Extended or Continuous Infusion of Carbapenems in Children with Severe Infections: A Systematic Review and Narrative Synthesis
Abstract
1. Introduction
2. Results
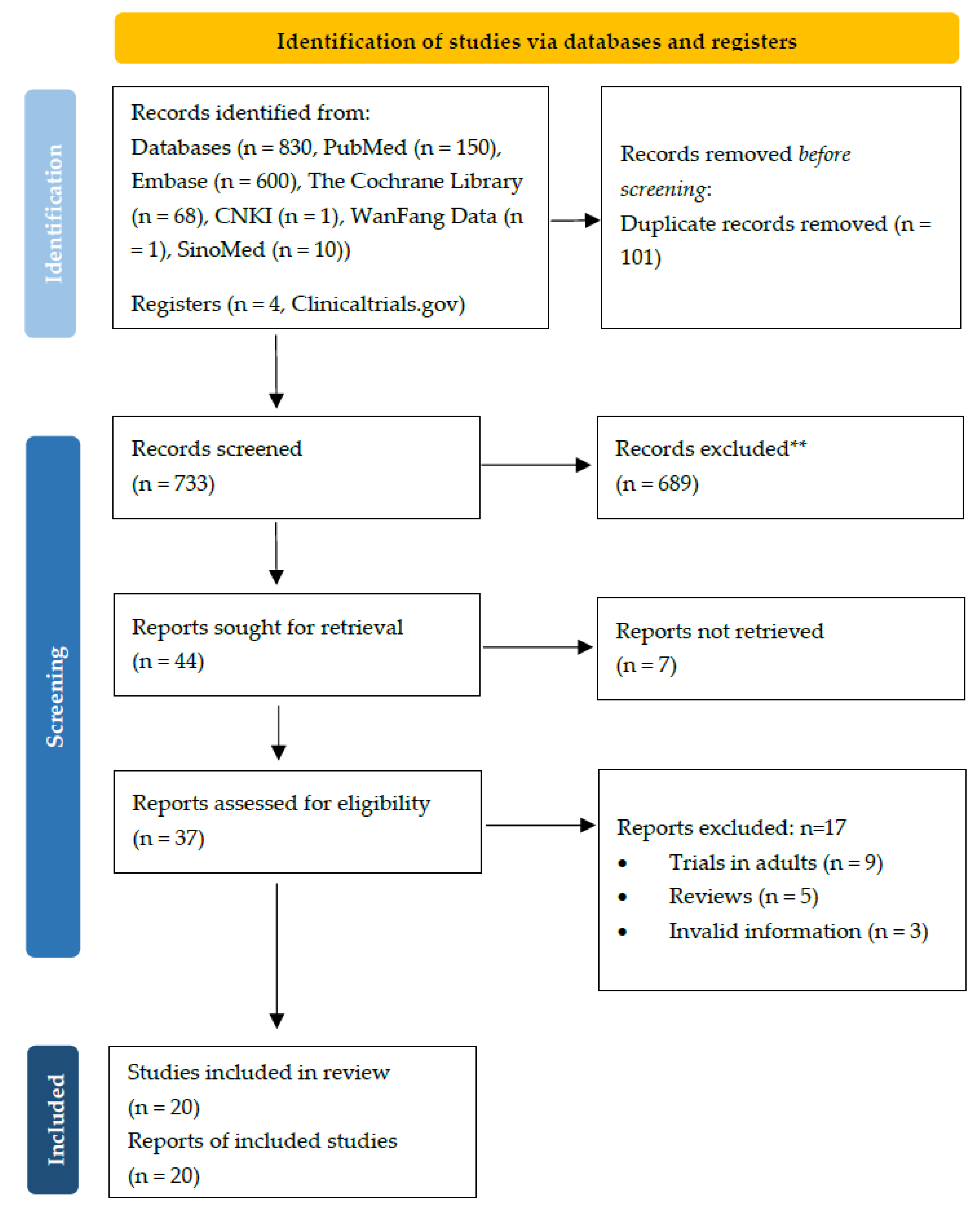
2.1. Description of Included Studies
2.2. RCTs
2.3. Observational Studies
2.4. PK/PPK Data
3. Discussion
4. Materials and Methods
4.1. Eligibility Criteria
4.2. Search Strategy
4.3. Data Extraction
4.4. Quality Assessment
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| EI | extended infusion |
| CI | continuous infusion |
| STI | short-term intravenous infusion |
| RCTs | randomized controlled trials |
| PK | pharmacokinetic |
| PPK | population pharmacokinetic |
| PD | pharmacodynamic |
| MER | meropenem |
| MDR | multi-drug resistant |
| MIC | minimum inhibitory concentration |
| %fT > MIC | the time at which free concentrations remain above the minimum inhibitory concentration |
| GRADE | grading of Recommendations Assessment, Development and Evaluation approach |
| LOS | late-onset sepsis |
| AEs | adverse events |
| PTA | probability of target attainment |
| CFR | cumulative fraction of response |
| PRISMA | the preferred reporting items for systematic review and meta-analyses |
| AMSTAR | a measurement tool to assess systematic reviews |
References
- Zhanel, G.G.; Wiebe, R.; Dilay, L.; Thomson, K.; Rubinstein, E.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Comparative Review of the Carbapenems. Drugs 2007, 67, 1027–1052. [Google Scholar] [CrossRef]
- Kattan, J.N.; Villegas, M.V.; Quinn, J.P. New developments in carbapenems. Clin. Microbiol. Infect. 2008, 14, 1102–1111. [Google Scholar] [CrossRef]
- Ayalew, K.; Nambiar, S.; Yasinskaya, Y.; Jantausch, B.A. Carbapenems in Pediatrics. Ther. Drug Monit. 2003, 25, 593–599. [Google Scholar] [CrossRef]
- Hsu, A.J.; Tamma, P.D. Treatment of Multidrug-Resistant Gram-Negative Infections in Children. Clin. Infect. Dis. 2014, 58, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P. Carbapenems: A potent class of antibiotics. Expert Opin. Pharmacother. 2007, 9, 23–37. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Shabaan, A.E.; Nour, I.; Elsayed Eldegla, H.; Nasef, N.; Shouman, B.; Abdel-Hady, H. Conventional Versus Prolonged Infusion of Meropenem in Neonates with Gram-negative Late-onset Sepsis: A Randomized Controlled Trial. Pediatr. Infect. Dis. J. 2017, 36, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ni, S.W.; Zhu, K.R.; Wang, Y.; Wang, L.L.; Deng, F. Efficacy of prolonged intravenous infusion duration of meropenem on neonatallate-onset-sepsis. Guangxi Med. J. 2018, 40, 372. [Google Scholar]
- Lu, S.; Chen, C.; Xue, H.; Zhang, B.H.; Fang, J.P. Evaluation of meropenem as initial therapy for nosocomial infections in children with leukemia and agranulocytosis. Chin. J. Infect. Chemother. 2010, 10, 277. [Google Scholar]
- Saito, J.; Shoji, K.; Oho, Y.; Aoki, S.; Matsumoto, S.; Yoshida, M.; Nakamura, H.; Kaneko, Y.; Hayashi, T.; Yamatani, A.; et al. Meropenem pharmacokinetics during extracorporeal membrane oxygenation and continuous haemodialysis: A case report. J. Glob. Antimicrob. Resist. 2020, 22, 651–655. [Google Scholar] [CrossRef]
- Cies, J.J.; Moore, W.S., 2nd; Dickerman, M.J.; Small, C.; Carella, D.; Chopra, A.; Parker, J. Pharmacokinetics of continu-ous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy 2014, 34, e175–e179. [Google Scholar] [CrossRef] [PubMed]
- Cies, J.; Moore, W.; Dickerman, M.; Small, C.; Carella, D.; Shea, P.; Parker, J.; Chopra, A. Pharmacokinetics of continuous infusion meropenem with extra-corporeal life support and crrt. Crit. Care Med. 2014, 42, A1630–A1631. [Google Scholar] [CrossRef]
- Falagas, M.E.; Siempos, I.I.; Tsakoumis, I. Cure of persistent, post-appendectomy klebsiella pneumoniae septicaemia with continuous intravenous administration of meropenem. Scand. J. Infect. Dis. 2006, 38, 807–810. [Google Scholar] [CrossRef]
- Cies, J.J.; Moore, W.S., 2nd; Calaman, S.; Brown, M.; Narayan, P.; Parker, J.; Chopra, A. Pharmacokinetics of continuous-infusion meropenem for the treatment of serratia marcescens ventriculitis in a pediatric patient. Pharmacotherapy 2015, 35, e32–e36. [Google Scholar] [CrossRef] [PubMed]
- Zobell, J.T.; Ferdinand, C.; Young, D.C. Continuous infusion meropenem and ticarcillin-clavulanate in pediatric cystic fibrosis patients. Pediatr. Pulmonol. 2014, 49, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Kongthavonsakul, K.; Lucksiri, A.; Eakanunkul, S.; Roongjang, S.; Na Ayuthaya, S.I.; Oberdorfer, P. Pharmacokinetics and pharmacodynamics of meropenem in children with severe infection. Int. J. Antimicrob. Agents 2016, 48, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Courter, J.D.; Kuti, J.L.; Girotto, J.E.; Nicolau, D.P. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr. Blood Cancer 2009, 53, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.S.; Neu, N.; Cies, J.J.; Lapin, C.; Muhlebach, M.S.; Novak, K.J.; Nguyen, S.T.; Saiman, L.; Nicolau, D.P.; Kuti, J.L. Population pharmacokinetics of meropenem administered as a prolonged infusion in children with cystic fibrosis. J. Antimicrob. Chemother. 2016, 71, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.E.; Ivaturi, V.; Gobburu, J.; Green, T.P. Dosage regimens for meropenem in children with pseudomonas infections do not meet serum concentration targets. Clin. Transl. Sci. 2020, 13, 301–308. [Google Scholar] [CrossRef] [PubMed]
- van den Anker, J.N.; Pokorna, P.; Kinzig-Schippers, M.; Martinkova, J.; de Groot, R.; Drusano, G.L.; Sorgel, F. Meropenem pharmacokinetics in the newborn. Antimicrob. Agents Chemother. 2009, 53, 3871–3879. [Google Scholar] [CrossRef]
- Padari, H.; Metsvaht, T.; Kõrgvee, L.T.; Germovsek, E.; Ilmoja, M.L.; Kipper, K.; Herodes, K.; Standing, J.F.; Oselin, K.; Lutsar, I. Short versus long infusion of meropenem in very-low-birth-weight neonates. Antimicrob. Agents Chemother. 2012, 56, 4760–4764. [Google Scholar] [CrossRef] [PubMed]
- Cies, J.; Moore, W.; Chopra, A. Population pharmacokinetics of meropenem in a pediatric icu population. Crit. Care Med. 2014, 42, A1516. [Google Scholar] [CrossRef]
- Rapp, M.; Urien, S.; Foissac, F.; Béranger, A.; Bouazza, N.; Benaboud, S.; Bille, E.; Zheng, Y.; Gana, I.; Moulin, F.; et al. Population pharmacokinetics of meropenem in critically ill children with different renal functions. Eur. J. Clin. Pharmacol. 2020, 76, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.; Maximova, N.; Pea, F. Pharmacokinetics and pharmacodynamics of continuous-infusion meropenem in pediatric hematopoietic stem cell transplant patients. Antimicrob. Agents Chemother. 2015, 59, 5535–5541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Chen, X.Y.; Bi, J.; Wang, M.Y.; Xu, B.P.; Tang, B.H.; Li, C.; Zhao, W.; Shen, A.D. Reappraisal of the optimal dose of meropenem in critically ill infants and children: A developmental pharmacokinetic-pharmacodynamic analysis. Antimicrob. Agents Chemother. 2020, 64, e00760-20. [Google Scholar]
- Germovsek, E.; Lutsar, I.; Kipper, K.; Karlsson, M.O.; Planche, T.; Chazallon, C.; Meyer, L.; Trafojer, U.M.; Metsvaht, T.; Fournier, I.; et al. Plasma and csf pharmacokinetics of meropenem in neonates and young infants: Results from the neomero studies. J. Antimicrob. Chemother. 2018, 73, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef]
- Breilh, D.; Texier-Maugein, J.; Allaouchiche, B.; Saux, M.C.; Boselli, E. Carbapenems. J. Chemother. 2013, 25, 1–17. [Google Scholar] [CrossRef]
- Chen, C.; Ying, Y.Q.; Yan, Y.Y.; Zhai, S.D. Efficacy and safety of extended or continuous intravenous infusion of carbapenems against severe infection: A systematic review. Chin. Hosp. Pharm. J. 2017, 37, 1622. [Google Scholar]
- Falagas, M.E.; Tansarli, G.S.; Ikawa, K.; Vardakas, K.Z. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: A systematic review and meta-analysis. Clin. Infect. Dis. 2013, 56, 272–282. [Google Scholar]
- Yu, Z.; Pang, X.; Wu, X.; Shan, C.; Jiang, S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PLoS ONE 2018, 13, e0201667. [Google Scholar] [CrossRef]
- Hartman, S.J.; Brüggemann, R.J.; Orriëns, L.; Dia, N.; Schreuder, M.F.; de Wildt, S.N. Pharmacokinetics and target attainment of antibiotics in critically ill children: A systematic review of current literature. Clin. Pharmacokinet. 2020, 59, 173–205. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.C.; Lam, W.M.; Manasco, K.B. Continuous and extended infusions of β-lactam antibiotics in the pediatric population. Ann. Pharmacother. 2012, 46, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; Marriott, J.F. Paediatric pharmacokinetics: Key considerations. Br. J. Clin. Pharmacol. 2015, 79, 395–404. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garnett, C.E.; Gobburu, J.V.; Bhattaram, V.A.; Brar, S.; Earp, J.C.; Jadhav, P.R.; Krudys, K.; Lesko, L.J.; Li, F.; et al. Impact of pharmacometric analyses on new drug approval and labelling decisions: A review of 198 submissions between 2000 and 2008. Clin. Pharmacokinet. 2011, 50, 627–635. [Google Scholar] [CrossRef]
- Manolis, E.; Osman, T.E.; Herold, R.; Koenig, F.; Tomasi, P.; Vamvakas, S.; Saint Raymond, A. Role of modeling and simulation in pediatric investigation plans. Paediatr. Anaesth. 2011, 21, 214–221. [Google Scholar] [CrossRef]
- Fawaz, S.; Barton, S.; Whitney, L.; Swinden, J.; Nabhani-Gebara, S. Stability of meropenem after reconstitution for administration by prolonged infusion. Hosp. Pharm. 2019, 54, 190–196. [Google Scholar] [CrossRef]
- Psathas, P.A.; Kuzmission, A.; Ikeda, K.; Yasuo, S. Stability of doripenem in vitro in representative infusion solutions and infusion bags. Clin. Ther. 2008, 30, 2075–2087. [Google Scholar] [CrossRef]
- Soman, R.; Gupta, N.; Shetty, A.; Rodrigues, C. Are prolonged/continuous infusions of β-lactams for all? Clin. Infect. Dis. 2013, 57, 323. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. Amstar 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
| Reference | Study Design | Country | Disease and Pathogen | Age (T/C) | Sample Size (T/C) | Dosage Regimen (Doses, Dosing Interval and Infusion Time) | Efficacy Outcomes and Evaluation Time | Safety Outcomes |
|---|---|---|---|---|---|---|---|---|
| Shabaan 2017 [7] | RCT | Egypt | Gram-negative late-onset sepsis; most frequently Klebsiell a species and E. coli | 33.5 w ± 3.8/ 34.3 w ± 3.5 | 102 (51/51) | meningitis infection: 20 mg/kg/dose q8 h; Pseudomonas infection: 40 mg/kg/dose q8 h T: MER > 4 h C: MER 0.5 h | primary outcome: clinical improvement rate; microbiologic eradication rate (D7) secondary outcomes: mortality; meropenem-related (MR) duration of mechanical ventilation; MR length of NICU stay; total length of NICU stay; duration of respiratory support; duration of mechanical ventilation, MR duration of inotropes; CRP concentrations (D7) | incidence of MR-AEs (D7): AKI, diarrhea, rash, seizures, nausea and vomiting and increased levels of liver transaminases |
| Wang 2018 [8] | RCT | China | LOS; NA | 30.3 w ± 2.1/ 30.5 ± 3.1 | 120 (60/60) | MER 20 mg/kg/dose q8 h T: 3 h C: 0.5 h | WBC count, N%, levels of CRP and PCT, duration of clinical symptoms remission; mortality; rate of assisted ventilation treatment | incidence of AKI, levels of ALT, AST, urea nitrogen and creatinine, skin rash and gastrointestinal symptoms |
| Lu 2010 [9] | Case series | China | nosocomial infections with leukemia and agranulocytosis; blood culture positive in 4 cases * | 9 mo~15 y (mean age: 5.4 y) | 41 | MER 20 mg/kg/dose q8 h over 3 h | clinical effective rate (D3) | incidence of AEs |
| Saito 2020 [10] | case report | Japan | ECMO with continuous hemodialysis for postoperative management; bacteremia; ESBL-E. coli | 19 mo | 1 | MER POD 2, 120 mg/kg/d q8 h 3 h; after two infusions, 200 mg/kg/d q8 h 3 h; POD 5, 300 mg/kg/d q8 h 3 h | blood culture result, clinical outcome | NA |
| Cies-1 2014 [11] | Case report | United states | ECLS-ECMO; Pseudomonas aeruginosa; MIC = 0.5 μg/mL | 8 mo | 1 | MER D13 40 mg/kg 0.5 h, followed by 200 mg/kg/d, CI | serum MER concentration (8 h, D3 and D9), blood culture result at D13–21 of ECLS or decannulation, PTA | NA |
| Cies-2 2014 [12] | Case report | United states | MIC = 0.25 μg/mL ECLS-CRRT, sepsis, Pseudomonas aeruginosa; MIC = 0.25 mcg/mL | 10 d | 1 | MER D12 40 mg/kg 0.5 h, followed by 240 mg/kg/d CI | serum MER concentration (D12 and D15), blood culture result (D13–15), PTA | NA |
| Falagas 2006 [13] | Case report | Greece | post-appendectomy septicaemia; Klebsiella pneumoniae; MIC = 8 mg/L (intermediately susceptible) | 15 y | 1 | MER D9-D10 1 g q8 h ; D11–D17 2 g q8 h; D18 6 g/d CI | temperature, WBC count; continuous evaluation | NA |
| Cies 2015 [14] | Case report | United states | ventriculitis; Serratia marcescens; MIC ≤ 0.25 μg/mL | 2 y | 1 | MER D26 40 mg/kg/dose q6 h over 0.5 h; D27 200 mg/kg/d CI | serum and CSF MER concentration, PTA | NA |
| Zobell 2014 [15] | Case report | United states | cystic fibrosis, MDR Inquilinus limosus; MIC = 4 mcg/mL | 13 y | 1 | MER 500 mg (51/mg/d) q8 h, 3 g–6 g 22.5~23.5 h CI, multiple hospitalization | improvement of pulmonary function test | blood counts, renal function, and hepatic function |
| References | Study Design | PK/PPK Studies | Monte-Carlo Simulation | |||||
|---|---|---|---|---|---|---|---|---|
| Subject | Number of Patients/Blood Samples | Dosage Regimen (Doses, Dosing Interval and Infusion Time) | Number of Simulations | Dosage Regimen (Doses, Dosing Interval, and Infusion Time) | MIC (mg/L) | PK/PD Target | ||
| Bolus vs. 0.5 h~1 h vs. 3 h EI | ||||||||
| Kongthavonsakul 2016 [16] | PPK modeling and simulation | children with severe infection | 14/84 | MER 20 mg/kg/dose q8 h i.v. bolus | 10,000 | MER 20, 30, 40 mg/kg/dose q8 h i.v. bolus/1 h/3 h EI | 1, 2, 4, 8, 16 | 40% fT > MIC |
| Courter 2009 [17] | simulation | children with bacterial meningitis or other infections | 99/425 | MER 10, 20, 40 mg/kg single dose over 5 min or 0.5 h imipenem/cilastatini: 25 mg/kg 15–20 min q6 h | 5000 | MER 20/40 mg/kg/dose q8 h 0.5 h/3 h EI imipenem/cilastatin: 15/25 mg/kg q6 h 0.5 h/3 h EI | 0.016–128 | 40% fT > MIC, PTA ≥ 90%, CFR ≥ 90% |
| Pettit 2016 [18] | PPK modeling and simulation | children with CF hospitalized for an acute pulmonary exacerbation | 30/120 | MER 40 mg/kg/dose q8 h 3 h | 5000 | MER 40 mg/kg/dose q8 h 0.5 h/3 h EI | 0.03–128 | 40% fT > MIC |
| Hassan 2020 # [19] | PPK modeling and simulation | children with infections | 288/NA | MER 10–40 mg/kg/dose q8 h–q12 h | 1000 | MER < 50 kg: 20 mg/kg/dose q8 h 0.5 h; 40 mg/kg/dose q8 h 0.5 h; 20 mg/kg/dose q6 h 0.5 h; 20 mg/kg/dose q8 h 3 h EI > 50 kg: 1 g/dose q8 h 0.5 h; 2 g/dose q8 h 0.5 h; 1 g/dose q6 h 0.5 h; 1 g/dose q8 h 3 h EI | 2, 4 | 40% fT > MIC |
| 0.5 h vs. 4 h EI | ||||||||
| Anker 2009 [20] | PPK modeling and simulation | pre-term and full-term neonates | 38/342 | MER 10, 20, 40 mg/kg single dose 0.5 h | 10000 | MER 20/40 mg/kg/dose q8 h/q12 h 0.5 h/4 h EI | Up to 8 | 40% fT > MIC |
| Padari 2012 [21] | PPK model verification | very-low-birth-weight neonates | 19/114 | MER 20 mg/kg/dose q12 h 0.5/4 h | NA | NA | 2 | fT > MIC; fT > 6.2 MIC |
| Cies 2014 [22] | PPK modeling and simulation | children in PICU | 11/(2–3 per child) | MER standard doses * | 1000 | MER 40 mg/kg;q8 h; 0.5 h/4 h EI | 0.25–32 | 40% fT > MIC;PTA > 90% |
| CI | ||||||||
| Rapp 2020 [23] | PPK modeling and simulation | children in PICU | 40/121 | MER 20 mg/kg/dose q8 h over 20 min/CI | 400 | MER 20 mg/kg q8 h 20 min/3 h EI; 40 mg/kg q8 h 20 min; 60 mg/kg/d CI; 120 mg/kg/d CI | Up to 8 mg/L | 50% fT > MIC; 100% fT > MIC |
| Cojutti 2015 [24] | Retrospective PK study and simulation | underwent hematopoietic stem cell transplantation with suspected or documented Gram-negative infection | 21/44 | MER Mean 92.69 mg/kg/d CI | 10,000 | MER 15, 30, 45, 60, 90 mg/kg/d CI | 0.25–256 | Css/MIC ≥ 1 and ≥ 4, PTA ≥ 90% |
| Wang 2020 [25] | PPK modeling and simulation | critically ill infants and children (bacterial meningitis, sepsis, severe pneumonia) | 57/135 | MER Mean 23.7 ± 8.59 mg/kg, most 0.5–1 h | 100 | MER 20–60 mg/kg/dose; q6 h/q8 h/q12 h; 2/4 h infusion, CI | 1, 2, 4, 8 | 70% fT > MIC, PTA70% |
| Germovask 2018 [26] | PPK modeling and simulation | neonates and infants (sepsis, or bacterial meningitis, or pleocytosis, or a positive Gram-stain from the CSF) | 167/401 | MER meningitis: 40 mg/kg; sepsis: 20 mg/kg <32 week’s GA and <2 week’s PNA q12 h, others q8 h > 0.5 h | 1000 | MER 20/40 mg/kg bolus/CI | 0.25, 0.5, 1, 2, 4, 8, 16 | Plasma (for sepsis) or CSF (for meningitis) 61%T > MIC |
| MIC (mg/L) | Dosage (mg/kg) | References | PTA 40 % fT > MIC | ||||
|---|---|---|---|---|---|---|---|
| Bolus | 0.5 h STI | 1 h EI | 3 h EI | 4 h EI | |||
| MER | |||||||
| 1 | 20 | Courter 2009 | 92.0 | 100.0 | |||
| Kongthavonsakul 2016 | 67.8 | 100.0 | |||||
| Saito 2020 | 46.3 | 63.8 | |||||
| 40 | Courter 2009 | 98.0 | 100.0 | ||||
| Cies 2014 | 98.0 | 100.0 | |||||
| Pettit 2016 | 87.6 | >99.0 | |||||
| Saito 2020 | 60.0 | 76.3 | |||||
| 66.67 | Saito 2020 | 100.0 | 100.0 | ||||
| 100 | Saito 2020 | 100.0 | 100.0 | ||||
| 2 | 20 | Courter 2009 | 72.0 | 100.0 | |||
| Kongthavonsakul 2016 | 40.0 | 67.3 | 100.0 | ||||
| Hassan 2020 | 68.4 | >97.5 | |||||
| Padari 2012 | 100.0 | 100.0 | |||||
| 40 | Courter 2009 | 92.0 | 100.0 | ||||
| Cies 2014 | 96.0 | 100.0 | |||||
| Pettit 2016 | 70.1 | >99.0 | |||||
| 4 | 20 | Courter 2009 | 33.0 | 97.0 | |||
| Kongthavonsakul 2016 | 15.5 | 99.9 | |||||
| Hassan 2020 | 41.7 | 90.7 | |||||
| 40 | Courter 2009 | 72.0 | 100.0 | ||||
| Cies 2014 | 90.0 | 100.0 | |||||
| Pettit 2016 | 35.4 | >99.0 | |||||
| 8 | 20 | Courter 2009 | 3.0 | 54.0 | |||
| 40 | Courter 2009 | 33.0 | 97.0 | ||||
| Cies 2014 | 71.5 | 99.6 | |||||
| Pettit 2016 | 10.0 | 82.8 | |||||
| CFR (%) | |||||||
| NA | 20 | Courter 2009 | 91.0 | 95.0 | |||
| 84.0 | 98.0 | ||||||
| 40 | 94.0 | 98.0 | |||||
| 93.0 | 98.0 | ||||||
| Imipenem and Cilastatin Sodium | |||||||
| 1 | 60 | Courter 2009 | 58.0 | 100.0 | |||
| 100 | 66.0 | 100.0 | |||||
| 2 | 60 | 45.0 | 95.0 | ||||
| 100 | 55.0 | 99.0 | |||||
| 4 | 60 | 31.0 | 74.0 | ||||
| 100 | 41.0 | 91.0 | |||||
| 8 | 60 | 18.0 | 38.0 | ||||
| 100 | 27.0 | 65.0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Zhang, Y.; Wang, Z.; Ying, Y.; Xing, Y.; Tong, X.; Zhai, S. Extended or Continuous Infusion of Carbapenems in Children with Severe Infections: A Systematic Review and Narrative Synthesis. Antibiotics 2021, 10, 1088. https://doi.org/10.3390/antibiotics10091088
Zhou P, Zhang Y, Wang Z, Ying Y, Xing Y, Tong X, Zhai S. Extended or Continuous Infusion of Carbapenems in Children with Severe Infections: A Systematic Review and Narrative Synthesis. Antibiotics. 2021; 10(9):1088. https://doi.org/10.3390/antibiotics10091088
Chicago/Turabian StyleZhou, Pengxiang, Yahui Zhang, Zhenhuan Wang, Yingqiu Ying, Yan Xing, Xiaomei Tong, and Suodi Zhai. 2021. "Extended or Continuous Infusion of Carbapenems in Children with Severe Infections: A Systematic Review and Narrative Synthesis" Antibiotics 10, no. 9: 1088. https://doi.org/10.3390/antibiotics10091088
APA StyleZhou, P., Zhang, Y., Wang, Z., Ying, Y., Xing, Y., Tong, X., & Zhai, S. (2021). Extended or Continuous Infusion of Carbapenems in Children with Severe Infections: A Systematic Review and Narrative Synthesis. Antibiotics, 10(9), 1088. https://doi.org/10.3390/antibiotics10091088





