and Their Potential Transmission to Humans. Appl. Environ. Microbiol. 2003, 69, 6489–6494. [Google Scholar] [CrossRef] [Green Version]
- Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017.
- Geitani, R.; Ayoub Moubareck, C.; Touqui, L.; Karam Sarkis, D. Cationic Antimicrobial Peptides: Alternatives and/or Adjuvants to Antibiotics Active against Methicillin-Resistant Staphylococcus Aureus and Multidrug-Resistant Pseudomonas Aeruginosa. BMC Microbiol. 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The Microbiome-Shaping Roles of Bacteriocins. Nat. Rev. Microbiol. 2021, 1–14. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Ciumac, D.; Gong, H.; Hu, X.; Lu, J.R. Membrane Targeting Cationic Antimicrobial Peptides. J. Colloid Interface Sci. 2019, 537, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hidalgo, M.; Montalbán-López, M.; Cebrián, R.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. AS-48 Bacteriocin: Close to Perfection. Cell. Mol. Life Sci. 2011, 68, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, R.; Arévalo, S.; Rubiño, S.; Arias-Santiago, S.; Rojo, M.D.; Montalbán-López, M.; Martínez-Bueno, M.; Valdivia, E.; Maqueda, M. Control of Propionibacterium Acnes by Natural Antimicrobial Substances: Role of the Bacteriocin AS-48 and Lysozyme. Sci. Rep. 2018, 8, 11766. [Google Scholar] [CrossRef] [Green Version]
- Perales-Adan, J.; Rubiño, S.; Martinez-Bueno, M.; Valdivia, E.; Montalban-Lopez, M.; Cebrian, R.; Maqueda, M. LAB Bacteriocins Controlling the Food Isolated (Drug-Resistant) Staphylococci. Front. Microbiol. 2018, 9, 1143. [Google Scholar] [CrossRef]
- Ananou, S.; Gálvez, A.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Synergistic Effect of Enterocin AS-48 in Combination with Outer Membrane Permeabilizing Treatments against Escherichia Coli O157:H7. J. Appl. Microbiol. 2005, 99, 1364–1372. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Cebrián, R.; Maqueda, M.; Romero, D.; Rosales, M.J.; Sánchez-Moreno, M.; Marín, C. Assessing the Effectiveness of AS-48 in Experimental Mice Models of Chagas’ Disease. J. Antimicrob. Chemother. 2020, 75, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Abengózar, M.Á.; Cebrián, R.; Saugar, J.M.; Gárate, T.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M.; Rivas, L. Enterocin AS-48 as Evidence for the Use of Bacteriocins as New Leishmanicidal Agents. Antimicrob. Agents Chemother. 2017, 61, e02288-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-García, M.; Bart, J.-M.; Campos-Salinas, J.; Valdivia, E.; Martínez-Bueno, M.; González-Rey, E.; Navarro, M.; Maqueda, M.; Cebrián, R.; Pérez-Victoria, J.M. Autophagic-Related Cell Death of Trypanosoma Brucei Induced by Bacteriocin AS-48. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 203–212. [Google Scholar] [CrossRef]
- Cebrián, R.; Rodríguez-Cabezas, M.E.; Martín-Escolano, R.; Rubiño, S.; Garrido-Barros, M.; Montalbán-López, M.; Rosales, M.J.; Sánchez-Moreno, M.; Valdivia, E.; Martínez-Bueno, M.; et al. Preclinical Studies of Toxicity and Safety of the AS-48 Bacteriocin. J. Adv. Res. 2019, 20, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Baños, A.; García, J.D.; Núñez, C.; Mut-Salud, N.; Ananou, S.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Subchronic Toxicity Study in BALBc Mice of Enterocin AS-48, an Anti-Microbial Peptide Produced by Enterococcus Faecalis UGRA10. Food Chem. Tox. 2019, 132, 110667. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Pérez-Vázquez, M.; Oliver, A.; Sánchez Del Saz, B.; Gutiérrez, M.O.; Martínez-Ferrer, M.; Baquero, F. Evaluation of the Wider System, a New Computer-Assisted Image-Processing Device for Bacterial Identification and Susceptibility Testing. J. Clin. Microbiol. 2000, 38, 1339–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.J. Antibiotic Resistance in Staphylococcus Aureus. Current Status and Future Prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Vogelaers, D.; Blot, S. The Rising Problem of Antimicrobial Resistance in the Intensive Care Unit. Ann. Intensive Care 2011, 1, 47. [Google Scholar] [CrossRef] [Green Version]
- Junie, L.M.; Homorodean, D. Methicillin-Resistant Staphylococcus Aureus (MRSA) and MLSB (Macrolide-Lincosamide-Stretogramin B) Isolated from Community and Hospital—Associated Infections (Ca/Ha-MRSA). Int. J. Infect. Dis. 2008, 12, e272–e273. [Google Scholar] [CrossRef] [Green Version]
- Mahdiyoun, S.M.; Kazemian, H.; Ahanjan, M.; Houri, H.; Goudarzi, M. Frequency of Aminoglycoside-Resistance Genes in Methicillin-Resistant Staphylococcus Aureus (MRSA) Isolates from Hospitalized Patients. Jundishapur J. Microbiol. 2016, 9, e35052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, R.; Okubo, T. Aminoglycosides resistance of methicillin-resistant Staphylococcus aureus. Nihon Rinsho 1992, 50, 1036–1041. [Google Scholar] [PubMed]
- Adhikari, R.P.; Shrestha, S.; Barakoti, A.; Amatya, R. Inducible Clindamycin and Methicillin Resistant Staphylococcus Aureus in a Tertiary Care Hospital, Kathmandu, Nepal. BMC Infect. Dis. 2017, 17, 483. [Google Scholar] [CrossRef] [PubMed]
- Epaud, R.; Delestrain, C.; Weaver, T.E.; Akinbi, H.T. Bacterial Killing Is Enhanced by Exogenous Administration of Lysozyme in the Lungs. Respir. Med. Res. 2019, 76, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pérez, C.; Gracia, B.; Rodrigues, L.; Vitoria, A.; Cebrián, R.; Deboosère, N.; Song, O.; Brodin, P.; Maqueda, M.; Aínsa, J.A. Synergy between Circular Bacteriocin AS-48 and Ethambutol against Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e00359-18. [Google Scholar] [CrossRef] [Green Version]
- Ananou, S.; Rivera, S.; Madrid, M.I.; Maqueda, M.; Martínez-Bueno, M.; Valdivia, E. Application of Enterocin AS-48 as Biopreservative in Eggs and Egg Fractions: Synergism through Lysozyme. LWT 2018, 89, 409–417. [Google Scholar] [CrossRef]
- Parastan, R.; Kargar, M.; Solhjoo, K.; Kafilzadeh, F. Staphylococcus Aureus Biofilms: Structures, Antibiotic Resistance, Inhibition, and Vaccines. Gene Rep. 2020, 20, 100739. [Google Scholar] [CrossRef]
- Lin, Q.; Sun, H.; Yao, K.; Cai, J.; Ren, Y.; Chi, Y. The Prevalence, Antibiotic Resistance and Biofilm Formation of Staphylococcus Aureus in Bulk Ready-To-Eat Foods. Biomolecules 2019, 9, 524. [Google Scholar] [CrossRef] [Green Version]
- Lamret, F.; Colin, M.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Antibiotic Tolerance of Staphylococcus Aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics 2020, 9, 547. [Google Scholar] [CrossRef]
- Conlon, B.P. Staphylococcus Aureus Chronic and Relapsing Infections: Evidence of a Role for Persister Cells: An Investigation of Persister Cells, Their Formation and Their Role in S. Aureus Disease. Bioessays 2014, 36, 991–996. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Valle, J.; Solano, C.; Arrizubieta, M.J.; Cucarella, C.; Lamata, M.; Amorena, B.; Leiva, J.; Penadés, J.R.; Lasa, I. The Enterococcal Surface Protein, Esp, Is Involved in Enterococcus Faecalis Biofilm Formation. Appl. Environ. Microbiol. 2001, 67, 4538–4545. [Google Scholar] [CrossRef] [Green Version]
- Zmantar, T.; Kouidhi, B.; Miladi, H.; Mahdouani, K.; Bakhrouf, A. A Microtiter Plate Assay for Staphylococcus Aureus Biofilm Quantification at Various PH Levels and Hydrogen Peroxide Supplementation. New Microbiol. 2010, 33, 137–145. [Google Scholar]
- Reffuveille, F.; Josse, J.; Vallé, Q.; Mongaret, C.; Gangloff, S.C. Staphylococcus Aureus Biofilms and Their Impact on the Medical Field; IntechOpen: London, UK, 2017; ISBN 978-953-51-2984-4. [Google Scholar]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranjec, C.; Ovchinnikov, K.V.; Grønseth, T.; Ebineshan, K.; Srikantam, A.; Diep, D.B. A Bacteriocin-Based Antimicrobial Formulation to Effectively Disrupt the Cell Viability of Methicillin-Resistant Staphylococcus Aureus (MRSA) Biofilms. NPJ Biofilms Microbiomes 2020, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Fighting Biofilms with Lantibiotics and Other Groups of Bacteriocins. NPJ Biofilms Microbiomes 2018, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Montalbán-López, M.; Scott, T.A.; Ramesh, S.; Rahman, I.R.; van Heel, A.J.; Viel, J.H.; Bandarian, V.; Dittmann, E.; Genilloud, O.; Goto, Y.; et al. New Developments in RiPP Discovery, Enzymology and Engineering. Nat. Prod. Rep. 2020, 38, 130–239. [Google Scholar] [CrossRef] [PubMed]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization of Tetrazolium Salt Assay for Pseudomonas Aeruginosa Biofilm Using Microtiter Plate Method. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Anderson, R.C.; Haverkamp, R.G.; Yu, P.-L. Investigation of Morphological Changes to Staphylococcus Aureus Induced by Ovine-Derived Antimicrobial Peptides Using TEM and AFM. FEMS Microbiol. Lett. 2004, 240, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Grande Burgos, M.J.; Kovács, Á.T.; Mirończuk, A.M.; Abriouel, H.; Gálvez, A.; Kuipers, O.P. Response of Bacillus Cereus ATCC 14579 to Challenges with Sublethal Concentrations of Enterocin AS-48. BMC Microbiol. 2009, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Escolano, R.; Cebrián, R.; Martín-Escolano, J.; Rosales, M.J.; Maqueda, M.; Sánchez-Moreno, M.; Marín, C. Insights into Chagas Treatment Based on the Potential of Bacteriocin AS-48. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Valdivia, E.; Maqueda, M.; Martínez-Bueno, M. Fast, Convenient, and Economical Method for Isolating Genomic DNA from Lactic Acid Bacteria Using a Modification of the Protein “Salting-out” Procedure. Anal. Biochem. 2007, 366, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Montalbán-López, M.; Cebrián, R.; Galera, R.; Mingorance, L.; Martín-Platero, A.M.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci. Antibiotics 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Ogier, J.-C.; Son, O.; Gruss, A.; Tailliez, P.; Delacroix-Buchet, A. Identification of the Bacterial Microflora in Dairy Products by Temporal Temperature Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2002, 68, 3691–3701. [Google Scholar] [CrossRef] [Green Version]
- Cebrián, R.; Baños, A.; Valdivia, E.; Pérez-Pulido, R.; Martínez-Bueno, M.; Maqueda, M. Characterization of Functional, Safety, and Probiotic Properties of Enterococcus Faecalis UGRA10, a New AS-48-Producer Strain. Food Microbiol. 2012, 30, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, R.; Martínez-Bueno, M.; Valdivia, E.; Albert, A.; Maqueda, M.; Sánchez-Barrena, M.J. The Bacteriocin AS-48 Requires Dimer Dissociation Followed by Hydrophobic Interactions with the Membrane for Antibacterial Activity. J. Struct. Biol. 2015, 190, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuenca, F.; Martínez-Martínez, L.; Pascual, A.; De Cueto, M.; Gutiérrez, O.; Nieto, J.; Perea, E.J. Evaluation of the WIDER I System for Antimicrobial Susceptibility Testing of Clinical Isolates of Haemophilus Influenzae and Streptococcus Pneumoniae. Clin. Microbiol. Infect. 2003, 9, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute, Ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015; ISBN 978-1-56238-987-1.
- Ananou, S.; Valdivia, E.; Martínez Bueno, M.; Gálvez, A.; Maqueda, M. Effect of Combined Physico-Chemical Preservatives on Enterocin AS-48 Activity against the Enterotoxigenic Staphylococcus Aureus CECT 976 Strain. J. Appl. Microbiol. 2004, 97, 48–56. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
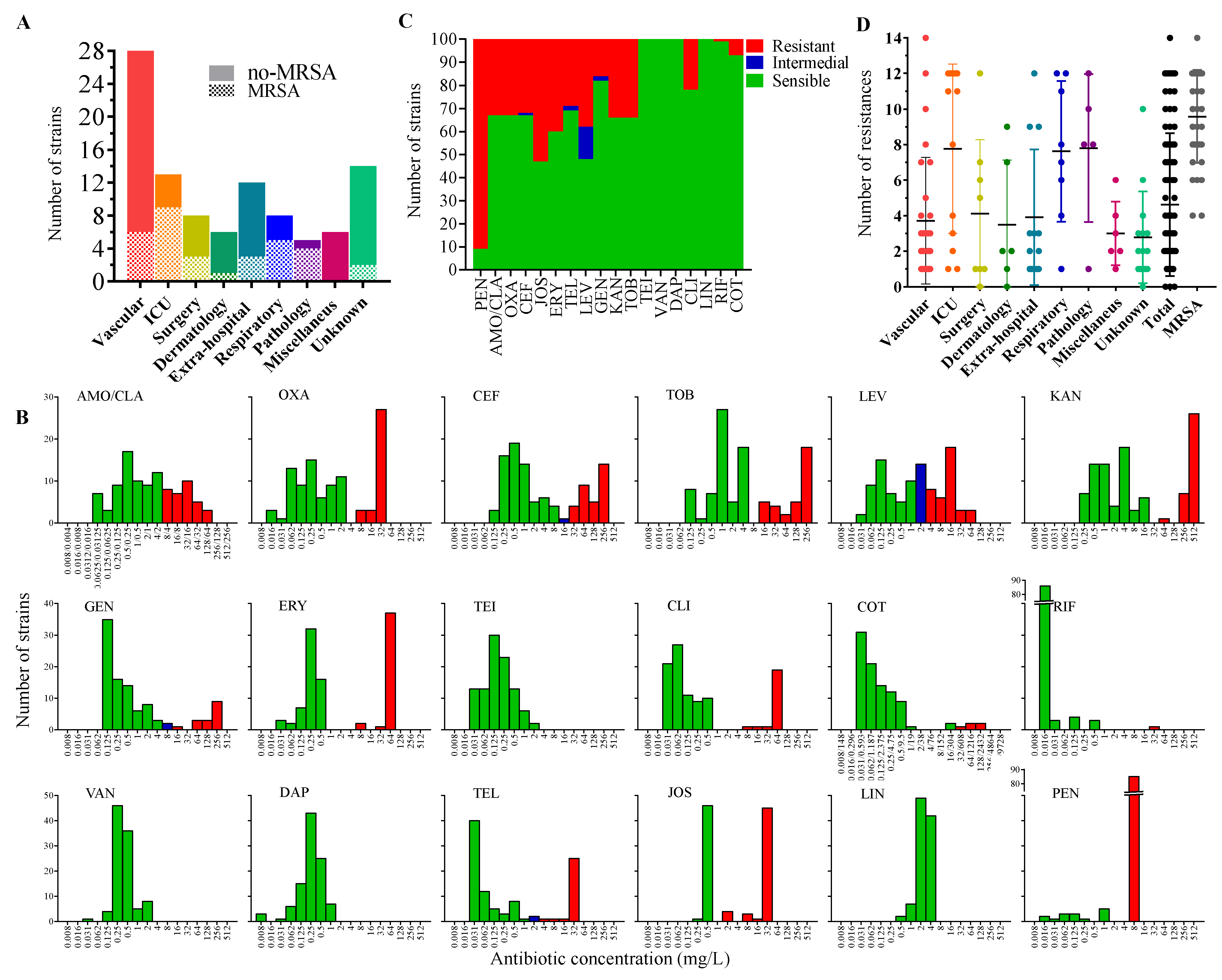

| Phenotype | Strain Numbers | Origin (Number of Strains) |
|---|---|---|
| No antibiotic resistance | 32, 72, 110 | Surgery (1), Dermatology (1), Unknown (1) |
| PEN | 4, 5, 6, 13, 21, 24, 35, 47, 48, 52, 55, 73, 81, 84, 85, 91, 97, 100, 101, 106, 120, 147, 160, 191, 207, 208, 221, | Vascular (10), Extra-hospital (4), Unknown (4), Surgery (3), ICU (2), Dermatology (1), Pathology (1), Miscellaneous (1) |
| LEV | 207 | Respiratory |
| PEN, JOS | 77, 113, 170, 190, 192 | Vascular (2), Dermatology (1), Extra-hospital (2), Miscellaneous (1) |
| PEN, TEL | 3, 62, 68, 105 | Unknown (2), ICU (1), Miscellaneous (1) |
| PEN, ERY | 23, 122 | Vascular |
| PEN, LEV | 193 | Unknown |
| PEN, COT | 49 | Extra-hospital |
| LEV, JOS | 12 | Dermatology |
| PEN, KAN, TOB | 14, 142, 148, 205, 220 | Extra-hospital (2), Vascular (1), ICU (1) |
| PEN, ERY, JOS | 76, 114, 175, 214, | Vascular (2), Miscellaneous (1), Unknown (1) |
| PEN, TEL, JOS | 118, 141 | Unknown |
| PEN, LEV, JOS | 218 | Vascular |
| ERY, JOS, COT | 29 | Vascular |
| PEN, ERY, JOS, CLIN | 16 | Unknown |
| PEN, AMO/CLA, OXA, LEV | 78 | Vascular |
| PEN, AMO/CLA, OXA, CEF | 145 | ICU |
| PEN, KAN, TOB, TEL | 153 | Miscellaneous |
| GEN, KAN, TOB, COT | 53 | Vascular |
| LEV, ERY, JOS, CLIN | 203 | Respiratory |
| PEN, TEL, ERY, JOS, CLIN | 103 | Surgery |
| PEN, GEN, KAN, TOB, JOS | 121 | Vascular |
| PEN, AMO/CLA, OXA, CEF, LEV, JOS | 19, 135, 185 | Unknown (1), Surgery (1), Respiratory (1) |
| PEN, KAN, TOB, TEL, JOS, ERY | 75 | Miscellaneous |
| PEN, AMO/CLA, OXA, CEF, LEV, ERY, JOS | 20, 87 | Surgery, vascular |
| PEN, LEV, TEL, ERY, JOS, CLI, COT | 28 | Vascular |
| PEN, GEN, KAN, TOB, TEL, ERY, JOS | 79 | Respiratory |
| KAN, TOB, LEV, TEL, ERY, JOS, CLI | 139 | Dermatology |
| PEN, AMO/CLA, OXA, CEF, LEV, TEL, ERY, JOS | 2 | Vascular |
| PEN, AMO/CLA, OXA, CEF, KAN, TOB, LEV, JOS | 119, 204 | Pathology, Respiratory |
| PEN, AMO/CLA, OXA, CEF, LEV, ERY, JOS, CLI | 174, 186 | Pathology, ICU |
| PEN, AMO/CLA, OXA, CEF, KAN, TOB, LEV, ERY, JOS | 33, 154, 171 | Extra-hospital (2), Dermatology (1) |
| PEN, AMO/CLA, OXA, CEF, GEN, KAN, TOB, ERY, JOS, CLI | 90 | Pathology |
| PEN, AMO/CLA, OXA, CEF, GEN, KAN, TOB, LEV, ERY, JOS | 176 | Vascular |
| PEN, AMO/CLA, OXA, CEF, KAN, TOB, LEV, TEL, ERY, JOS | 215 | Unknown |
| PEN, AMO/CLA, OXA, CEF, KAN, TOB, LEV, TEL, ERY, JOS, CLI | 1, 152, 155, | ICU (2), Respiratory (1) |
| PEN, AMO/CLA, OXA, CEF, GEN, KAN, TOB, LEV, TEL, ERY, JOS, CLI | 17, 30, 80, 96, 104, 111, 112, 136, 219 | ICU (5), Respiratory (1), Vascular (1), Surgery (1), Pathology (1) |
| PEN, AMO/CLA, OXA, CEF, GEN, KAN, TOB, LEV, TEL, ERY, JOS, COT | 95 | Extra-hospital |
| PEN, AMO/CLA, OXA, CEF, KAN, TOB, LEV, TEL, ERY, JOS, CLI, COT | 54 | Respiratory |
| PEN, AMO/CLA, OXA, CEF, GEN, KAN, TOB, LEV, TEL, ERY, JOS, COT | 80 | Extra-hospital |
| PEN, AMO/CLA, OXA, CEF, GEN, KAN, TOB, LEV, TEL, ERY, JOS, CLI, COT, RIF | 94 | Vascular |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Suárez, C.; Cebrián, R.; Gasca-Capote, C.; Sorlózano-Puerto, A.; Gutiérrez-Fernández, J.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Antimicrobial Activity of the Circular Bacteriocin AS-48 against Clinical Multidrug-Resistant Staphylococcus aureus. Antibiotics 2021, 10, 925. https://doi.org/10.3390/antibiotics10080925
Velázquez-Suárez C, Cebrián R, Gasca-Capote C, Sorlózano-Puerto A, Gutiérrez-Fernández J, Martínez-Bueno M, Maqueda M, Valdivia E. Antimicrobial Activity of the Circular Bacteriocin AS-48 against Clinical Multidrug-Resistant Staphylococcus aureus. Antibiotics. 2021; 10(8):925. https://doi.org/10.3390/antibiotics10080925
Chicago/Turabian StyleVelázquez-Suárez, Cristina, Rubén Cebrián, Carmen Gasca-Capote, Antonio Sorlózano-Puerto, José Gutiérrez-Fernández, Manuel Martínez-Bueno, Mercedes Maqueda, and Eva Valdivia. 2021. "Antimicrobial Activity of the Circular Bacteriocin AS-48 against Clinical Multidrug-Resistant Staphylococcus aureus" Antibiotics 10, no. 8: 925. https://doi.org/10.3390/antibiotics10080925
APA StyleVelázquez-Suárez, C., Cebrián, R., Gasca-Capote, C., Sorlózano-Puerto, A., Gutiérrez-Fernández, J., Martínez-Bueno, M., Maqueda, M., & Valdivia, E. (2021). Antimicrobial Activity of the Circular Bacteriocin AS-48 against Clinical Multidrug-Resistant Staphylococcus aureus. Antibiotics, 10(8), 925. https://doi.org/10.3390/antibiotics10080925







