In Vitro Activity of Propolis on Oral Microorganisms and Biofilms
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity against Planktonic Microorganisms
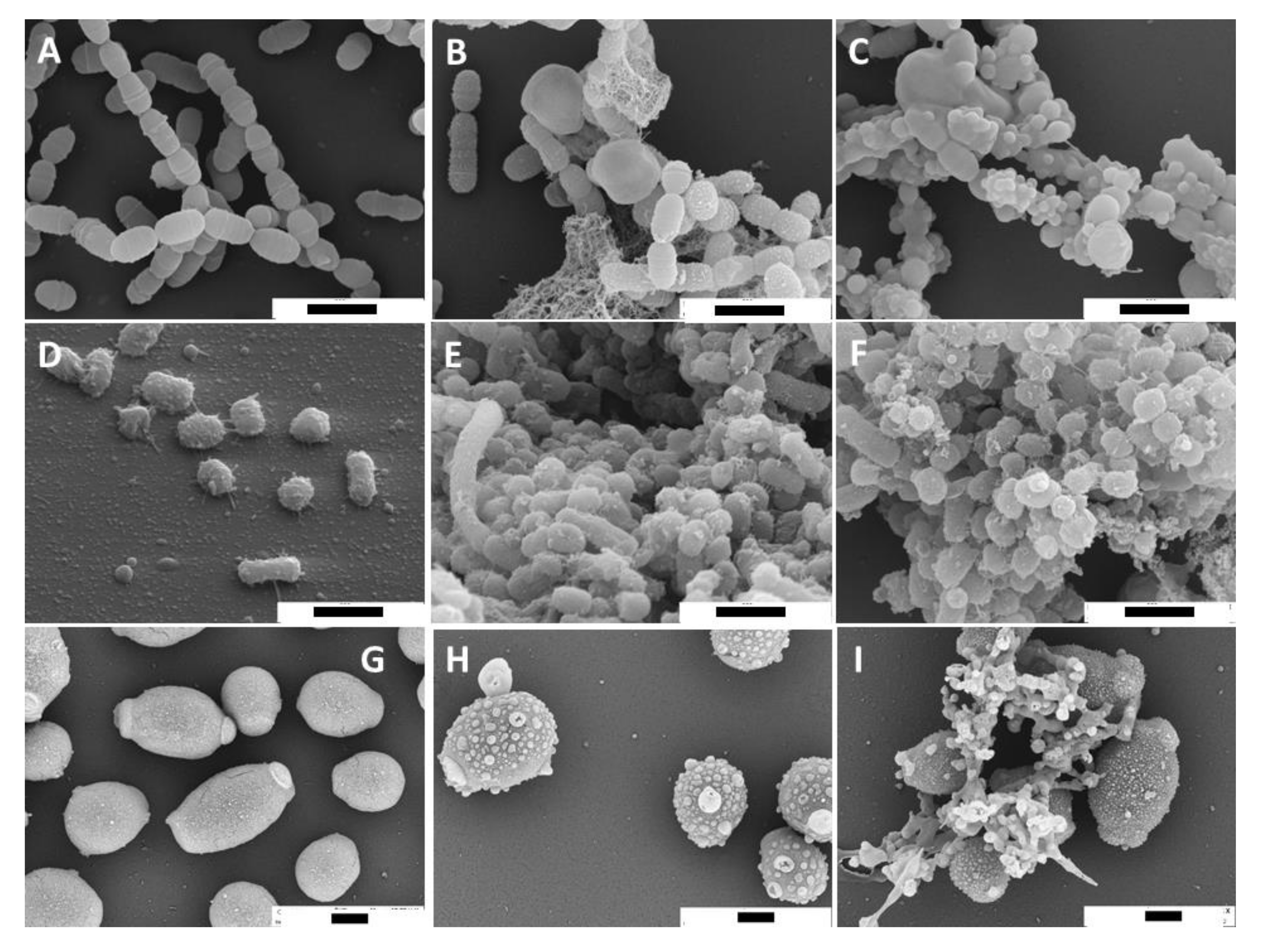
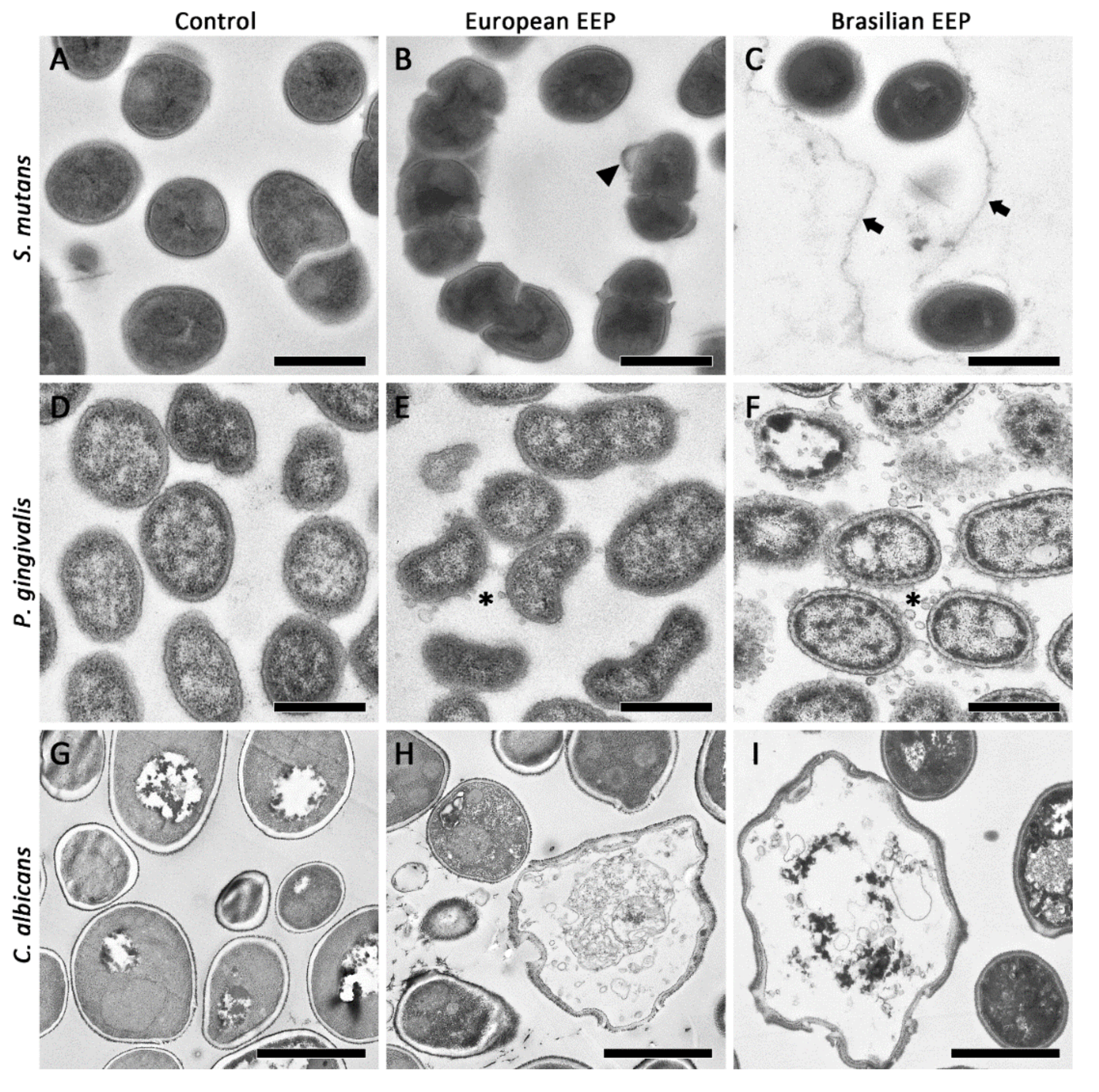
2.2. SEM and TEM Images of Microorganisms Exposed to Propolis
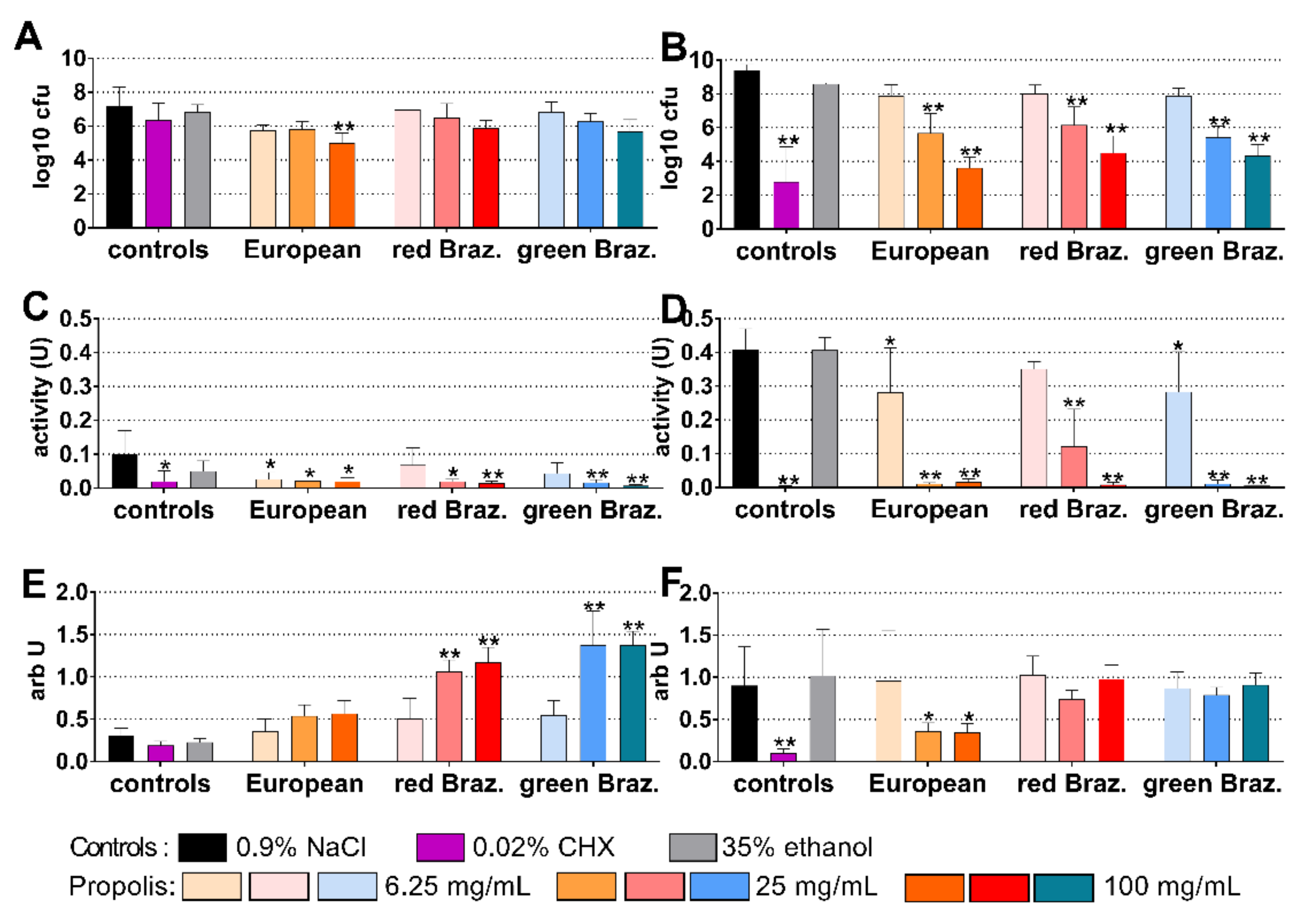
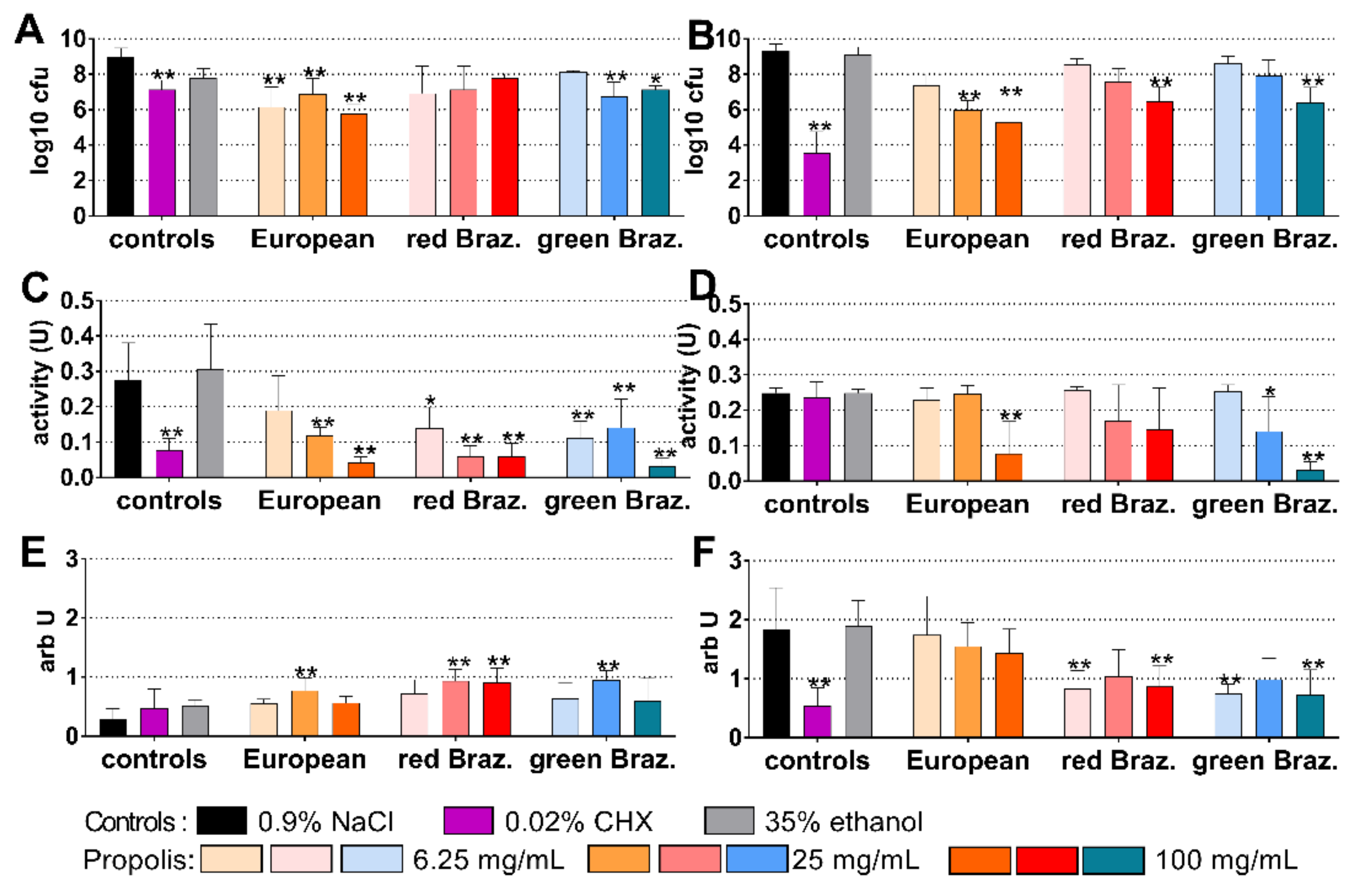
2.3. Cariogenic Biofilm
2.4. Periodontal Biofilm
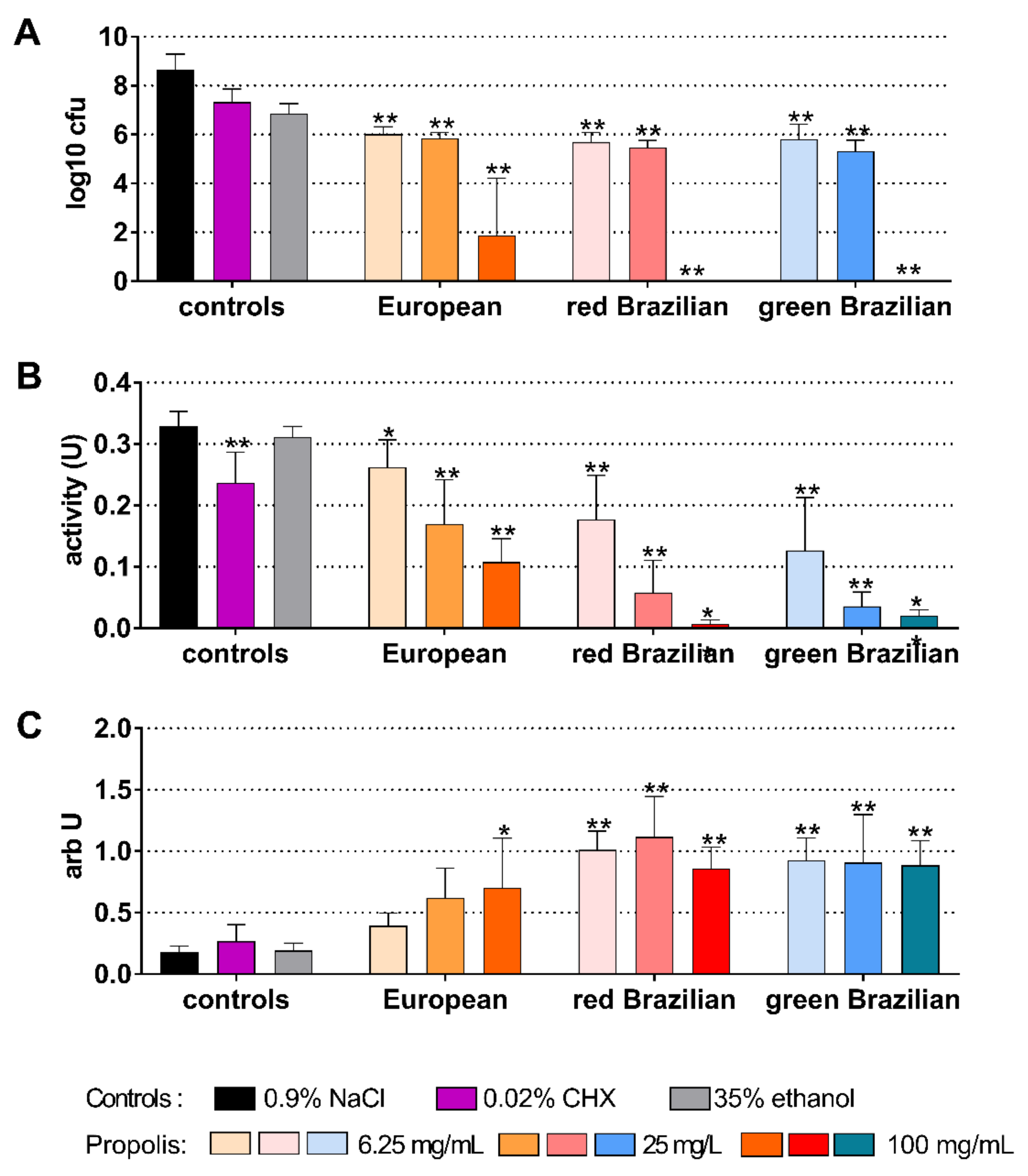
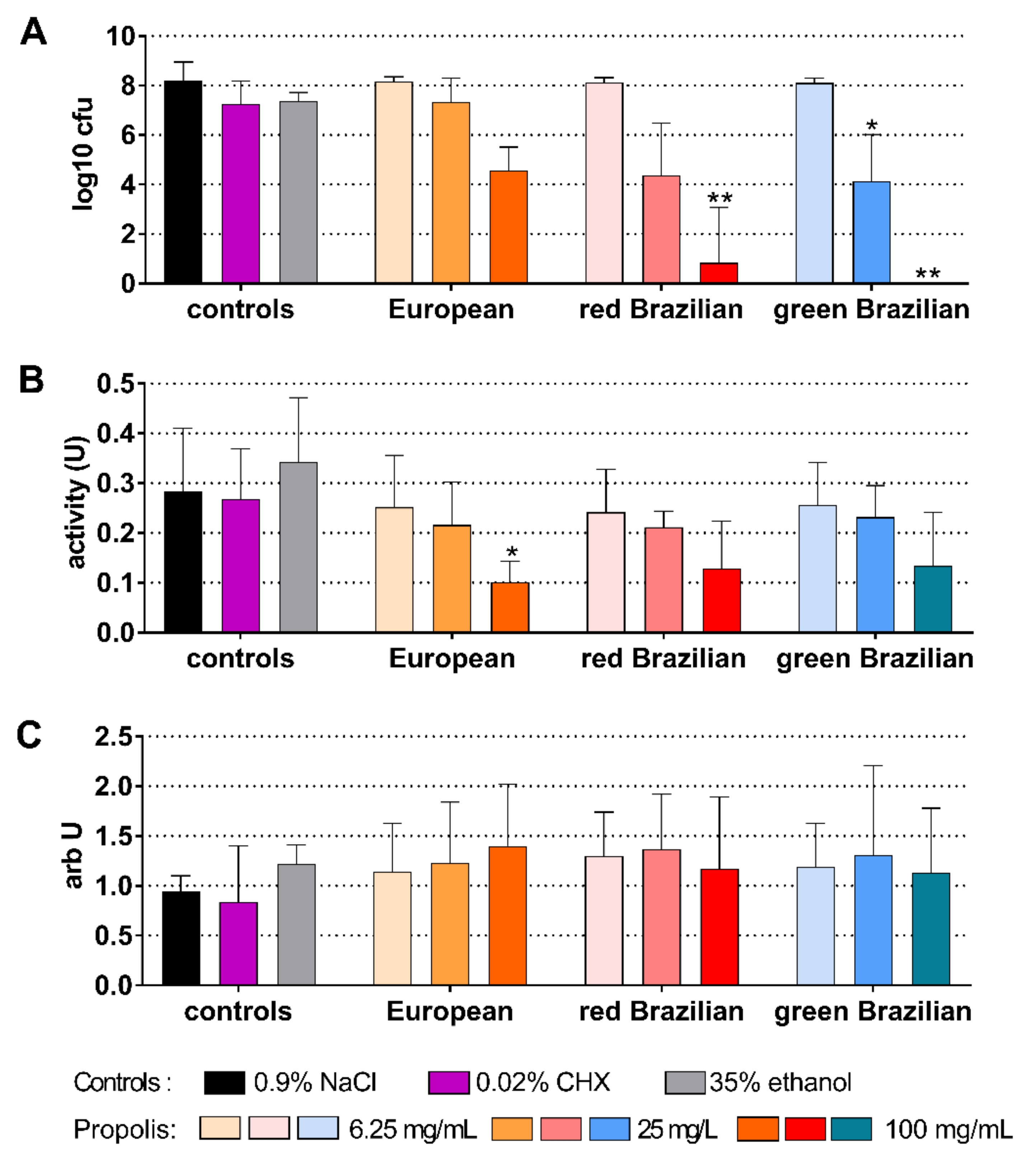
2.5. Candida Biofilm
2.6. Chemical Analysis of Propolis Extracts
3. Discussion
4. Materials and Methods
4.1. Propolis Preparations
4.2. Microorganisms
- Streptococcus gordonii ATCC 10558
- Actinomyces naeslundii ATCC 12104
- S. mutans ATCC 25175
- S. sobrinus ATCC 33478
- Lactobacillus acidophilus ATCC 11975
- Fusobacterium nucleatum ATCC 25586
- Campylobacter rectus ATCC 33238
- Parvimonas micra ATCC 33270
- Eikenella corrodens ATCC 23834
- Prevotella intermedia ATCC 2561
- Capnocytophaga gingivalis ATCC 33624
- Porphyromonas gingivalis ATCC 33277
- Tannerella forsythia ATCC 43037
- Filifactor alocis ATCC 33099
- Treponema denticola ATCC 35405
- Candida albicans ATCC 76615.
4.3. Susceptibility Tests: Determination of MICs
4.4. Visualization of Mode of Action of Propolis
4.5. Activity on Biofilms
4.6. Statistical Analysis
4.7. Chemical Analysis of Propolis by LC–HRMS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nyvad, B.; Crielaard, W.; Mira, A.; Takahashi, N.; Beighton, D. Dental caries from a molecular microbiological perspective. Caries Res. 2013, 47, 89–102. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- Lombardi, A.; Ouanounou, A. Fungal infections in dentistry: Clinical presentations, diagnosis, and treatment alternatives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, A.; Leone, P.; Solimando, A.G.; Fasano, R.; Malerba, E.; Prete, M.; Corrente, M.; Prati, C.; Vacca, A.; Racanelli, V. Antibiotics or No Antibiotics, That Is the Question: An Update on Efficient and Effective Use of Antibiotics in Dental Practice. Antibiotics 2021, 10, 550. [Google Scholar] [CrossRef]
- Guentsch, A. Antibiotics against Periodontal Biofilms. Monogr. Oral Sci. 2021, 29, 119–132. [Google Scholar] [CrossRef]
- Guerra, F.; Pasqualotto, D.; Rinaldo, F.; Mazur, M.; Corridore, D.; Nofroni, I.; Ottolenghi, L.; Nardi, G.M. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: Performance-related evaluation of mouthwashes added with Anti-Discoloration System and cetylpyridinium chloride. Int. J. Dent. Hyg. 2019, 17, 229–236. [Google Scholar] [CrossRef]
- Muller, H.D.; Eick, S.; Moritz, A.; Lussi, A.; Gruber, R. Cytotoxicity and Antimicrobial Activity of Oral Rinses In Vitro. Biomed. Res. Int. 2017, 2017, 4019723. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, V.R.; Caiaffa, K.S.; Oliveira, W.C.; Pereira, J.A.; Abuna, G.F.; Polaquini, C.R.; Regasini, L.O.; Guiotti, A.M.; Duque, C. Cytotoxicity and effects of curcumin and cinnamaldehyde hybrids on biofilms of oral pathogens. Biofouling 2021, 37, 591–605. [Google Scholar] [CrossRef]
- Cheng, P.C.; Wong, G. Honey bee propolis: Prospects in medicine. Bee World 1996, 77, 8–15. [Google Scholar] [CrossRef]
- Przybylek, I.; Karpinski, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxidative Med. Cell Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Condor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharm. 2020, 131, 110622. [Google Scholar] [CrossRef] [PubMed]
- RojaRamya, K.S.; Vinay, C.; Uloopi, K.S.; Chandrasekhar, R. In vivo evaluation of zinc oxide-propolis mixture as root canal filling material in the primary molars: A 24-month follow-up randomized controlled trial. J. Indian Soc. Pedod. Prev. Dent. 2020, 38, 171–176. [Google Scholar] [CrossRef] [PubMed]
- El-Allaky, H.S.; Wahba, N.A.; Talaat, D.M.; Zakaria, A.S. Antimicrobial effect of propolis administered through two different vehicles in high caries risk children: A randomized clinical trial. J. Clin. Pediatr. Dent. 2020, 44, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Senpuku, H.; Ohnishi, M.; Takai, H.; Ogata, Y. Effect of topical administration of propolis in chronic periodontitis. Odontology 2020, 108, 704–714. [Google Scholar] [CrossRef]
- Pina, G.M.; Lia, E.N.; Berretta, A.A.; Nascimento, A.P.; Torres, E.C.; Buszinski, A.F.; de Campos, T.A.; Coelho, E.B.; Martins, V.P. Efficacy of propolis on the denture stomatitis treatment in older adults: A multicentric randomized trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 8971746. [Google Scholar] [CrossRef]
- de Figueiredo, K.A.; da Silva, H.D.P.; Miranda, S.L.F.; Goncalves, F.; de Sousa, A.P.; de Figueiredo, L.C.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Is as Effective as Amoxicillin in Controlling Red-Complex of Multispecies Subgingival Mature Biofilm In Vitro. Antibiotics 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Nakagawa, T.; Maruyama, K.; Dono, Y.; Katsuragi, H.; Sato, S. Effect of Brazilian green propolis on oral pathogens and human periodontal fibroblasts. J. Oral Biosci. 2016, 58, 50–54. [Google Scholar] [CrossRef]
- Martins, M.L.; Monteiro, A.S.N.; Guimaraes, J.E.C.; Guimaraes, M.B.D.T.; da Silva, R.F.; Cabral, L.M.; Farah, A.; dePaula, J.; Romanos, M.T.V.; Maia, L.C.; et al. Cytotoxic and antibacterial effect of a red propolis mouthwash, with or without fluoride, on the growth of a cariogenic biofilm. Arch. Oral Biol. 2019, 107, 104512. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.R.; Bobis, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Koru, O.; Toksoy, F.; Acikel, C.H.; Tunca, Y.M.; Baysallar, M.; Uskudar Guclu, A.; Akca, E.; Ozkok Tuylu, A.; Sorkun, K.; Tanyuksel, M.; et al. In vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe 2007, 13, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, R.; Borgonovo, G.; Leoni, V.; Giupponi, L.; Ceciliani, G.; Sala, S.; Bassoli, A.; Giorgi, A. Effectiveness of different analytical methods for the characterization of propolis: A case of study in Northern Italy. Molecules 2020, 25, 504. [Google Scholar] [CrossRef] [Green Version]
- Regueira, M.S.N.; Tintino, S.R.; da Silva, A.R.P.; Costa, M.D.S.; Boligon, A.A.; Matias, E.F.F.; de Queiroz Balbino, V.; Menezes, I.R.A.; Melo Coutinho, H.D. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar] [CrossRef]
- Stahli, A.; Maheen, C.U.; Strauss, F.J.; Eick, S.; Sculean, A.; Gruber, R. Caffeic acid phenethyl ester protects against oxidative stress and dampens inflammation via heme oxygenase 1. Int. J. Oral Sci. 2019, 11, 6. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial effect of caffeic acid phenethyl ester on cariogenic bacteria and Streptococcus mutans biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and antibiofilm activity against Streptococcus mutans of individual and mixtures of the main polyphenolic compounds found in Chilean propolis. Biomed. Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef] [Green Version]
- Ristivojevic, P.; Stevic, T.; Starovic, M.; Pavlovic, S.; Ozcan, M.M.; Beric, T.; Dimkic, I. Phenolic composition and biological activities of geographically different type of propolis and black cottonwood resins against oral streptococci, vaginal microbiota and phytopathogenic Fusarium species. J. Appl. Microbiol. 2020, 129, 296–310. [Google Scholar] [CrossRef]
- da Silva Barboza, A.; Aitken-Saavedra, J.P.; Ferreira, M.L.; Fabio Aranha, A.M.; Lund, R.G. Are propolis extracts potential pharmacological agents in human oral health? - A scoping review and technology prospecting. J. Ethnopharmacol. 2021, 271, 113846. [Google Scholar] [CrossRef]
- Grecka, K.; Kus, P.M.; Okinczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anti-cariogenic and anti-biofilms activity of Tunisian propolis extract and its potential protective effect against cancer cells proliferation. Anaerobe 2010, 16, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Gomes, B.P.; Rosalen, P.L.; Ambrosano, G.M.; Park, Y.K.; Cury, J.A. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch. Oral Biol. 2000, 45, 141–148. [Google Scholar] [CrossRef]
- Gucwa, K.; Kusznierewicz, B.; Milewski, S.; Van Dijck, P.; Szweda, P. Antifungal Activity and Synergism with Azoles of Polish Propolis. Pathogens 2018, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Barik, S.; Muralitharan, G.; Busi, S. Ferulic acid encapsulated chitosan-tripolyphosphate nanoparticles attenuate quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa PAO1. IET Nanobiotechnol. 2018, 12, 1056–1061. [Google Scholar] [CrossRef]
- Reda, B.; Hollemeyer, K.; Trautmann, S.; Hannig, M.; Volmer, D.A. Determination of chlorhexidine retention in different oral sites using matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Arch. Oral Biol. 2020, 110, 104623. [Google Scholar] [CrossRef]
- Tan, S.; Gao, J.; Li, Q.; Guo, T.; Dong, X.; Bai, X.; Yang, J.; Hao, S.; He, F. Synergistic effect of chlorogenic acid and levofloxacin against Klebsiella pneumonia infection in vitro and in vivo. Sci. Rep. 2020, 10, 20013. [Google Scholar] [CrossRef] [PubMed]
- Vanni, R.; Waldner-Tomic, N.M.; Belibasakis, G.N.; Attin, T.; Schmidlin, P.R.; Thurnheer, T. Antibacterial efficacy of a propolis toothpaste and mouthrinse against a supragingival multispecies biofilm. Oral Health Prev. Dent. 2015, 13, 531–535. [Google Scholar] [CrossRef]
- Miranda, S.L.F.; Damasceno, J.T.; Faveri, M.; Figueiredo, L.; da Silva, H.D.; Alencar, S.M.A.; Rosalen, P.L.; Feres, M.; Bueno-Silva, B. Brazilian red propolis reduces orange-complex periodontopathogens growing in multispecies biofilms. Biofouling 2019, 35, 308–319. [Google Scholar] [CrossRef]
- Zarnowski, R.; Westler, W.M.; Lacmbouh, G.A.; Marita, J.M.; Bothe, J.R.; Bernhardt, J.; Lounes-Hadj Sahraoui, A.; Fontaine, J.; Sanchez, H.; Hatfield, R.D.; et al. Novel entries in a fungal biofilm matrix encyclopedia. mBio 2014, 5, e01314–e01333. [Google Scholar] [CrossRef] [Green Version]
- Bhat, N.; Bapat, S.; Asawa, K.; Tak, M.; Chaturvedi, P.; Gupta, V.V.; George, P.P. The antiplaque efficacy of propolis-based herbal toothpaste: A crossover clinical study. J. Nat. Sci. Biol. Med. 2015, 6, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Bretz, W.A.; Paulino, N.; Nor, J.E.; Moreira, A. The effectiveness of propolis on gingivitis: A randomized controlled trial. J. Altern. Complement. Med. 2014, 20, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Giammarinaro, E.; Marconcini, S.; Genovesi, A.; Poli, G.; Lorenzi, C.; Covani, U. Propolis as an adjuvant to non-surgical periodontal treatment: A clinical study with salivary anti-oxidant capacity assessment. Minerva Stomatol. 2018, 67, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Machorowska-Pieniazek, A.; Skucha-Nowak, M.; Mertas, A.; Tanasiewicz, M.; Niedzielska, I.; Morawiec, T.; Baron, S. Effects of Brazilian propolis on dental plaque and gingiva in patients with oral cleft malformation treated with multibracket and removable appliances: A comparative study. Evid.-Based Complement. Altern. Med. 2016, 2016, 2038407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawiec, T.; Dziedzic, A.; Niedzielska, I.; Mertas, A.; Tanasiewicz, M.; Skaba, D.; Kasperski, J.; Machorowska-Pieniazek, A.; Kucharzewski, M.; Szaniawska, K.; et al. The biological activity of propolis-containing toothpaste on oral health environment in patients who underwent implant-supported prosthodontic rehabilitation. Evid.-Based Complement. Altern. Med. 2013, 2013, 704947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, A. Honeybee propolis extract in periodontal treatment: A clinical and microbiological study of propolis in periodontal treatment. Indian J. Dent. Res. 2012, 23, 294. [Google Scholar] [CrossRef]
- El-Sharkawy, H.M.; Anees, M.M.; Van Dyke, T.E. Propolis improves periodontal status and glycemic control in patients with type 2 Diabetes mellitus and chronic periodontitis: A randomized clinical trial. J. Periodontol. 2016, 87, 1418–1426. [Google Scholar] [CrossRef]
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Fitoterapia 2020, 147, 104775. [Google Scholar] [CrossRef]
- Wozniak, M.; Mrowczynska, L.; Kwasniewska-Sip, P.; Waskiewicz, A.; Nowak, P.; Ratajczak, I. Effect of the solvent on propolis phenolic profile and its antifungal, antioxidant, and in vitro cytoprotective activity in human erythrocytes under oxidative stress. Molecules 2020, 25, 4266. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Wusteman, M.C.; Armitage, J.W.; Wang, L.H.; Busza, A.L.; Pegg, D.E. Cryopreservation studies with porcine corneas. Curr. Eye Res. 1999, 19, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kwasny, S.M.; Opperman, T.J. Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr. Protoc. Pharm. 2010, 50, 13A-8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettit, R.K.; Weber, C.A.; Kean, M.J.; Hoffmann, H.; Pettit, G.R.; Tan, R.; Franks, K.S.; Horton, M.L. Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 2005, 49, 2612–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliasson, B.; Liakopoulos, V.; Franzen, S.; Naslund, I.; Svensson, A.M.; Ottosson, J.; Gudbjornsdottir, S. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: A nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol. 2015, 3, 847–854. [Google Scholar] [CrossRef]


| Strain | CHX | Ethanol | European EEP | Red Brazilian EEP | Green Brazilian EEP |
|---|---|---|---|---|---|
| Streptococcus gordonii ATCC 10558 | ≤0.0004 | 17.5 | 0.2 | ≤0.1 | ≤0.1 |
| Actinomyces naeslundii ATCC 12104 | 0.0016 | 8.75 | 50 | ≤0.1 | ≤0.1 |
| S. mutans ATCC 25175 | 0.0016 | 17.5 | 0.2 | 3.13 | 0.2 |
| Fusobacterium nucleatum ATCC 25586 | 0.0008 | 17.5 | 3.13 | 0.2 | 0.2 |
| Prevotella intermedia ATCC 25611 | ≤0.0004 | 4.38 | 12.5 | ≤0.1 | ≤0.1 |
| Porphyromonas gingivalis ATCC 33277 | 0.0016 | 35.0 | 0.2 | 0.2 | 0.2 |
| Parvimonas micra ATCC 33270 | 0.0031 | 35.0 | ≤0.1 | ≤0.1 | ≤0.1 |
| Candida albicans ATCC 76615 | ≤0.0004 | 17.5 | 6.25 | 3.13 | 3.13 |
| European Propolis | Peak | RT (min) | Compound | Brazilian Propolis | Peak | RT (min) | Compound |
|---|---|---|---|---|---|---|---|
| P1 | 1 | 28.01 | Ferulic acid | P2, P3 | 1 | 14.02 | Chlorogenic acid |
| P1 | 2 | 29.16 | 3-Hydroxy-4-methoxycinnamic acid | P2, P3 | 2 | 21.9 | 4-Hydroxycinnamic acid |
| P1 | 3 | 36.06 | 3,4-Dimethoxycinnamic acid | P2, P3 | 3 | 27.28 | Luteolin 7-rutinoside |
| P1 | 4 | 36.59 | DMCA isomer | P2, P3 | 4 | 29.45 | Baccharin |
| P1 | 5 | 38.57 | Pinobanksin-methyl ether isomer | P2, P3 | 5 | 30.18 | ND |
| P1 | 6 | 40.79 | Pinobanksin 5-methyl ether | P2, P3 | 6 | 31.98 | Aromadendrin 4’-methyl ether 7-rhamnoside |
| P1 | 7 | 44.12 | Quercetin 3-methyl ether | P2, P3 | 7 | 34.85 | 3,4-Dicaffeoylquinic acid |
| P1 | 8 | 45.51 | Chrysin 5-methyl ether | P2, P3 | 8 | 35.89 | 3,5-Dicaffeoylquinic acid |
| P1 | 9 | 45.93 | Apigenin | P2, P3 | 9 | 37.36 | 1,5-Dicaffeoylquinic acid |
| P1 | 10 | 46.09 | Kaempferol | P2, P3 | 10 | 38.61 | Kaempferol 7-methyl ether 4’-glucoside |
| P1 | 11 | 46.55 | Isorhamnetin | P2, P3 | 11 | 43.95 | Di-Caffeoyl quinic acid isomer |
| P1 | 12 | 46.97 | Luteolin 5-methyl ether | P2, P3 | 12 | 45.04 | Quercetin |
| P1 | 13 | 47.34 | Quercetin 5,3’-dimethyl ether | P2, P3 | 13 | 47.54 | Luteolin 5-methyl ether |
| P1 | 14 | 47.85 | Galangin-5-methylether | P2, P3 | 14 | 47.96 | Pinocembrin |
| P1 | 15 | 48.91 | Quercetin 3,7-dimethyl ether | P2, P3 | 15 | 48.49 | Drupanin |
| P1 | 16 | 49.19 | Prenyl caffeate | P2, P3 | 16 | 49.08 | Viscidone |
| P1 | 17 | 49.73 | Chrysin | P2, P3 | 17 | 49.67 | Chrysin |
| P1 | 18 | 50.17 | Benzyl caffeate | P2, P3 | 18 | 49.87 | Pinocembrin-5-methyl ether |
| P1 | 19 | 50.3 | Pinobanksin 3-butyrate | P2, P3 | 19 | 50.17 | Benzyl caffeate |
| P1 | 20 | 50.69 | Caffeic acid phenethyl ester CAPE | P2, P3 | 20 | 50.33 | Kaempferol-7-methyl ether |
| P1 | 1 | 28.01 | Ferulic acid | P2, P3 | 1 | 14.02 | Chlorogenic acid |
| P1 | 2 | 29.16 | 3-Hydroxy-4-methoxycinnamic acid | P2, P3 | 2 | 21.9 | 4-Hydroxycinnamic acid |
| P1 | 3 | 36.06 | 3,4-Dimethoxycinnamic acid | P2, P3 | 3 | 27.28 | Luteolin 7-rutinoside |
| P1 | 4 | 36.59 | DMCA isomer | P2, P3 | 4 | 29.45 | Baccharin |
| P1 | 5 | 38.57 | Pinobanksin-methyl ether isomer | P2, P3 | 5 | 30.18 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In Vitro Activity of Propolis on Oral Microorganisms and Biofilms. Antibiotics 2021, 10, 1045. https://doi.org/10.3390/antibiotics10091045
Stähli A, Schröter H, Bullitta S, Serralutzu F, Dore A, Nietzsche S, Milia E, Sculean A, Eick S. In Vitro Activity of Propolis on Oral Microorganisms and Biofilms. Antibiotics. 2021; 10(9):1045. https://doi.org/10.3390/antibiotics10091045
Chicago/Turabian StyleStähli, Alexandra, Hannah Schröter, Simonetta Bullitta, Francesca Serralutzu, Antonio Dore, Sandor Nietzsche, Egle Milia, Anton Sculean, and Sigrun Eick. 2021. "In Vitro Activity of Propolis on Oral Microorganisms and Biofilms" Antibiotics 10, no. 9: 1045. https://doi.org/10.3390/antibiotics10091045







