Antimicrobial Peptides: A Potent Alternative to Antibiotics
Abstract
:1. Introduction
2. Classification and Mode of Action of Antimicrobial Peptides
2.1. Classification
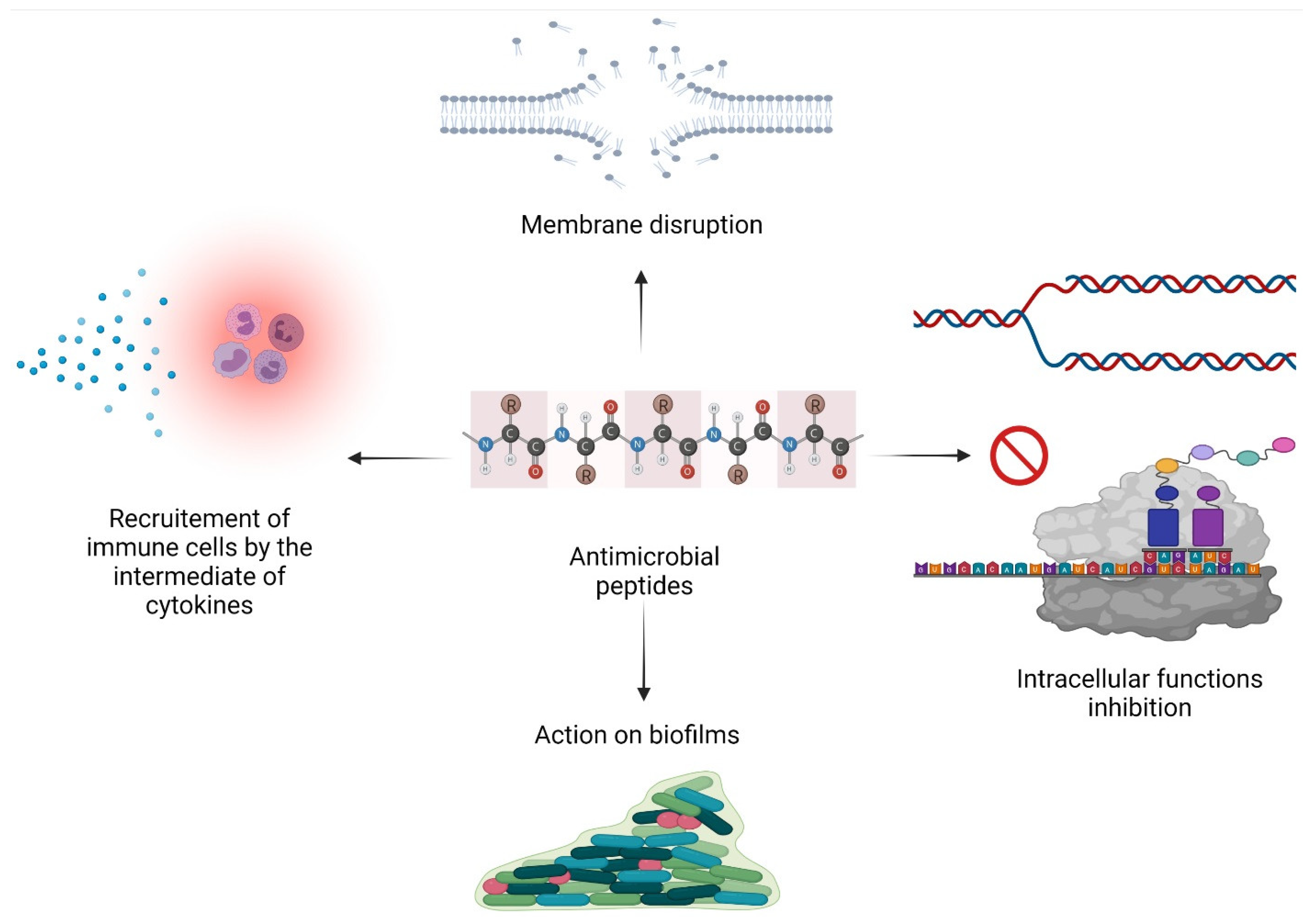
2.2. Mode of Action
2.2.1. Membrane Permeabilization
2.2.2. Inhibition of Intracellular Functions
2.2.3. Immunomodulatory Activity
2.2.4. Action on Biofilms
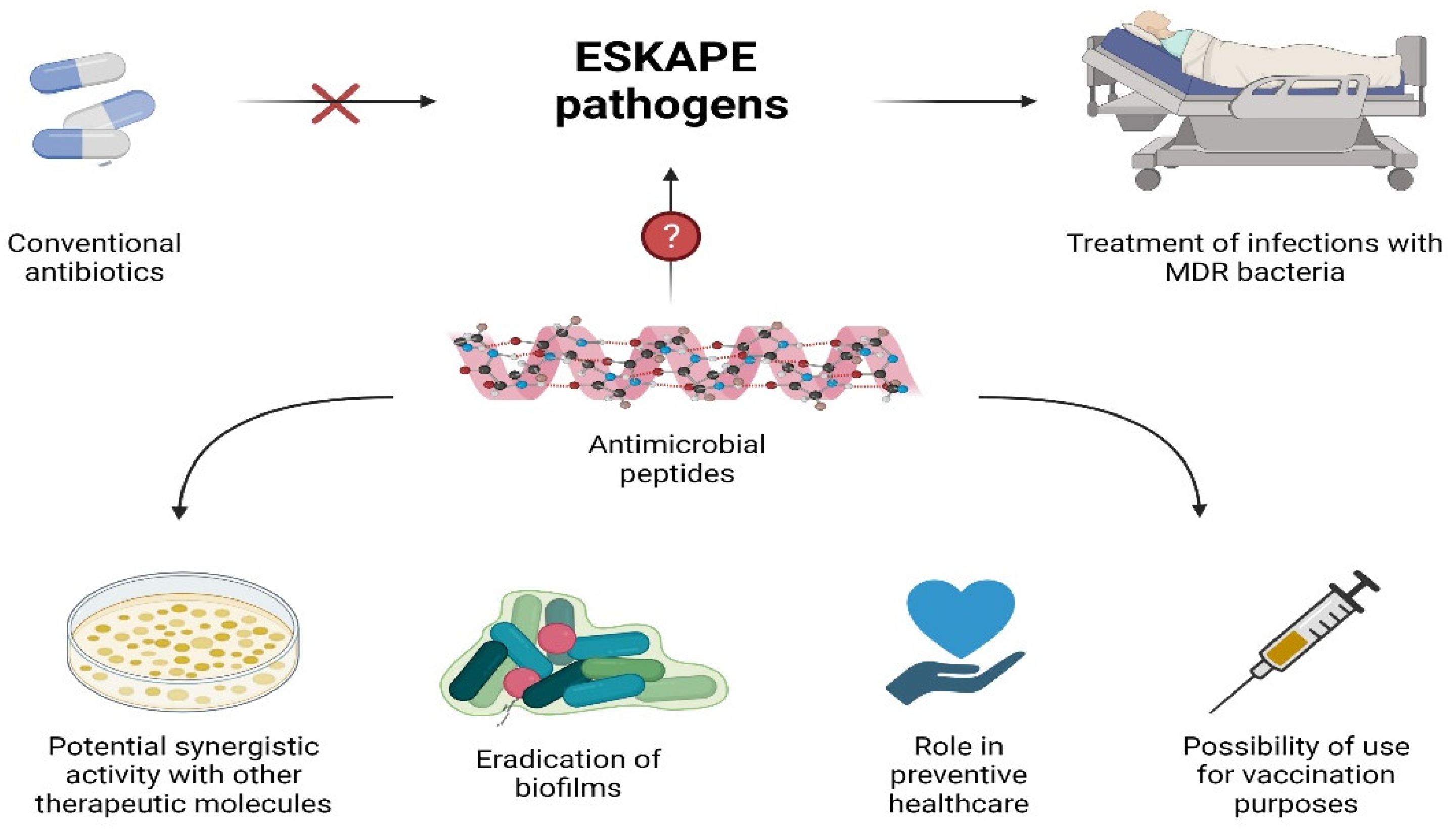
3. AMPs as Potential Alternatives to Antibiotics
3.1. Activity against MDR Bacteria and ESKAPE
3.2. Synergistic AMPs
3.3. AMPs Role in Preventive Healthcare
3.3.1. Prophylaxis
3.3.2. Biofilm Treatment
3.3.3. Vaccination: AMP-Based Vaccines
4. AMPs for Diagnostic Purposes
5. AMPs Facing 2019 Pandemic: SARS-CoV-2
6. Limitations and Challenges of the Therapeutic Use of AMPs
6.1. Clinical Trials of AMPs
6.2. How to Overcome Some of These Limitations?
6.3. Synthetic AMPs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gots, J.S. Production of extracellular penicillin-inactivating substances associated with penicillin resistance in Staphylococcus aureus. Proceedings of the Society for Experimental Biology and Medicine. Soc. Exp. Biol. Med. 1945, 60, 165–168. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clinical microbiology and infection: The official publication of the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Predicting drug resistance evolution: Insights from antimicrobial peptides and antibiotics. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, P. Multifunctional host defense peptides: Intracellular-targeting antimicrobial peptides. FEBS J. 2009, 276, 6483–6496. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.B.G. Antimicrobial Peptides Discovery, Design and Novel Therapeutic Strategies, 2nd ed.; University of Nebraska Medical Center: Omaha, NE, USA, 2017. [Google Scholar]
- The Antimicrobial Peptide Database. Available online: https://wangapd3.com/ (accessed on 1 July 2021).
- Ramos-Martín, F.; Annaval, T.; Buchoux, S.; Sarazin, C.; D’Amelio, N. ADAPTABLE: A comprehensive web platform of antimicrobial peptides tailored to the user’s research. Life Sci. Alliance 2019, 2, e201900512. [Google Scholar] [CrossRef] [Green Version]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [Green Version]
- Patocka, J.; Nepovimova, E.; Klimova, B.; Wu, Q.; Kuca, K. Antimicrobial Peptides: Amphibian Host Defense Peptides. Curr. Med. Chem. 2018, 25, 5924–5946. [Google Scholar] [CrossRef]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Salnikov, E.S.; Raya, J.; De Zotti, M.; Zaitseva, E.; Peggion, C.; Ballano, G.; Toniolo, C.; Raap, J.; Bechinger, B. Alamethicin Supramolecular Organization in Lipid Membranes from (19)F Solid-State NMR. Biophys. J. 2016, 111, 2450–2459. [Google Scholar] [CrossRef] [Green Version]
- Shai, Y.; Oren, Z. From ”carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides 2001, 22, 1629–1641. [Google Scholar] [CrossRef]
- Aisenbrey, C.; Marquette, A.; Bechinger, B. The mechanisms of action of cationic antimicrobial peptides refined by novel concepts from biophysical investigations. In Antimicrobial Peptides: Basics for Clinical Application; Matsuzaki, K., Ed.; Springer: Singapore, 2019; pp. 33–64. [Google Scholar]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechinger, B. The SMART model: Soft Membranes Adapt and Respond, also Transiently, in the presence of antimicrobial peptides. J. Pept. Sci. 2015, 21, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulay, G.; Leborgne, C.; Mason, A.J.; Aisenbrey, C.; Kichler, A.; Bechinger, B. Histidine-rich designer peptides of the LAH4 family promote cell delivery of a multitude of cargo. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2017, 23, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Lin, J.S.; Kapoor, R.; Wetzler, M.; Rea, J.A.C.; Didwania, M.K.; Contag, C.H.; Barron, A.E. Intracellular biomass flocculation as a key mechanism of rapid bacterial killing by cationic, amphipathic antimicrobial peptides and peptoids. Sci. Rep. 2017, 7, 16718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Camargo, C.; Salazar, V.A.; Barrero-Guevara, L.; Camargo, S.; Mosquera, A.; Groot, H.; Boix, E. Unveiling the Multifaceted Mechanisms of Antibacterial Activity of Buforin II and Frenatin 2.3S Peptides from Skin Micro-Organs of the Orinoco Lime Treefrog (Sphaenorhynchus lacteus). Int. J. Mol. Sci. 2018, 19, 2170. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Kou, Z.; Jiang, F.; Sun, X.; Shang, D. Interactions of Designed Trp-Containing Antimicrobial Peptides with DNA of Multidrug-Resistant Pseudomonas aeruginosa. DNA Cell Biol. 2021, 40, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Pérébaskine, N.; Benincasa, M.; Gambato, S.; Hofmann, S.; Huter, P.; Müller, C.; Hilpert, K.; Innis, C.A.; Tossi, A.; et al. The Dolphin Proline-Rich Antimicrobial Peptide Tur1A Inhibits Protein Synthesis by Targeting the Bacterial Ribosome. Cell Chem. Biol. 2018, 25, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Jangra, M.; Kaur, M.; Tambat, R.; Rana, R.; Maurya, S.K.; Khatri, N.; Ghafur, A.; Nandanwar, H. Tridecaptin M, a New Variant Discovered in Mud Bacterium, Shows Activity against Colistin- and Extremely Drug-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e00338-19. [Google Scholar] [CrossRef] [Green Version]
- Steinstraesser, L.; Kraneburg, U.; Jacobsen, F.; Al-Benna, S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 2011, 216, 322–333. [Google Scholar] [CrossRef]
- Liu, C.; Qi, J.; Shan, B.; Gao, R.; Gao, F.; Xie, H.; Yuan, M.; Liu, H.; Jin, S.; Wu, F.; et al. Pretreatment with cathelicidin-BF ameliorates Pseudomonas aeruginosa pneumonia in mice by enhancing NETosis and the autophagy of recruited neutrophils and macrophages. Int. Immunopharmacol. 2018, 65, 382–391. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. MMBR 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial Peptides as Potential Alternatives to Antibiotics in Food Animal Industry. Int. J. Mol. Sci. 2016, 17, 603. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.K.O.; Lovdahl, T.; Simonovic, D.; Hansen, K.O.; Andersen, A.J.C.; Devold, H.; Richard, C.S.M.; Andersen, J.H.; Strom, M.B.; Haug, T. Antimicrobial Activity of Small Synthetic Peptides Based on the Marine Peptide Turgencin A: Prediction of Antimicrobial Peptide Sequences in a Natural Peptide and Strategy for Optimization of Potency. Int. J. Mol. Sci. 2020, 21, 460. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-C.; Park, Y.; Hahm, K.-S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [Green Version]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2020, 49, D288–D297. [Google Scholar] [CrossRef]
- Lee, H.-R.; You, D.-G.; Kim, H.K.; Sohn, J.W.; Kim, M.J.; Park, J.K.; Lee, G.Y.; Yoo, Y.D. Romo1-Derived Antimicrobial Peptide Is a New Antimicrobial Agent against Multidrug-Resistant Bacteria in a Murine Model of Sepsis. mBio 2020, 11, e03258-19. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Development of a novel hybrid antimicrobial peptide for targeted killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020, 185, 111814. [Google Scholar] [CrossRef] [PubMed]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Sheard, D.E.; O’Brien-Simpson, N.M.; Wade, J.D.; Separovic, F. Combating bacterial resistance by combination of antibiotics with antimicrobial peptides. Pure Appl. Chem. 2019, 91, 199–209. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, X.; Zhang, M.; Zhong, S. Cerocin, a novel piscidin-like antimicrobial peptide from black seabass, Centropristis striata. Fish. Shellfish Immunol. 2021, 110, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, J.; Zhang, L.; Zhou, J.; He, Y.; Lu, Y.; Liu, K.; Yan, W.; Wang, K. Multiple action mechanism and in vivo antimicrobial efficacy of antimicrobial peptide Jelleine-I. J. Pept. Sci. An. Off. Publ. Eur. Pept. Soc. 2021, 27, e3294. [Google Scholar] [CrossRef]
- Li, F.; Gao, Z.; Wang, K.; Zhao, Y.; Wang, H.; Zhao, M.; Zhao, Y.; Bai, L.; Yu, Z.; Yang, X. A novel defensin-like peptide contributing to antimicrobial and antioxidant capacity of the tick Dermacentor silvarum (Acari: Ixodidae). Exp. Appl. Acarol. 2021, 83, 271–283. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Wu, C.-L.; Hsueh, J.-Y.; Yip, B.-S.; Chih, Y.-H.; Peng, K.-L.; Cheng, J.-W. Antimicrobial Peptides Display Strong Synergy with Vancomycin against Vancomycin-Resistant E. faecium, S. aureus, and Wild-Type E. coli. Int. J. Mol. Sci. 2020, 21, 4578. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, M. Molecular insight into how MRSA is becoming increasingly dangerous. Virulence 2012, 3, 521–523. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Jiang, W.; Xie, J.; Zhang, W.; Ji, Z.; Zou, J.; Cong, Z.; Xiao, X.; Gu, J.; Liu, R. Peptide-Mimicking Poly(2-oxazoline)s Displaying Potent Antimicrobial Properties. ChemMedChem 2021, 16, 309–315. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Suwalsky, M.; Patiño-González, E.; Fandiño-Devia, E.; Jemioła-Rzemińska, M.; Strzałka, K. Interaction of the antimicrobial peptide ∆M3 with the Staphylococcus aureus membrane and molecular models. Biochim. Biophys. Acta BBA Biomembr. 2021, 1863, 183498. [Google Scholar] [CrossRef]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Fleeman, R.M.; Macias, L.A.; Brodbelt, J.S.; Davies, B.W. Defining principles that influence antimicrobial peptide activity against capsulated Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2020, 117, 27620–27626. [Google Scholar] [CrossRef]
- Van der Weide, H.; Vermeulen-de Jongh, D.M.C.; van der Meijden, A.; Boers, S.A.; Kreft, D.; Ten Kate, M.T.; Falciani, C.; Pini, A.; Strandh, M.; Bakker-Woudenberg, I.; et al. Antimicrobial activity of two novel antimicrobial peptides AA139 and SET-M33 against clinically and genotypically diverse Klebsiella pneumoniae isolates with differing antibiotic resistance profiles. Int. J. Antimicrob. Agents 2019, 54, 159–166. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial Activity of Selected Antimicrobial Peptides Against Planktonic Culture and Biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins 2019, 11, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wu, Z.; Mao, C.; Guo, G.; Zeng, Z.; Fei, Y.; Wan, S.; Peng, J.; Wu, J. Antimicrobial Peptide Cec4 Eradicates the Bacteria of Clinical Carbapenem-Resistant Acinetobacter baumannii Biofilm. Front. Microbiol. 2020, 11, 1532. [Google Scholar] [CrossRef]
- Neshani, A.; Sedighian, H.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Jahangiri, A. Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb. Pathog. 2020, 146, 104238. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-J.; Liao, Y.-D.; Hsu, C.-C.; Huang, T.-Y.; Chuang, Y.-C.; Chen, J.-W.; Kuo, Y.-M.; Chia, J.-S. Identification of potential therapeutic antimicrobial peptides against Acinetobacter baumannii in a mouse model of pneumonia. Sci. Rep. 2021, 11, 7318. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorr, S.-U.; Brigman, H.; Anderson, J.; Hirsch, E. The antimicrobial peptide DGL13K is active against resistant gram-negative bacteria and subinhibitory concentrations stimulate bacterial growth without causing resistance. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hirt, H.; Gorr, S.-U. Antimicrobial peptide GL13K is effective in reducing biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4903–4910. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, C.; Harmouche, N.; Bechinger, B.; Haldar, J. Aryl-Alkyl-Lysines Interact with Anionic Lipid Components of Bacterial Cell Envelope Eliciting Anti-Inflammatory and Antibiofilm Properties. ACS Omega 2018, 3, 9182–9190. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [Green Version]
- Bechinger, B.; Juhl, D.W.; Glattard, E.; Aisenbrey, C. Revealing the Mechanisms of Synergistic Action of Two Magainin Antimicrobial Peptides. Front. Med Technol. 2020, 2, hal-03108924f. [Google Scholar] [CrossRef]
- Desbois, A.P.; Gemmell, C.G.; Coote, P.J. In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 2010, 35, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Jangra, M.; Raka, V.; Nandanwar, H. In Vitro Evaluation of Antimicrobial Peptide Tridecaptin M in Combination with Other Antibiotics against Multidrug Resistant Acinetobacter baumannii. Molecules 2020, 25, 3255. [Google Scholar] [CrossRef]
- Vanhoye, D.; Bruston, F.; Nicolas, P.; Amiche, M. Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 2003, 270, 2068–2081. [Google Scholar] [CrossRef]
- Zaïri, A.; Ferrières, L.; Latour-Lambert, P.; Beloin, C.; Tangy, F.; Ghigo, J.M.; Hani, K. In vitro activities of dermaseptins K4S4 and K4K20S4 against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa planktonic growth and biofilm formation. Antimicrob. Agents Chemother. 2014, 58, 2221–2228. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Heeney, D.; Srisengfa, Y.; Golomb, B.; Griffey, S.; Marco, M. Bacteriocin biosynthesis contributes to the anti-inflammatory capacities of probiotic Lactobacillus plantarum. Benef. Microbes 2018, 9, 333–344. [Google Scholar] [CrossRef]
- Wu, M.; Hancock, R.E. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 1999, 274, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oo, T.Z.; Cole, N.; Garthwaite, L.; Willcox, M.D.; Zhu, H. Evaluation of synergistic activity of bovine lactoferricin with antibiotics in corneal infection. J. Antimicrob. Chemother. 2010, 65, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, D.; Seisling, N.; Cotter, P.D.; Ross, R.P.; Hill, C. Synergistic Nisin-Polymyxin Combinations for the Control of Pseudomonas Biofilm Formation. Front. Microbiol. 2016, 7, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangiri, A.; Neshani, A.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Sedighian, H. Synergistic effect of two antimicrobial peptides, Nisin and P10 with conventional antibiotics against extensively drug-resistant Acinetobacter baumannii and colistin-resistant Pseudomonas aeruginosa isolates. Microb. Pathog. 2021, 150, 104700. [Google Scholar] [CrossRef] [PubMed]
- Portelinha, J.; Angeles-Boza, A.M. The Antimicrobial Peptide Gad-1 Clears Pseudomonas aeruginosa Biofilms under Cystic Fibrosis Conditions. Chembiochem Eur. J. Chem. Biol. 2021, 22, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.A. What is Preventive Medicine? Can. Fam Physician 1974, 20, 65–68. [Google Scholar] [PubMed]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Goffi, L.; Mocchegiani, F.; Riva, A.; Scalise, G.; Saba, V. Polycationic peptides as prophylactic agents against methicillin-susceptible or methicillin-resistant Staphylococcus epidermidis vascular graft infection. Antimicrob. Agents Chemother. 2000, 44, 3306–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateescu, M.; Baixe, S.; Garnier, T.; Jierry, L.; Ball, V.; Haikel, Y.; Metz-Boutigue, M.H.; Nardin, M.; Schaaf, P.; Etienne, O.; et al. Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. PLoS ONE 2015, 10, e0145143. [Google Scholar] [CrossRef]
- Monteiro, C.; Costa, F.; Pirttilä, A.M.; Tejesvi, M.V.; Martins, M.C.L. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci. Rep. 2019, 9, 10753. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.-L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef]
- Volejníková, A.; Melicherčík, P.; Nešuta, O.; Vaňková, E.; Bednárová, L.; Rybáček, J.; Čeřovský, V. Antimicrobial peptides prevent bacterial biofilm formation on the surface of polymethylmethacrylate bone cement. J. Med. Microbiol. 2019, 68, 961–972. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.C.; Oliva, M.d.l.M.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdiero, E.; Lombardi, L.; Falanga, A.; Libralato, G.; Guida, M.; Carotenuto, R. Biofilms: Novel Strategies Based on Antimicrobial Peptides. Pharmaceutics 2019, 11, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.H.; Park, H.; Cho, Y.B.; Choi, C.H. Effect of vancomycin-coated tympanostomy tubes on methicillin-resistant Staphylococcus aureus biofilm formation: In vitro study. J. Laryngol. Otol. 2010, 124, 594–598. [Google Scholar] [CrossRef]
- Wongkaewkhiaw, S.; Taweechaisupapong, S.; Thanaviratananich, S.; Bolscher, J.G.M.; Nazmi, K.; Anutrakunchai, C.; Chareonsudjai, S.; Kanthawong, S. D-LL-31 enhances biofilm-eradicating effect of currently used antibiotics for chronic rhinosinusitis and its immunomodulatory activity on human lung epithelial cells. PLoS ONE 2020, 15, e0243315. [Google Scholar] [CrossRef]
- Wongkaewkhiaw, S.; Taweechaisupapong, S.; Anutrakunchai, C.; Nazmi, K.; Bolscher, J.G.M.; Wongratanacheewin, S.; Kanthawong, S. D-LL-31 in combination with ceftazidime synergistically enhances bactericidal activity and biofilm destruction in Burkholderia pseudomallei. Biofouling 2019, 35, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Wang, Y.; Tan, T.; Ji, Y.; Hu, J.; Zhang, Y. Antimicrobial d-Peptide Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 1703–1712. [Google Scholar] [CrossRef]
- Li, M.; Yu, D.H.; Cai, H. The synthetic antimicrobial peptide KLKL5KLK enhances the protection and efficacy of the combined DNA vaccine against Mycobacterium tuberculosis. DNA Cell Biol. 2008, 27, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, T.A.; Lingnau, K.; Nagy, E.; Jonsdottir, I. IC31, a two-component novel adjuvant mixed with a conjugate vaccine enhances protective immunity against pneumococcal disease in neonatal mice. Scand. J. Immunol. 2009, 69, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Baindara, P.; Mandal, S.M. Antimicrobial Peptides and Vaccine Development to Control Multi-drug Resistant Bacteria. Protein Pept. Lett. 2019, 26, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Pardoux, É.; Boturyn, D. Antimicrobial Peptides as Probes in Biosensors Detecting Whole Bacteria: A Review. Molecules 2020, 25, 1998. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Imran, M.B.; Nadeem, M.A.; Shahid, A. Antimicrobial peptides as infection imaging agents: Better than radiolabeled antibiotics. Int. J. Pept. 2012, 2012, 965238. [Google Scholar] [CrossRef] [Green Version]
- Mazaheri Tehrani, M.; Erfani, M.; Amirmozafari, N. [(99m) Tc-HYNIC/EDDA]-MccJ25 antimicrobial peptide analog as a potential radiotracer for detection of infection. Chem. Biol. Drug Des. 2021, 97, 904–913. [Google Scholar] [CrossRef]
- Database of Antimicrobial Activity and Structure of Peptides. Available online: https://dbaasp.org/ (accessed on 21 June 2021).
- Balmeh, N.; Mahmoudi, S.; Fard, N.A. Manipulated bio antimicrobial peptides from probiotic bacteria as proposed drugs for COVID-19 disease. Inform. Med. Unlocked 2021, 23, 100515. [Google Scholar] [CrossRef]
- Mahendran, A.S.K.; Lim, Y.S.; Fang, C.-M.; Loh, H.-S.; Le, C.F. The Potential of Antiviral Peptides as COVID-19 Therapeutics. Front. Pharmacol. 2020, 11, 1475. [Google Scholar] [CrossRef]
- Bakovic, A.; Risner, K.; Bhalla, N.; Alem, F.; Chang, T.L.; Weston, W.; Harness, J.A.; Narayanan, A. Brilacidin, a COVID-19 Drug Candidate, Exhibits Potent In Vitro Antiviral Activity Against SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Elnagdy, S.; AlKhazindar, M. The Potential of Antimicrobial Peptides as an Antiviral Therapy against COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Liscano, Y.; Oñate-Garzón, J.; Ocampo-Ibáñez, I.D. In Silico Discovery of Antimicrobial Peptides as an Alternative to Control SARS-CoV-2. Molecules 2020, 25, 5535. [Google Scholar] [CrossRef]
- Svendsen, J.S.M.; Grant, T.M.; Rennison, D.; Brimble, M.A. Very Short and Stable Lactoferricin-Derived Antimicrobial Peptides: Design Principles and Potential Uses. Acc. Chem. Res. 2019, 52, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Moncla, B.J.; Pryke, K.; Rohan, L.C.; Graebing, P.W. Degradation of naturally occurring and engineered antimicrobial peptides by proteases. Adv. Biosci. Biotechnol. 2011, 2, 404–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böttger, R.; Hoffmann, R.; Knappe, D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef]
- Pujarini, D.; Santasabuj, D. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets Against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2016, 16, 99–129. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Atefyekta, S.; Blomstrand, E.; Rajasekharan, A.K.; Svensson, S.; Trobos, M.; Hong, J.; Webster, T.J.; Thomsen, P.; Andersson, M. Antimicrobial Peptide-Functionalized Mesoporous Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Boto, A.; Pérez de la Lastra, J.M.; González, C.C. The Road from Host-Defense Peptides to a New Generation of Antimicrobial Drugs. Molecules 2018, 23, 311. [Google Scholar] [CrossRef] [Green Version]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides—Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Nuding, S.; Gersemann, M.; Hosaka, Y.; Konietzny, S.; Schaefer, C.; Beisner, J.; Schroeder, B.O.; Ostaff, M.J.; Saigenji, K.; Ott, G.; et al. Gastric antimicrobial peptides fail to eradicate Helicobacter pylori infection due to selective induction and resistance. PLoS ONE 2013, 8, e73867. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.-S.; Fu, C.-I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philosophical transactions of the Royal Society of London. Ser. B Biol. Sci. 2016, 371, 20150292. [Google Scholar] [CrossRef] [Green Version]
- Min, C.; Ohta, K.; Kajiya, M.; Zhu, T.; Sharma, K.; Shin, J.; Mawardi, H.; Howait, M.; Hirschfeld, J.; Bahammam, L.; et al. The antimicrobial activity of the appetite peptide hormone ghrelin. Peptides 2012, 36, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Obuobi, S.; Tay, H.K.-L.; Tram, N.D.T.; Selvarajan, V.; Khara, J.S.; Wang, Y.; Ee, P.L.R. Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J. Control. Release 2019, 313, 120–130. [Google Scholar] [CrossRef]
- Molhoek, E.M.; van Dijk, A.; Veldhuizen, E.J.; Haagsman, H.P.; Bikker, F.J. Improved proteolytic stability of chicken cathelicidin-2 derived peptides by D-amino acid substitutions and cyclization. Peptides 2011, 32, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Moorcroft, S.C.T.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Evans, S.D. Nanoparticle-Loaded Hydrogel for the Light-Activated Release and Photothermal Enhancement of Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2020, 12, 24544–24554. [Google Scholar] [CrossRef]
- Sun, C.; Gu, L.; Hussain, M.A.; Chen, L.; Lin, L.; Wang, H.; Pang, S.; Jiang, C.; Jiang, Z.; Hou, J. Characterization of the Bioactivity and Mechanism of Bactenecin Derivatives Against Food-Pathogens. Front. Microbiol. 2019, 10, 2593. [Google Scholar] [CrossRef] [Green Version]
- Veltri, D.; Kamath, U.; Shehu, A. Improving Recognition of Antimicrobial Peptides and Target Selectivity through Machine Learning and Genetic Programming. IEEE/ACM Trans. Comput. Biol. Bioinform. 2017, 14, 300–313. [Google Scholar] [CrossRef]
- Yurkova, M.S.; Zenin, V.A.; Sadykhov, E.G.; Fedorov, A.N. Dimerization of Antimicrobial Peptide Polyphemusin I into One Polypeptide Chain: Theoretical and Practical Consequences. Appl. Biochem. Microbiol. 2020, 56, 893–897. [Google Scholar] [CrossRef]
- Irazazabal, L.N.; Porto, W.F.; Ribeiro, S.M.; Casale, S.; Humblot, V.; Ladram, A.; Franco, O.L. Selective amino acid substitution reduces cytotoxicity of the antimicrobial peptide mastoparan. Biochim. Biophys. Acta 2016, 1858, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Drayton, M.; Kizhakkedathu, J.N.; Straus, S.K. Towards Robust Delivery of Antimicrobial Peptides to Combat Bacterial Resistance. Molecules 2020, 25, 3048. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.; Mahendiratta, S.; Prakash, A.; Medhi, B. Specifically targeted antimicrobial peptides: A new and promising avenue in selective antimicrobial therapy. Indian J. Pharm. 2018, 50, 1–3. [Google Scholar] [CrossRef]
- Guo, L.; Edlund, A. Targeted Antimicrobial Peptides: A Novel Technology to Eradicate Harmful Streptococcus Mutans. J. Calif Dent. Assoc. 2017, 45, 557–564. [Google Scholar]
- Peng, J.; Qiu, S.; Jia, F.; Zhang, L.; He, Y.; Zhang, F.; Sun, M.; Deng, Y.; Guo, Y.; Xu, Z.; et al. The introduction of L-phenylalanine into antimicrobial peptide protonectin enhances the selective antibacterial activity of its derivative phe-Prt against Gram-positive bacteria. Amino Acids 2021, 53, 23–32. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, T.; Chen, L.; Zhou, J.; Wang, C. Analogs of the Cathelicidin-Derived Antimicrobial Peptide PMAP-23 Exhibit Improved Stability and Antibacterial Activity. Probiotics Antimicrob. Proteins 2021, 13, 273–286. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wu, Y.; Wang, L.; Ma, C.; Xi, X.; Bininda-Emonds, O.R.P.; Shaw, C.; Chen, T.; Zhou, M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Biol. Sci. 2018, 14, 599–607. [Google Scholar] [CrossRef]

| AMPs | Source | Synergistic Molecule | Target | Refs. |
|---|---|---|---|---|
| PGLa | Frog skin | Magainin 2 | E. coli and S. aureus | [67] |
| Ranalexin | -Bullfrog R. catesbeiana -Staphylococcus simulans | Endopeptidase lysostaphin | S. aureus (MRSA) | [68] |
| Tridecaptin M | Mud bacterium | Rifampicin, vancomycin, and ceftazidime | Extremely drug-resistant A. baumannii | [69] |
| Dermaseptin | Amphibians skin | Dermaseptin | E.coli, P. aeruginosa, S. aureus | [70,71] |
| Bactenecin | Lactic acid bacteria | Bactenecin | E. coli, P. aeruginosa, S. Typhimurium | [72,73] |
| Lactoferricin | Mammalians | Ciprofloxacin, ceftazidime | P. aeruginosa | [74] |
| Nisin | Lactococcus lactis | Colistin | Pseudomonas biofilms | [75,76] |
| P10 | Ceftazidim/doripenem | MDR A. baumannii and colistin-resistant P. aeruginosa | [76] | |
| Gad-1 | Fish | Kanamycin, ciprofloxacin | P. aeruginosa | [77] |
| AMP | Amino Acid Sequence | Synthesis | Target Group |
|---|---|---|---|
| Cathelicidin-BF | KFFRKLKKSVKKRAKEFFKKPRVIGVSIPF | Ribosomal | GP and GN |
| Defensin | GFGCPLDQMQCHRHCQTITGRSGGYCSGPLKLTCTCYR | Ribosomal | GP and GN |
| Nisin-P | VXxKXLXxPGXKxGILMXXAIKxAxXGXHFG | Ribosomal | GP and GN |
| Gramicidin S | VXLfPVXLfP | Nonribosomal | GP, GN, parasites, fungus, cancer cells, and mammalian cells |
| Polymyxin Colistin | XTXXXlLXXT | Synthetic | GN |
| Mellitin | GIGAVLKWLPALIKRKRQQ | Synthetic | GP, GN, and mammalian cells |
| Sm-Piscidin | KGARQAWKDYKYNRNMQKMNQGYGQQGG | Ribosomal | GP, GN, and fungus |
| Jellein-1 | PFKISIHL | Ribosomal | GP, GN, and mammalian cells |
| Aurein-1.1 | GLFDIIKKIAESI | Ribosomal | GP and GN |
| Citropin-1.1 | GLFDVIKKVASVIGGL | Ribosomal | GP, GN, fungus, mammalian cells, and mollicute |
| Omiganan | ILRWPWWPWRRK | Synthetic | GP, GN, fungus, and mammalian cells |
| ZY4 | VCKRWKKWKRKWKKWCV | Synthetic | GP, GN, fungus, and mammalian cells |
| GL13K | GKIIKLKASLKLL | Synthetic | GP, GN, insects, and mammalian cells |
| Chain201D | KWIVWRWRFKR | Synthetic | GP, GN, and fungus |
| SAAP-148 | LKRVWKRVFKLLKRYWRQLKKPVR | Synthetic | GP and GN |
| Lactococcin-G | GTWDDIGQGIGRVAYWVGKAMGNMSDVNQASRINRKKKHKKWGWLAWVEPAGEFLKGFGKGAIKEGNKDKWKNI | Ribosomal | GP |
| Caerin-1.10 | GLLSVLGSVAKHVLPHVVPVIAEKL | Ribosomal | GP, GN, viruses, and mammalian cells |
| Murepavadin | LSYXXXXWXXASPP | Synthetic | GP, GN, cancer cells, and mammalian cells |
| Protonectin | ILGTILPLLKGL | Ribosomal | GP, GN, cancer cells, fungus, and mammalian cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. https://doi.org/10.3390/antibiotics10091095
Rima M, Rima M, Fajloun Z, Sabatier J-M, Bechinger B, Naas T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics. 2021; 10(9):1095. https://doi.org/10.3390/antibiotics10091095
Chicago/Turabian StyleRima, Mariam, Mohamad Rima, Ziad Fajloun, Jean-Marc Sabatier, Burkhard Bechinger, and Thierry Naas. 2021. "Antimicrobial Peptides: A Potent Alternative to Antibiotics" Antibiotics 10, no. 9: 1095. https://doi.org/10.3390/antibiotics10091095
APA StyleRima, M., Rima, M., Fajloun, Z., Sabatier, J.-M., Bechinger, B., & Naas, T. (2021). Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics, 10(9), 1095. https://doi.org/10.3390/antibiotics10091095








