The Potential of Probiotics to Eradicate Gut Carriage of Pathogenic or Antimicrobial-Resistant Enterobacterales
Abstract
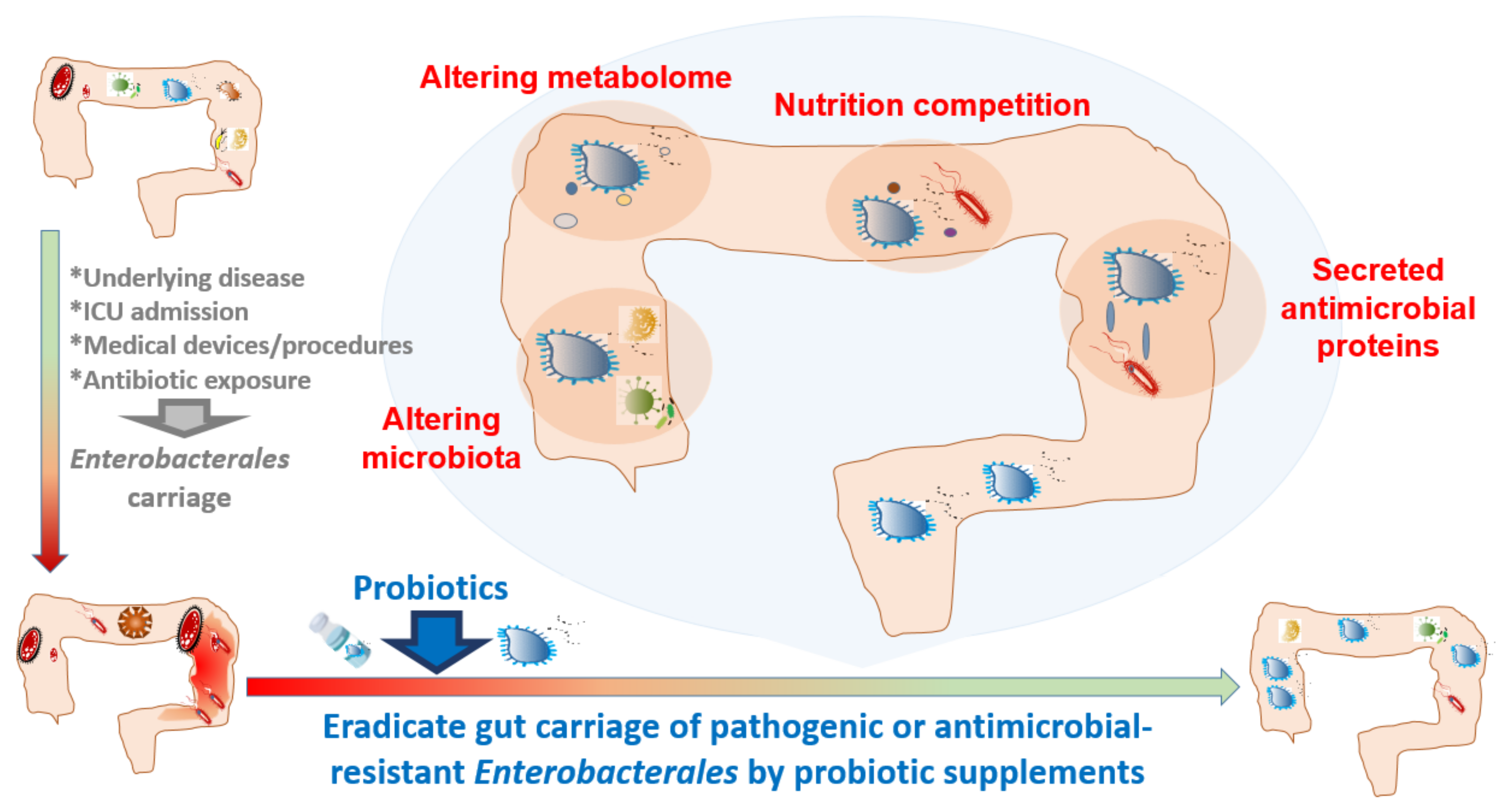
:1. Introduction
1.1. Rationale for Probiotic Supplements to Eradicate Enterobacterales Carriage in the Gut
1.2. Probiotic Supplements to Decrease Gut Carriage of Enterobacterales in Livestock or Domesticated Animals
1.3. The Selection of Probiotics to Decrease Gut Colonization of Enterobacterales in Humans
1.4. Probiotic Supplementation to Decrease Potential Gut Pathogenic Enterobacterales from Infants to Children
1.5. Probiotic Supplementation to Decrease Gut Pathogenic or Antimicrobial-Resistant Enterobacterales Colonization in Adults
1.6. Possible Mechanisms by Which Probiotic Supplementation Decreases Gut Enterobacterales Carriage
1.7. Improve the Effect of Probiotics in Eradicating Enterobacterales
1.8. Clinical Trials of Probiotics or Synbiotics to Improve Gut Health
1.9. Clinical Safety Issue of Probiotics
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greer, R.; Dong, X.; Morgun, A.; Shulzhenko, N. Investigating a holobiont: Microbiota perturbations and transkingdom networks. Gut Microbes 2016, 7, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Ochman, H.; Worobey, M.; Kuo, C.H.; Ndjango, J.B.; Peeters, M.; Hahn, B.H.; Hugenholtz, P. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol. 2010, 8, e1000546. [Google Scholar] [CrossRef] [PubMed]
- El Kafsi, H.; Gorochov, G.; Larsen, M. Host genetics affect microbial ecosystems via host immunity. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 10. [Google Scholar] [CrossRef]
- Grech, A.; Collins, C.E.; Holmes, A.; Lal, R.; Duncanson, K.; Taylor, R.; Gordon, A. Maternal exposures and the infant gut microbiome: A systematic review with meta-analysis. Gut Microbes 2021, 13, 1–30. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Stanton, C.; Ross, R.P.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium and Lactobacillus Composition at Species Level and Gut Microbiota Diversity in Infants before 6 Weeks. Int. J. Mol. Sci. 2019, 20, 3306. [Google Scholar] [CrossRef] [Green Version]
- Rivard-Yazigi, L.; Zahar, J.R.; Le Guillou, S.; Chalouhi, C.; Lecuyer, H.; Bureau, C.; Nassif, X.; Gendrel, D.; Abadie, V. Risk factors associated with extended-spectrum beta-lactamase-producing Enterobacteriaceae carriage at admission in an infant cohort at a tertiary teaching hospital in France. Am. J. Infect. Control 2013, 41, 844–845. [Google Scholar] [CrossRef]
- Hurrell, E.; Kucerova, E.; Loughlin, M.; Caubilla-Barron, J.; Hilton, A.; Armstrong, R.; Smith, C.; Grant, J.; Shoo, S.; Forsythe, S. Neonatal enteral feeding tubes as loci for colonisation by members of the Enterobacteriaceae. BMC Infect. Dis. 2009, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, F.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; et al. Cesarean Section, Formula Feeding, and Infant Antibiotic Exposure: Separate and Combined Impacts on Gut Microbial Changes in Later Infancy. Front. Pediatr. 2017, 5, 200. [Google Scholar] [CrossRef]
- Rondanelli, M.; Giacosa, A.; Faliva, M.A.; Perna, S.; Allieri, F.; Castellazzi, A.M. Review on microbiota and effectiveness of probiotics use in older. World J. Clin. Cases 2015, 3, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Korach-Rechtman, H.; Hreish, M.; Fried, C.; Gerassy-Vainberg, S.; Azzam, Z.S.; Kashi, Y.; Berger, G. Intestinal Dysbiosis in Carriers of Carbapenem-Resistant Enterobacteriaceae. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Quinones, M.; Thirakittiwatthana, W.; Yungyuen, T.; Otto, M.; Kiratisin, P. Composition of the intestinal microbiota in extended-spectrum beta-lactamase-producing Enterobacteriaceae carriers and non-carriers in Thailand. Int. J. Antimicrob. Agents 2019, 53, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Hung, Y.P.; Lin, W.T.; Dai, W.; Huang, Y.L.; Ko, W.C. Risk factors and clinical impact of bacteremia due to carbapenem-nonsusceptible Enterobacteriaceae: A multicenter study in southern Taiwan. J. Microbiol. Immunol. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, H.; Ishibashi, N.; Wada, K.; Oka, K.; Takahashi, M.; Yamasaki, Y.; Aoyagi, T.; Takemura, H.; Kitagawa, M.; Kaku, M. The effect of gut microbiota and probiotic organisms on the properties of extended spectrum beta-lactamase producing and carbapenem resistant Enterobacteriaceae including growth, beta-lactamase activity and gene transmissibility. J. Infect. Chemother. 2019, 25, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Catho, G.; Huttner, B.D. Strategies for the eradication of extended-spectrum beta-lactamase or carbapenemase-producing Enterobacteriaceae intestinal carriage. Expert Rev. Anti-Infect. Ther. 2019, 17, 557–569. [Google Scholar] [CrossRef]
- Weng, Y.J.; Jiang, D.X.; Liang, J.; Ye, S.C.; Tan, W.K.; Yu, C.Y.; Zhou, Y. Effects of Pretreatment with Bifidobacterium bifidum Using 16S Ribosomal RNA Gene Sequencing in a Mouse Model of Acute Colitis Induced by Dextran Sulfate Sodium. Med. Sci. Monit. 2021, 27, e928478. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Chen, Y.; Yang, Y. Probiotic Lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition 2020, 78, 110863. [Google Scholar] [CrossRef]
- Sasaki, K.; Sasaki, D.; Inoue, J.; Hoshi, N.; Maeda, T.; Yamada, R.; Kondo, A. Bacillus coagulans SANK 70258 suppresses Enterobacteriaceae in the microbiota of ulcerative colitis in vitro and enhances butyrogenesis in healthy microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 3859–3867. [Google Scholar] [CrossRef]
- Yi, R.; Tan, F.; Liao, W.; Wang, Q.; Mu, J.; Zhou, X.; Yang, Z.; Zhao, X. Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice. Microorganisms 2019, 7, 530. [Google Scholar] [CrossRef] [Green Version]
- Whittemore, J.C.; Stokes, J.E.; Price, J.M.; Suchodolski, J.S. Effects of a synbiotic on the fecal microbiome and metabolomic profiles of healthy research cats administered clindamycin: A randomized, controlled trial. Gut Microbes 2019, 10, 521–539. [Google Scholar] [CrossRef] [Green Version]
- Park, H.E.; Kim, Y.J.; Do, K.H.; Kim, J.K.; Ham, J.S.; Lee, W.K. Effects of Queso Blanco Cheese Containing Bifidobacterium longum KACC 91563 on the Intestinal Microbiota and Short Chain Fatty Acid in Healthy Companion Dogs. Korean J. Food Sci. Anim. Resour. 2018, 38, 1261–1272. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Li, J.; Chen, Y.; Yang, Y. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int. J. Mol. Med. 2019, 43, 1139–1148. [Google Scholar] [CrossRef]
- Berreta, A.; Kopper, J.J.; Alexander, T.L.; Kogan, C.J.; Burbick, C.R. Effect of an In Vitro Proximal Gastrointestinal Tract on Viability of Commercially Available Equine Probiotics. J. Equine Vet. Sci. 2021, 104, 103671. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, R.; Grayson, A.G.; Schena, I.; Amanze, O.; Suwintono, K.; Quinn, R.A. Microbial Transformations of Organically Fermented Foods. Metabolites 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jimenez-Colmenero, F.; Pintado, T.; de Menezes, C.R.; Fries, L.L.M. Effect of different strategies of Lactobacillus plantarum incorporation in chorizo sausages. J. Sci. Food Agric. 2019, 99, 6706–6712. [Google Scholar] [CrossRef] [PubMed]
- Linninge, C.; Xu, J.; Bahl, M.I.; Ahrne, S.; Molin, G. Lactobacillus fermentum and Lactobacillus plantarum increased gut microbiota diversity and functionality, and mitigated Enterobacteriaceae, in a mouse model. Benef. Microbes 2019, 10, 413–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de La Cochetiere, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Nagata, S. Beneficial microbes for premature infants, and children with malignancy undergoing chemotherapy. Benef. Microbes 2010, 1, 357–365. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, H.Y.; Feng, J.Y.; Zhang, M.M.; Zhou, Y.; Wu, X.T. Use of pro-/synbiotics as prophylaxis in patients undergoing colorectal resection for cancer: A meta-analysis of randomized controlled trials. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Saliu, E.M.; Ren, H.; Boroojeni, F.G.; Zentek, J.; Vahjen, W. The Impact of Direct-Fed Microbials and Phytogenic Feed Additives on Prevalence and Transfer of Extended-Spectrum Beta-Lactamase Genes in Broiler Chicken. Microorganisms 2020, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.R.; Wilson, K.M.; Trombetta, M.; Briggs, W.N.; Duff, A.F.; Chasser, K.M.; Bottje, W.G.; Bielke, L. A Proteomic View of the Cross-Talk Between Early Intestinal Microbiota and Poultry Immune System. Front. Physiol. 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.H.; Teng, P.Y.; Lee, T.T.; Yu, B. Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. enterica. Asian-Australas. J. Anim. Sci. 2020, 33, 1797–1808. [Google Scholar] [CrossRef]
- Luise, D.; Bertocchi, M.; Motta, V.; Salvarani, C.; Bosi, P.; Luppi, A.; Fanelli, F.; Mazzoni, M.; Archetti, I.; Maiorano, G.; et al. Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 2019, 10, 74. [Google Scholar] [CrossRef]
- Nakphaichit, M.; Sobanbua, S.; Siemuang, S.; Vongsangnak, W.; Nakayama, J.; Nitisinprasert, S. Protective effect of Lactobacillus reuteri KUB-AC5 against Salmonella Enteritidis challenge in chickens. Benef. Microbes 2019, 10, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ni, X.; Wen, B.; Duan, L.; Sun, H.; Yang, M.; Zou, F.; Lin, Y.; Liu, Q.; Zeng, Y.; et al. Appropriate dose of Lactobacillus buchneri supplement improves intestinal microbiota and prevents diarrhoea in weaning Rex rabbits. Benef. Microbes 2018, 9, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Arreguin-Nava, M.A.; Graham, B.D.; Adhikari, B.; Agnello, M.; Selby, C.M.; Hernandez-Velasco, X.; Vuong, C.N.; Solis-Cruz, B.; Hernandez-Patlan, D.; Latorre, J.D.; et al. Evaluation of in ovo Bacillus spp. based probiotic administration on horizontal transmission of virulent Escherichia coli in neonatal broiler chickens. Poult. Sci. 2019, 98, 6483–6491. [Google Scholar] [CrossRef]
- Wilson, K.M.; Rodrigues, D.R.; Briggs, W.N.; Duff, A.F.; Chasser, K.M.; Bielke, L.R. Evaluation of the impact of in ovo administered bacteria on microbiome of chicks through 10 days of age. Poult. Sci. 2019, 98, 5949–5960. [Google Scholar] [CrossRef]
- Slizewska, K.; Chlebicz, A. Synbiotics impact on dominant faecal microbiota and short-chain fatty acids production in sows. FEMS Microbiol. Lett. 2019, 366, i133–i146. [Google Scholar] [CrossRef] [Green Version]
- Villagran-de la Mora, Z.; Nuno, K.; Vazquez-Paulino, O.; Avalos, H.; Castro-Rosas, J.; Gomez-Aldapa, C.; Angulo, C.; Ascencio, F.; Villarruel-Lopez, A. Effect of a Synbiotic Mix on Intestinal Structural Changes, and Salmonella Typhimurium and Clostridium Perfringens Colonization in Broiler Chickens. Animals 2019, 9, 777. [Google Scholar] [CrossRef] [Green Version]
- Veljovic, K.; Dinic, M.; Lukic, J.; Mihajlovic, S.; Tolinacki, M.; Zivkovic, M.; Begovic, J.; Mrvaljevic, I.; Golic, N.; Terzic-Vidojevic, A. Promotion of Early Gut Colonization by Probiotic Intervention on Microbiota Diversity in Pregnant Sows. Front. Microbiol. 2017, 8, 2028. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Vahjen, W.; Dadi, T.; Saliu, E.M.; Boroojeni, F.G.; Zentek, J. Synergistic Effects of Probiotics and Phytobiotics on the Intestinal Microbiota in Young Broiler Chicken. Microorganisms 2019, 7, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cesare, A.; Caselli, E.; Lucchi, A.; Sala, C.; Parisi, A.; Manfreda, G.; Mazzacane, S. Impact of a probiotic-based cleaning product on the microbiological profile of broiler litters and chicken caeca microbiota. Poult. Sci. 2019, 98, 3602–3610. [Google Scholar] [CrossRef]
- Marti, M.; Spreckels, J.E.; Ranasinghe, P.D.; Wejryd, E.; Marchini, G.; Sverremark-Ekstrom, E.; Jenmalm, M.C.; Abrahamsson, T. Effects of Lactobacillus reuteri supplementation on the gut microbiota in extremely preterm infants in a randomized placebo-controlled trial. Cell Rep. Med. 2021, 2, 100206. [Google Scholar] [CrossRef]
- Wieers, G.; Verbelen, V.; Van Den Driessche, M.; Melnik, E.; Vanheule, G.; Marot, J.C.; Cani, P.D. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota With Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front. Public Health 2020, 8, 578089. [Google Scholar] [CrossRef]
- Zhou, Y.; Ni, X.; Duan, L.; Niu, L.; Liu, Q.; Zeng, Y.; Wang, Q.; Wang, J.; Khalique, A.; Pan, K.; et al. Lactobacillus plantarum BSGP201683 Improves the Intestinal Barrier of Giant Panda Microbiota-Associated Mouse Infected by Enterotoxigenic Escherichia coli K88. Probiotics Antimicrob. Proteins 2021, 13, 664–676. [Google Scholar] [CrossRef]
- Ljungquist, O.; Kampmann, C.; Resman, F.; Riesbeck, K.; Tham, J. Probiotics for intestinal decolonization of ESBL-producing Enterobacteriaceae: A randomized, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2020, 26, 456–462. [Google Scholar] [CrossRef]
- Nguyen, M.; Holdbrooks, H.; Mishra, P.; Abrantes, M.A.; Eskew, S.; Garma, M.; Oca, C.G.; McGuckin, C.; Hein, C.B.; Mitchell, R.D.; et al. Impact of Probiotic B. infantis EVC001 Feeding in Premature Infants on the Gut Microbiome, Nosocomially Acquired Antibiotic Resistance, and Enteric Inflammation. Front. Pediatr. 2021, 9, 618009. [Google Scholar] [CrossRef]
- Ramos-Ramos, J.C.; Lazaro-Perona, F.; Arribas, J.R.; Garcia-Rodriguez, J.; Mingorance, J.; Ruiz-Carrascoso, G.; Borobia, A.M.; Pano-Pardo, J.R.; Herruzo, R.; Arnalich, F. Proof-of-concept trial of the combination of lactitol with Bifidobacterium bifidum and Lactobacillus acidophilus for the eradication of intestinal OXA-48-producing Enterobacteriaceae. Gut Pathog. 2020, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.F.; Zhu, C.R.; Gong, X.L.; Li, H.L.; Xiong, L.K.; Wang, K.J.; Liu, G.S. Beneficial Effects of Probiotic Treatment on Gut Microbiota in Very Low Birth Weight Infants. Gastroenterol. Res. Pract. 2019, 2019, 3682836. [Google Scholar] [CrossRef]
- Ishizeki, S.; Sugita, M.; Takata, M.; Yaeshima, T. Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administered bifidobacteria: A comparison between one-species and three-species administration. Anaerobe 2013, 23, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.T.; Borghese, R.A.; Kalanetra, K.M.; Mirmiran, M.; Mills, D.A.; Underwood, M.A. Probiotic Administration in Infants With Gastroschisis: A Pilot Randomized Placebo-Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 852–857. [Google Scholar] [CrossRef]
- Wang, C.; Nagata, S.; Asahara, T.; Yuki, N.; Matsuda, K.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Yamashiro, Y. Intestinal Microbiota Profiles of Healthy Pre-School and School-Age Children and Effects of Probiotic Supplementation. Ann. Nutr. Metab. 2015, 67, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.B.; Yang, Y.; Xu, X.; Wang, W.P. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J. Pediatr. 2016, 12, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Fornasero, S.; Ceratto, S.; De Marco, A.; Mandras, N.; Roana, J.; Tullio, V.; Amisano, G. Probiotics and gut health in infants: A preliminary case-control observational study about early treatment with Lactobacillus reuteri DSM 17938. Clin. Chim. Acta 2015, 451, 82–87. [Google Scholar] [CrossRef]
- Umenai, T.; Shime, N.; Asahara, T.; Nomoto, K.; Itoi, T. A pilot study of Bifidobacterium breve in neonates undergoing surgery for congenital heart disease. J. Intensive Care 2014, 2, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R.; Koebnick, C.; Schildt, J.; Schmidt, S.; Mueller, M.; Possner, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: A double-blind, placebo-controlled, randomized study. J. Clin. Microbiol. 2006, 44, 4025–4031. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowska-Liszewska, D.; Seliga-Siwecka, J.; Kornacka, M.K. The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Hum. Dev. 2012, 88, 57–60. [Google Scholar] [CrossRef]
- Szajewska, H.; Guandalini, S.; Morelli, L.; Van Goudoever, J.B.; Walker, A. Effect of Bifidobacterium animalis subsp lactis supplementation in preterm infants: A systematic review of randomized controlled trials. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La-Ongkham, O.; Nakphaichit, M.; Leelavatcharamas, V.; Keawsompong, S.; Nitisinprasert, S. Distinct gut microbiota of healthy children from two different geographic regions of Thailand. Arch. Microbiol. 2015, 197, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Dall, L.B.; Lausch, K.R.; Gedebjerg, A.; Fuursted, K.; Storgaard, M.; Larsen, C.S. Do probiotics prevent colonization with multi-resistant Enterobacteriaceae during travel? A randomized controlled trial. Travel Med. Infect. Dis. 2019, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Arnbjerg, C.J.; Vestad, B.; Hov, J.R.; Pedersen, K.K.; Jespersen, S.; Johannesen, H.H.; Holm, K.; Halvorsen, B.; Fallentin, E.; Hansen, A.E.; et al. Effect of Lactobacillus rhamnosus GG Supplementation on Intestinal Inflammation Assessed by PET/MRI Scans and Gut Microbiota Composition in HIV-Infected Individuals. J. Acquir. Immune Defic. Syndr. 2018, 78, 450–457. [Google Scholar] [CrossRef]
- Nagino, T.; Kaga, C.; Kano, M.; Masuoka, N.; Anbe, M.; Moriyama, K.; Maruyama, K.; Nakamura, S.; Shida, K.; Miyazaki, K. Effects of fermented soymilk with Lactobacillus casei Shirota on skin condition and the gut microbiota: A randomised clinical pilot trial. Benef. Microbes 2018, 9, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; Puri, P.; Sterling, R.K.; Luketic, V.; Stravitz, R.T.; Siddiqui, M.S.; Fuchs, M.; et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment. Pharmacol. Ther. 2014, 39, 1113–1125. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gobel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin. Nutr. 2013, 32, 935–940. [Google Scholar] [CrossRef]
- Mangell, P.; Thorlacius, H.; Syk, I.; Ahrne, S.; Molin, G.; Olsson, C.; Jeppsson, B. Lactobacillus plantarum 299v does not reduce enteric bacteria or bacterial translocation in patients undergoing colon resection. Dig. Dis. Sci. 2012, 57, 1915–1924. [Google Scholar] [CrossRef]
- Oudhuis, G.J.; Bergmans, D.C.; Dormans, T.; Zwaveling, J.H.; Kessels, A.; Prins, M.H.; Stobberingh, E.E.; Verbon, A. Probiotics versus antibiotic decontamination of the digestive tract: Infection and mortality. Intensive Care Med. 2011, 37, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.S.; Macfie, J.; Gatt, M.; Larsen, C.N.; Jensen, S.S.; Leser, T.D. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br. J. Surg. 2007, 94, 546–554. [Google Scholar] [CrossRef] [PubMed]
- More, M.I.; Swidsinski, A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis—A review. Clin. Exp. Gastroenterol. 2015, 8, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Andrejcakova, Z.; Sopkova, D.; Vlckova, R.; Hertelyova, Z.; Gancarcikova, S.; Nemcova, R. The Application of Lactobacillus reuteri CCM 8617 and Flaxseed Positively Improved the Health of Mice Challenged with Enterotoxigenic E. coli O149:F4. Probiotics Antimicrob. Proteins 2020, 12, 937–951. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Nuccio, S.P.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, R.A.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef]
- Litvak, Y.; Mon, K.K.Z.; Nguyen, H.; Chanthavixay, G.; Liou, M.; Velazquez, E.M.; Kutter, L.; Alcantara, M.A.; Byndloss, M.X.; Tiffany, C.R.; et al. Commensal Enterobacteriaceae Protect against Salmonella Colonization through Oxygen Competition. Cell Host Microbe 2019, 25, 128–139. [Google Scholar] [CrossRef] [Green Version]
- D’Inca, R.; Barollo, M.; Scarpa, M.; Grillo, A.R.; Brun, P.; Vettorato, M.G.; Castagliuolo, I.; Sturniolo, G.C. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig. Dis. Sci. 2011, 56, 1178–1187. [Google Scholar] [CrossRef]
- Palmer, J.D.; Piattelli, E.; McCormick, B.A.; Silby, M.W.; Brigham, C.J.; Bucci, V. Engineered Probiotic for the Inhibition of Salmonella via Tetrathionate-Induced Production of Microcin H47. ACS Infect. Dis. 2018, 4, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Zaheer, R.; Adator, E.H.; Barbieri, R.; Reuter, T.; McAllister, T.A. Bacteriocin Occurrence and Activity in Escherichia coli Isolated from Bovines and Wastewater. Toxins 2019, 11, 475. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 23 August 2021).
- Giuffre, M.; Campigotto, M.; Campisciano, G.; Comar, M.; Croce, L.S. A story of liver and gut microbes: How does the intestinal flora affect liver disease? A review of the literature. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G889–G906. [Google Scholar] [CrossRef]
- Vyas, V.; Mian, S.; Paolino, K.; Siddique, Z. Lactobacillus masticator abscess after probiotics consumption. Bayl. Univ. Med. Cent. Proc. 2020, 34, 93–94. [Google Scholar] [CrossRef]
- Chiang, M.C.; Chen, C.L.; Feng, Y.; Chen, C.C.; Lien, R.; Chiu, C.H. Lactobacillus rhamnosus sepsis associated with probiotic therapy in an extremely preterm infant: Pathogenesis and a review for clinicians. J. Microbiol. Immunol. Infect. 2020, 54, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Sendil, S.; Shrimanker, I.; Mansoora, Q.; Goldman, J.; Nookala, V.K. Lactobacillus rhamnosus Bacteremia in an Immunocompromised Renal Transplant Patient. Cureus 2020, 12, e6887. [Google Scholar] [CrossRef] [Green Version]
- Pasala, S.; Singer, L.; Arshad, T.; Roach, K. Lactobacillus endocarditis in a healthy patient with probiotic use. IDCases 2020, 22, e00915. [Google Scholar] [CrossRef] [PubMed]
- Egervarn, M.; Lindmark, H.; Olsson, J.; Roos, S. Transferability of a tetracycline resistance gene from probiotic Lactobacillus reuteri to bacteria in the gastrointestinal tract of humans. Antonie Van Leeuwenhoek 2010, 97, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Antibiotic resistance propagation through probiotics. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
| First Author | Country | Publish Year | Patient Population/Number | Probiotics | Main Findings after Probiotic Supplementation | References |
|---|---|---|---|---|---|---|
| Mohan R | Germany | 2006 | Preterm infants/69 | Bifidobacterium lactis Bb12 | Lower viable counts of Enterobacterales | [57] |
| Chrzanowska-Liszewska D | Poland | 2012 | Bottle fed preterm/60 | Lactobacillus rhamnosus GG (LGG) | Increase number of Enterobacterales in gut | [58] |
| Umenai T | Japan | 2014 | Neonates undergoing cardiac surgery/21 | B. breve strain Yakult (BBG-01) | Significantly fewer Enterobacterales in gut | [56] |
| Savino F | Italy | 2015 | Hospitalized infant/60 | L. reuteri DSM 17938 | Less colonization by diarrheagenic E. coli. | [55] |
| Wang C | Japan | 2015 | In preschool and school-age children/23 | L. casei strain Shirota | Increased population levels of Bifidobacterium and total Lactobacillus, decreased Enterobacterales, Staphylococcus and Clostridium perfringens | [53] |
| Wu BB | China | 2016 | Healthy newborns/300 | B. longum BB536 | Higher Bifidobacterium/Enterobacterales ratio and increased the ratio of IFN-γ/IL-4 secretion cells | [54] |
| Powell WT | USA | 2016 | Infants/24 | B. longum subsp. infantis | Overall, microbial communities were not significantly influenced, with trends only toward lower Enterobacterales | [52] |
| Li YF | China | 2019 | Low birth weight infants/36 | L. plantarum LK006, B. longum LK014, and B. bifidum LK012 | Increase in Streptococcaceae and Lactobacillaceae and decrease in Enterobacterales | [50] |
| Nguyen M | USA | 2021 | Infants/77 | B. infantis | Reduced abundance of antibiotic resistance genes among Enterobacterales and Staphylococcaceae | [48] |
| Martí M | Sweden | 2021 | First month/132 | L. reuteri | Lower abundance of Enterobacterales and Staphylococcaceae | [44] |
| First Author | Country | Publish Year | Patient Number | Probiotics | Main Findings after Probiotic Supplementation | References |
|---|---|---|---|---|---|---|
| Mangell P | Sweden | 2012 | 75 | Lactobacillus plantarum 299v | Increased Enterobacterales and Gram-negative anaerobes in the colon 1 week after probiotics without change in the incidence of bacterial translocation and postoperative complications | [66] |
| Larsen N | Denmark | 2013 | 50 | L. salivarius Ls-33 | No significant influence on Clostridium cluster I, Clostridium cluster IV, Faecalibacterium prausnitzii, Enterobacterales, Enterococcus, the Lactobacillus group, and Bifidobacterium | [65] |
| Bajaj JS | USA | 2014 | 30 | L. rhamnosus GG | Among cirrhotic patients with minimal hepatic encephalopathy, reduced Enterobacterales and increased Clostridiales Family XIV Incertae Sedis and Lachnospiraceae relative abundance, but no change in cognition | [64] |
| Nagino T | Japan | 2018 | 60 | L. casei Shirota | Consecutive intake of fermented soymilk (containing isoflavone), and L. casei Shirota decreased the levels of Enterobacterales | [63] |
| Arnbjerg CJ | Denmark | 2018 | 45 | L. rhamnosus GG | Decrease in intestinal inflammation, along with a reduction of Enterobacterales in the gut microbiome among human- immunodeficiency-virus-infected individuals | [62] |
| Dall LB | Denmark | 2019 | 31 | L. rhamnosus GG | No effect on the risk of colonization with extended spectrum β-lactamase (ESBL)-Enterobacterales | [61] |
| Ljungquist O | Sweden | 2020 | 80 | Vivomixx® 1 | No support of Vivomixx® as being superior to the placebo for intestinal decolonization in adult patients with chronic colonization of ESBL-producing Enterobacterales | [47] |
| Ramos-Ramos JC | Spain | 2020 | 8 | B. bifidum and L. acidophilus (Infloran®) | Three weeks of a combination of prebiotics and probiotics decreased the intestinal load of OXA-48-producing Enterobacterales | [49] |
| Wieërs G | Belgium | 2021 | 120 | Bactiol duo® 2 | Colonization with AmpC-producing Enterobacterales declined after the probiotic intervention | [45] |
| ClinicalTrials.gov Identifier | Official Title | First Posted | Study Design/Case Number | Probiotic Strain | Location | Outcome Measures | Status |
|---|---|---|---|---|---|---|---|
| NCT 00722410 | Safety and efficacy study of eradication of carbapenem- resistant Klebsiella pneumoniae from the gastrointestinal tract by probiotics | 25 July 2008 | Open-label, randomized/60 | VSL#3® 1 | Jerusalem, Israel | Negative stool culture for carbapenem-resistant Klebsiella pneumoniae | Not yet recruiting |
| NCT 03967301 | Prevention and decolonization of multidrug-resistant bacteria with probiotics | 30 May 2019 | Double-blinded, randomized/228 | Bioflora® 2 | Buenos Aires, Argentina | Risk of colonization and/or infection by carbapenem-resistant Enterobacterales | Not yet recruiting |
| NCT 04431934 | Open-label, randomized study to assess the efficacy of a probiotic or fecal microbiota transplantation (FMT) on the eradication of rectal multidrug-resistant Gram-negative bacilli (MDR-GNB) carriage (PROFTMDECOL) | 16 June 2020 | Open-label, randomized/437 | Vivomixx® 3 | Barcelona, Spain | Eradication of rectal multidrug-resistant Gram-negative bacilli carriage | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-P.; Lee, C.-C.; Lee, J.-C.; Tsai, P.-J.; Hsueh, P.-R.; Ko, W.-C. The Potential of Probiotics to Eradicate Gut Carriage of Pathogenic or Antimicrobial-Resistant Enterobacterales. Antibiotics 2021, 10, 1086. https://doi.org/10.3390/antibiotics10091086
Hung Y-P, Lee C-C, Lee J-C, Tsai P-J, Hsueh P-R, Ko W-C. The Potential of Probiotics to Eradicate Gut Carriage of Pathogenic or Antimicrobial-Resistant Enterobacterales. Antibiotics. 2021; 10(9):1086. https://doi.org/10.3390/antibiotics10091086
Chicago/Turabian StyleHung, Yuan-Pin, Ching-Chi Lee, Jen-Chieh Lee, Pei-Jane Tsai, Po-Ren Hsueh, and Wen-Chien Ko. 2021. "The Potential of Probiotics to Eradicate Gut Carriage of Pathogenic or Antimicrobial-Resistant Enterobacterales" Antibiotics 10, no. 9: 1086. https://doi.org/10.3390/antibiotics10091086







