Antibiotic Prescribing Patterns in Paediatric Primary Care in Italy: Findings from 2012–2018
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Prescribing Trend over Years and by Age Class
2.2. Antibiotic Prescribing Trend by Diagnosis Class
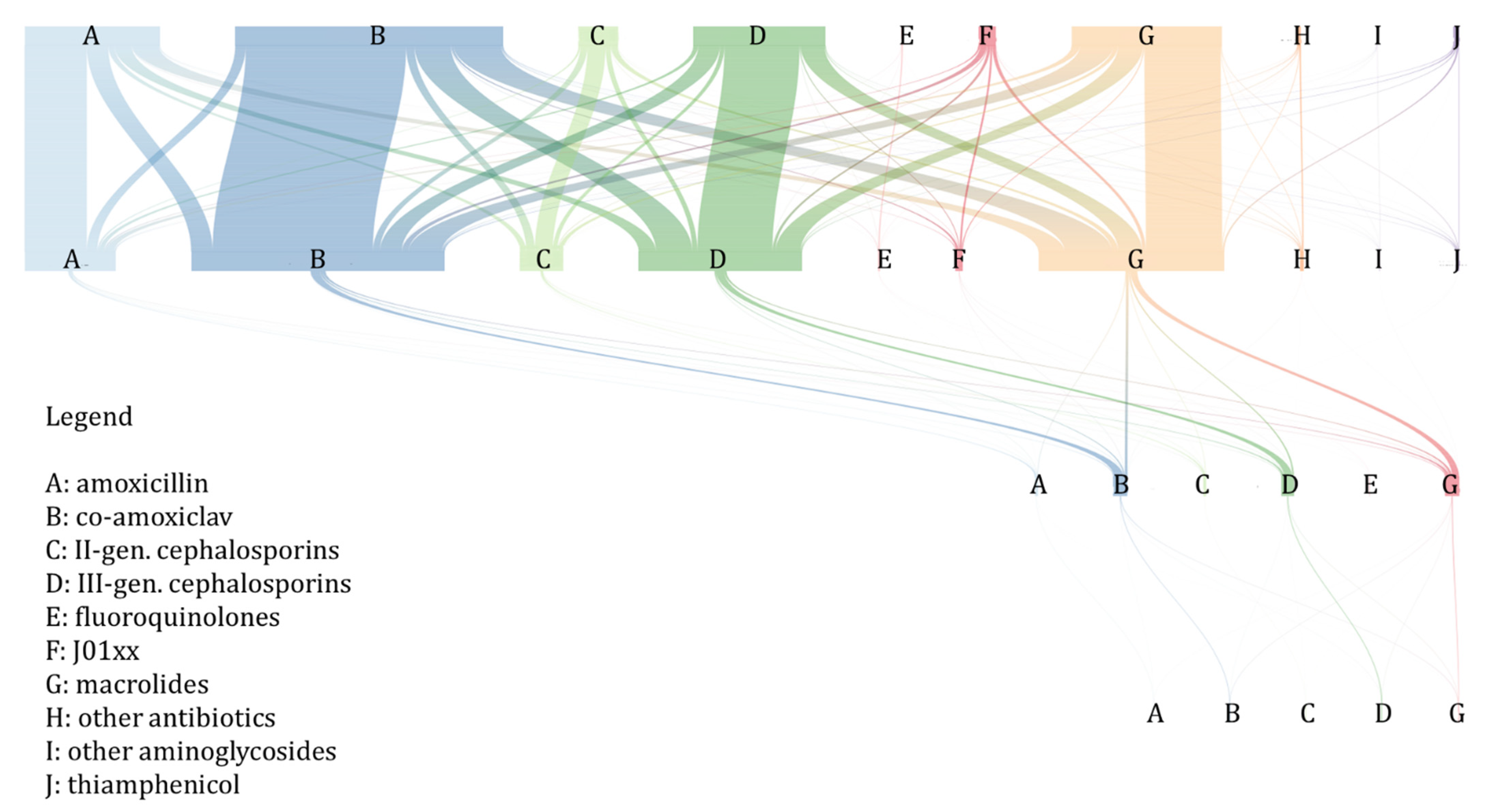
2.3. Antibiotic Treatment Switching and Prolongation
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Inclusion and Exclusion Criteria
4.3. Measures and Outcomes
- Prescription: all prescriptions of the same antibiotic class were grouped if they occurred on the same visit (e.g., in case of two prescriptions for amoxicillin on the same visit, just one is counted as a prescription).
- Antibiotic index (AI): the number of antibiotic prescriptions per person-year.
- Treatment episode: all prescriptions occurring within 14 days of the first antibiotic prescription.
- Switch: treatment episode with a second prescription different in class from the first [27]
- Early switch: the first switch occurring within 1–3 days of the first prescription [27]
- Late switch: the first switch occurring within 4–14 days of the first prescription [27]
- Treatment prolongation: treatment episode with a second prescription.
- Day of switching: difference in days between the date of the second prescription and the date of the first prescription in a switch.
4.4. Statistical Analyses
4.5. Ethics
5. Conclusions and Future Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youngster, I.; Avorn, J.; Belleudi, V.; Cantarutti, A.; Diez-Domingo, J.; Kirchmayer, U.; Park, B.-J.; Peiró, S.; Sanfélix-Gimeno, G.; Schröder, H.; et al. Antibiotic Use in Children—A Cross-National Analysis of 6 Countries. J. Pediatr. 2017, 182, 239–244.e1. [Google Scholar] [CrossRef]
- Holstiege, J.; Schink, T.; Molokhia, M.; Mazzaglia, G.; Innocenti, F.; Oteri, A.; Bezemer, I.; Poluzzi, E.; Puccini, A.; Ulrichsen, S.P.; et al. Systemic antibiotic prescribing to paediatric outpatients in 5 European countries: A population-based cohort study. BMC Pediatr. 2014, 14, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Maat, J.; van de Voort, E.; Mintegi, S.; Gervaix, A.; Nieboer, D.; Moll, H.; Oostenbrink, R.; van Veen, M.; Noordzij, J.G.; Smit, F.; et al. Antibiotic prescription for febrile children in European emergency departments: A cross-sectional, observational study. Lancet Infect. Dis. 2019, 19, 382–391. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018; ECDC: Stockholm, Sweden, 2019.
- The Medicines Utilisation Monitoring Centre. National Report on Antibiotics Use in Italy. Year 2018; Italian Medicines Agency: Rome, Italy, 2019.
- Di Mario, S.; Gagliotti, C.; Buttazzi, R.; Cisbani, L.; Di Girolamo, C.; Brambilla, A.; Moro, M.L.; The Regional Working Group. “Progetto ProBA-Progetto Bambini e Antibiotici-2014”. Observational pre-post study showed that a quality improvement project reduced paediatric antibiotic prescribing rates in primary care. Acta Paediatr. 2018, 107, 1805–1809. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M., Jr.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shively, N.R.; Buehrle, D.J.; Clancy, C.J.; Decker, B.K. Prevalence of Inappropriate Antibiotic Prescribing in Primary Care Clinics within a Veterans Affairs Health Care System. Antimicrob. Agents Chemother. 2018, 62, e00337-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crotty, M.P.; Meyers, S.; Hampton, N.; Bledsoe, S.; Ritchie, D.J.; Buller, R.S.; Storch, G.A.; Kollef, M.H.; Micek, S.T. Impact of antibacterials on subsequent resistance and clinical outcomes in adult patients with viral pneumonia: An opportunity for stewardship. Crit. Care 2015, 19, 404. [Google Scholar] [CrossRef] [Green Version]
- Ebell, M.H.; Radke, T. Antibiotic use for viral acute respiratory tract infections remains common. Am. J. Manag. Care 2015, 21, e567–e575. [Google Scholar] [PubMed]
- Donà, D.; Luise, D.; Barbieri, E.; Masiero, N.; Maita, S.; Antoniello, L.; Zaoutis, T.; Giaquinto, C.; Gamba, P. Effectiveness and Sustainability of an Antimicrobial Stewardship Program for Perioperative Prophylaxis in Pediatric Surgery. Pathogens 2020, 9, 490. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horikoshi, Y.; Suwa, J.; Higuchi, H.; Kaneko, T.; Furuichi, M.; Aizawa, Y.; Fukuoka, K.; Okazaki, K.; Ito, K.; Shoji, T. Sustained pediatric antimicrobial stewardship program with consultation to infectious diseases reduced carbapenem resistance and infection-related mortality. Int. J. Infect. Dis. 2017, 64, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliotti, C.; Buttazzi, R.; Di Mario, S.; Morsillo, F.; Moro, M.L. A regionwide intervention to promote appropriate antibiotic use in children reversed trends in erythromycin resistance to Streptococcus pyogenes. Acta Paediatr. 2015, 104, e422–e424. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Clavenna, A.; Cartabia, M.; Piovani, D.; Bortolotti, A.; Fortino, I.; Merlino, L.; Bonati, M. Antibiotic prescription in the outpatient paediatric population attending emergency departments in Lombardy, Italy: A retrospective database review. BMJ Paediatr. Open 2019, 3, e000546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uso di Antibiotici e Resistenze Antimicrobiche in Età Pediatrica. Rapporto Emilia-Romagna . 2019. Available online: https://assr.regione.emilia-romagna.it/pubblicazioni/rapporti-documenti/antibiotici-pediatria-2019 (accessed on 17 September 2020).
- Barchitta, M.; Quattrocchi, A.; Maugeri, A.; La Rosa, M.C.; La Mastra, C.; Sessa, L.; Cananzi, P.; Murolo, G.; Oteri, A.; Basile, G.; et al. Antibiotic Consumption and Resistance during a 3-Year Period in Sicily, Southern Italy. Int. J. Environ. Res. Public Health 2019, 16, 2253. [Google Scholar] [CrossRef] [Green Version]
- Piovani, D.; Clavenna, A.; Cartabia, M.; Bortolotti, A.; Fortino, I.; Merlino, L.; Bonati, M. Assessing the quality of paediatric antibiotic prescribing by community paediatricians: A database analysis of prescribing in Lombardy. BMJ Paediatr. Open 2017, 1, e000169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, M.; Lallo, A.; Kirchmayer, U.; Davoli, M.; Fusco, D. Prevalence of antibiotic prescription in pediatric outpatients in Italy: The role of local health districts and primary care physicians in determining variation. A multilevel design for healthcare decision support. BMC Public Health 2017, 17, 886. [Google Scholar] [CrossRef]
- Orlando, V.; Monetti, V.M.; Juste, A.M.; Russo, V.; Mucherino, S.; Trama, U.; Guida, A.; Menditto, E. Drug Utilization Pattern of Antibiotics: The Role of Age, Sex and Municipalities in Determining Variation. Risk Manag. Healthc. Policy 2020, 13, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, J.; Trifirò, G.; Ientile, V.; Fontana, A.; Rossi, F.; Capuano, A.; Ferrajolo, C. Traceability of Pediatric Antibiotic Purchasing Pathways in Italy: A Nationwide Real-World Drug Utilization Analysis. Front. Pharmacol. 2020, 11, 1232. [Google Scholar] [CrossRef]
- Bronzwaer, S.L.; Cars, O.; Buchholz, U.; Mölstad, S.; Goettsch, W.G.; Veldhuijzen, I.K.; Kool, J.; Sprenger, M.J.; Degener, J.E.; Participants in the European Antimicrobial Resistance Surveillance System. The Relationship between Antimicrobial Use and Antimicrobial Resistance. Emerg. Infect. Dis. 2002, 8, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M.; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Seppälä, H.; Klaukka, T.; Vuopio-Varkila, J.; Muotiala, A.; Helenius, H.; Lager, K.; Huovinen, P. The Effect of Changes in the Consumption of Macrolide Antibiotics on Erythromycin Resistance in Group A Streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N. Engl. J. Med. 1997, 337, 441–446. [Google Scholar] [CrossRef]
- Gardner, T.L.; Dovey, S.M.; Tilyard, M.W.; Gurr, E. Differences between prescribed and dispensed medications. N. Z. Med. J. 1996, 109, 69–72. [Google Scholar]
- Van Katwyk, S.R.; Grimshaw, J.M.; Nkangu, M.; Nagi, R.; Mendelson, M.; Taljaard, M.; Hoffman, S.J. Government policy interventions to reduce human antimicrobial use: A systematic review and evidence map. PLoS Med. 2019, 16, e1002819. [Google Scholar] [CrossRef] [Green Version]
- Reilev, M.; Thomsen, R.; Aabenhus, R.; Sydenham, R.V.; Hansen, J.G.; Pottegård, A. Switching Between Antibiotics Among Danish Children 0–4 Years of Age. Pediatr. Infect. Dis. J. 2018, 37, 1112–1117. [Google Scholar] [CrossRef]
- Steffensen, F.H.; Schønheyder, H.C.; Mortensen, J.T.; Nielsen, K.; Sørensen, H.T. Changes in reimbursement policy for antibiotics and prescribing patterns in general practice. Clin. Microbiol. Infect. 1997, 3, 653–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsia, Y.; Sharland, M.; Jackson, C.; Wong, I.C.K.; Magrini, N.; Bielicki, J.A. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: An analysis of sales data from 70 middle-income and high-income countries. Lancet Infect. Dis. 2019, 19, 67–75. [Google Scholar] [CrossRef]
- Fürst, J.; Čižman, M.; Mrak, J.; Kos, D.; Campbell, S.; Coenen, S.; Gustafsson, L.L.; Fürst, L.; Godman, B. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: Findings and implications. Expert Rev. Anti-Infect. Ther. 2015, 13, 279–289. [Google Scholar] [CrossRef]
- Formoso, G.; Paltrinieri, B.; Marata, A.M.; Gagliotti, C.; Pan, A.; Moro, M.L.; Capelli, O.; Magrini, N. Feasibility and effectiveness of a low cost campaign on antibiotic prescribing in Italy: Community level, controlled, non-randomised trial. BMJ 2013, 347, f5391. [Google Scholar] [CrossRef] [Green Version]
- Barchitta, M.; Quattrocchi, A.; Maugeri, A.; La Rosa, M.C.; La Mastra, C.; Basile, G.; Giuffrida, G.; Rinaldi, F.M.; Murolo, G.; Agodi, A. The “Obiettivo Antibiotico” Campaign on Prudent Use of Antibiotics in Sicily, Italy: The Pilot Phase. Int. J. Environ. Res. Public Health 2020, 17, 3077. [Google Scholar] [CrossRef] [PubMed]
- AIFA. Available online: https://www.aifa.gov.it/-/oggi-giornata-europea-della-consapevolezza-sugli-antibiotici-aifa-lancia-un-quiz-sui-social (accessed on 17 September 2021).
- Baldo, V.; Cocchio, S.; Gallo, T.; Furlan, P.; Clagnan, E.; Del Zotto, S.; Saia, M.; Bertoncello, C.; Buja, A.; Baldovin, T. Impact of pneumococcal conjugate vaccination: A retrospective study of hospitaliza-tion for pneumonia in North-East Italy. J. Prev. Med. Hyg. 2016, 57, E61–E68. [Google Scholar] [PubMed]
- Fortunato, F.; Martinelli, D.; Cappelli, M.G.; Cozza, V.; Prato, R. Impact of Pneumococcal Conjugate Universal Routine Vaccination on Pneumococcal Disease in Italian Children. J. Immunol. Res. 2015, 2015, 206757. [Google Scholar] [CrossRef] [Green Version]
- In Vitro Diagnostics Quality Control Market Size, Share & Trends Analysis Report by Application, by Type (Quality Control, Quality Assurance Services), by End Use (Lab, Home-Care, Hospital), and Segment Forecasts, 2019–2026. Available online: https://www.grandviewresearch.com/industry-analysis/in-vitro-diagnostics-ivd-quality-control-market (accessed on 17 September 2020).
- Van Hoof, V.; Barglazan, D.; Blairon, L.; Braekevelt, B.; Debois, R.; De Vos, N.V.; Gruson, D.; Jonckheere, J.; Lanckmans, K.; Moens, M.; et al. Organisation and quality monitoring for point-of-care testing (POCT) in Belgium: Proposal for an expansion of the legal framework for POCT into primary health care. Acta Clin. Belg. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Donà, D.; Cantarutti, A.; Lundin, R.; Scamarcia, A.; Corrao, G.; Giaquinto, C. Antibiotic prescriptions in acute otitis media and pharyngitis in Italian pediatric outpatients. Ital. J. Pediatr. 2019, 45, 103. [Google Scholar] [CrossRef] [PubMed]
- Machowska, A.; Lundborg, C.S. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [Green Version]
- Dabernat, H.; Geslin, P.; Megraud, F.; Bégué, P.; Boulesteix, J.; Dubreuil, C.; De La Roque, F.; Trinh, A.; Scheimberg, A. Effects of cefixime or co-amoxiclav treatment on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children with acute otitis media. J. Antimicrob. Chemother. 1998, 41, 253–258. [Google Scholar] [CrossRef]
- Cohen, R. Clinical efficacy of cefpodoxime in respiratory tract infection. J. Antimicrob. Chemother. 2002, 50, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.L.; Gooch, W.M.; Oddo, L.P. Comparison of the palatability of the oral suspension of cefdinir vs. amoxicillin/clavulanate potassium, cefprozil and azithromycin in pediatric patients. Pediatr. Infect. Dis. J. 2000, 19, S174–S180. [Google Scholar] [CrossRef]
- Donà, D.; Luise, D.; Da Dalt, L.; Giaquinto, C. Treatment of Community-Acquired Pneumonia: Are All Countries Treating Children in the Same Way? A Literature Review. Int. J. Pediatr. 2017, 2017, 4239268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matera, M.G.; Rogliani, P.; Ora, J.; Cazzola, M. Current pharmacotherapeutic options for pediatric lower respiratory tract infections with a focus on antimicrobial agents. Expert Opin. Pharmacother. 2018, 19, 2043–2053. [Google Scholar] [CrossRef]
- Hamacher, J.; Luepke, J.; Reidenberg, B.E.; Nord, C.E.; Borner, K.; Koeppe, P.; Bristol, D.; Lode, H. Changes in fecal flora and comparative multiple-dose pharmacokinetics of ceftibuten, cefpodoxime proxetil and amoxycillin/clavulanate. Clin. Microbiol. Infect. 1999, 5, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Cantarutti, A.; Giaquinto, C. Pedianet Database. In Databases for Pharmaco-Epidemiological Research; Sturkenboom, M., Schink, T., Eds.; Springer Series on Epidemiology and Public Health; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Corsello, G.; Ferrara, P.; Chiamenti, G.; Nigri, L.; Campanozzi, A.; Mantovani, M.P. The Child Health Care System in Italy. J. Pediatr. 2016, 177, S116–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liese, J.G.; Silfverdal, S.A.; Giaquinto, C.; Carmona, A.; Larcombe, J.H.; Garcia-Sicilia, J.; Fuat, A.; Garces-Sanchez, M.; Basanta, M.L.A.; Hiraldo, E.M.; et al. Incidence and clinical presentation of acute otitis media in children aged . Epidemiol. Infect. 2014, 142, 1778–1788. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, E.; Cantarutti, A.; Cavagnis, S.; Cantarutti, L.; Baraldi, E.; Giaquinto, C.; Donà, D. Impact of bronchiolitis guidelines publication on primary care prescriptions in the Italian pediatric population. NPJ Prim. Care Respir. Med. 2021, 31, 15. [Google Scholar] [CrossRef] [PubMed]
- ISTAT. Glossario 2011. Available online: https://www.istat.it/it/files/2012/07/glossario_.pdf (accessed on 27 November 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 10 July 2020).
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef]
- Pierce, J.; Apisarnthanarak, A.; Schellack, N.; Cornistein, W.; Al Maani, A.; Adnan, S.; Stevens, M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries. Int. J. Infect. Dis. 2020, 96, 621–629. [Google Scholar] [CrossRef] [PubMed]





| Relative Risk | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI | Amoxicillin | Co-Amoxiclav | III-Gen. Cephalosporins | II-Gen. Cephalosporins | Macrolides | Lincosamides | Fluoroquinolones | J01XX | Thiamphenicol | Other Aminoglycosides | Other | |
| Time in year | 0.96 (0.96–0.97) | 1.02 (1.01–1.02) | 1.01 (1.01–1.02) | 1 (1–1) | 0.9 (0.89–0.9) | 0.99 (0.99–0.99) | 1.03 (0.99–1.07) | 0.91 (0.85–0.96) | 0.98 (0.96–1) | 1.02 (1.01–1.03) | 0.95 (0.94–0.96) | 0.83 (0.8–0.86) |
| Patients’ age in years | 0.87 (0.87–0.87) | 0.94 (0.94–0.94) | 1.01 (1.01–1.01) | 1.01 (1.01–1.01) | 0.93 (0.93–0.94) | 1.04 (1.04–1.04) | 1.2 (1.18–1.23) | 1.13 (1.09–1.17) | 1.17 (1.16–1.18) | 0.94 (0.93–0.94) | 1.14 (1.13–1.15) | 1.07 (1.05–1.09) |
| Male sex | 1.05 (1.04–1.06) | 0.99 (0.97–1) | 1.04 (1.03–1.05) | 0.97 (0.96–0.99) | 0.91 (0.89–0.94) | 1.03 (1.02–1.04) | 1.32 (1.13–1.54) | 1.38 (1.08–1.76) | 1.02 (0.94–1.1) | 0.55 (0.52–0.57) | 1 (0.94–1.05) | 1.11 (0.97–1.26) |
| Geographical location | ||||||||||||
| North | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Centre | 1.72 (1.71–1.74) | 0.35 (0.35–0.36) | 1.8 (1.78–1.83) | 1.37 (1.35–1.4) | 1.59 (1.53–1.65) | 0.92 (0.9–0.94) | 0.38 (0.28–0.5) | 4.4 (3.01–6.41) | 0.51 (0.46–0.56) | 1.5 (1.4–1.61) | 0.71 (0.66–0.77) | 0.84 (0.72–0.99) |
| South and islands | 1.75 (1.74–1.76) | 0.28 (0.28–0.29) | 1.33 (1.31–1.34) | 1.96 (1.93–1.99) | 1.88 (1.83–1.94) | 1.33 (1.32–1.35) | 0.88 (0.75–1.03) | 3.22 (2.27–4.57) | 0.27 (0.25–0.3) | 1.98 (1.87–2.09) | 0.81 (0.76–0.86) | 0.23 (0.19–0.28) |
| Diagnosis | Treatment Switch | N of Total Switches (% of Overall Switches) | N of Total Switches (% of Overall Switches by Diagnosis) | N of Early Switches (% of Overall Early Switches by Diagnosis) | N of Late Switches (% of Overall Late Switches by Diagnosis) | Mean (SD) | Median (IQR) |
|---|---|---|---|---|---|---|---|
| AOM | Amoxicillin–co-amoxiclav | 220 (1.3) | 220 (9.6) | 20 (5.1) | 200 (10.2) | 9.4 (3.7) | 10 (6) |
| Amoxicillin–III-gen. cephalosporins | 215 (1.3) | 215 (9.4) | 35 (8.9) | 180 (9.2) | 8.8 (4.3) | 10 (8) | |
| Amoxicillin–macrolides | 144 (0.8) | 144 (6.3) | 22 (5.6) | 122 (6.2) | 8.7 (4.1) | 9.5 (6.2) | |
| Co-amoxiclav–III-gen. cephalosporins | 382 (2.2) | 382 (16.7) | 68 (17.3) | 314 (16) | 8.5 (4.2) | 9 (7) | |
| Co-amoxiclav–macrolides | 186 (1.1) | 186 (8.1) | 22 (5.6) | 164 (8.3) | 9.2 (4.1) | 10 (7) | |
| III-gen. cephalosporins–co-amoxiclav | 215 (1.3) | 215 (9.4) | 36 (9.2) | 179 (9.1) | 8.4 (4.1) | 9 (7) | |
| III-gen. cephalosporins–Macrolides | 150 (0.9) | 150 (6.6) | 17 (4.3) | 133 (6.8) | 8.7 (3.8) | 9 (5.5) | |
| Bronchitis/bronchiolitis | Amoxicillin–macrolides | 299 (1.7) | 299 (11.2) | 44 (8.2) | 255 (11.7) | 7.6 (3.7) | 7 (5.5) |
| Co-amoxiclav–macrolides | 480 (2.8) | 480 (18) | 80 (14.9) | 400 (18.3) | 7.6 (3.8) | 7 (7) | |
| III-gen. cephalosporins–macrolides | 247 (1.4) | 247 (9.3) | 37 (6.9) | 210 (9.6) | 7.9 (3.8) | 8 (6) | |
| Macrolides–amoxicillin | 148 (0.9) | 148 (5.6) | 39 (7.3) | 109 (5) | 7 (4.2) | 6 (8) | |
| Macrolides–co-amoxiclav | 427 (2.5) | 427 (16) | 87 (16.2) | 340 (15.5) | 6.9 (3.9) | 7 (6) | |
| Macrolides–III-gen. cephalosporins | 409 (2.4) | 409 (15.3) | 86 (16) | 323 (14.8) | 7.1 (3.9) | 7 (6) | |
| Pharyngitis | Amoxicillin–co-amoxiclav | 285 (1.7) | 285 (8.8) | 33 (4.6) | 252 (9.6) | 9.4 (4) | 10 (6) |
| Amoxicillin–III-gen. cephalosporins | 248 (1.4) | 248 (7.6) | 50 (6.9) | 198 (7.6) | 8.8 (4.5) | 10 (9) | |
| Amoxicillin–macrolides | 275 (1.6) | 275 (8.5) | 48 (6.6) | 227 (8.7) | 8.4 (4.2) | 9 (8) | |
| Co-amoxiclav–III-gen. cephalosporins | 411 (2.4) | 411 (12.7) | 123 (17) | 288 (11) | 7 (4.6) | 7 (8) | |
| Co-amoxiclav–macrolides | 406 (2.4) | 406 (12.5) | 72 (10) | 334 (12.7) | 8.3 (4.2) | 8 (7) | |
| III-gen. cephalosporins–co-amoxiclav | 249 (1.5) | 249 (7.7) | 51 (7.1) | 198 (7.6) | 8.3 (4.4) | 9 (9) | |
| III-gen. cephalosporins–macrolides | 268 (1.6) | 268 (8.3) | 28 (3.9) | 240 (9.2) | 8.7 (3.9) | 9 (7) | |
| URTI | Amoxicillin–co-amoxiclav | 230 (1.3) | 230 (5.1) | 30 (4.2) | 200 (5.1) | 8.5 (4) | 8.5 (8) |
| Amoxicillin–macrolides | 359 (2.1) | 359 (8) | 54 (7.6) | 305 (7.8) | 7.7 (3.7) | 7 (6) | |
| Co-amoxiclav–III-gen. cephalosporins | 338 (2) | 338 (7.5) | 75 (10.5) | 263 (6.7) | 7.9 (4.3) | 8 (7.8) | |
| Co-amoxiclav–macrolides | 566 (3.3) | 566 (12.6) | 85 (11.9) | 481 (12.2) | 7.9 (3.9) | 8 (6) | |
| III-gen. cephalosporins–co-amoxiclav | 234 (1.4) | 234 (5.2) | 37 (5.2) | 197 (5) | 8.7 (4) | 9 (6) | |
| III-gen. cephalosporins–macrolides | 275 (1.6) | 275 (6.1) | 30 (4.2) | 245 (6.2) | 8.4 (3.8) | 8 (6) | |
| Macrolides–co-amoxiclav | 664 (3.9) | 664 (14.8) | 87 (12.2) | 577 (14.7) | 8 (3.8) | 7 (7) | |
| Macrolides–III-gen. cephalosporins | 470 (2.7) | 470 (10.4) | 79 (11.1) | 391 (10) | 7.7 (4) | 7 (7) | |
| NA | Amoxicillin–co-amoxiclav | 139 (0.8) | 139 (5.4) | 25 (4.8) | 114 (5.4) | 8.3 (4.2) | 8 (7.5) |
| Amoxicillin–macrolides | 144 (0.8) | 144 (5.6) | 24 (4.6) | 120 (5.7) | 7.5 (3.8) | 7 (7) | |
| Co-amoxiclav–III-gen. cephalosporins | 261 (1.5) | 261 (10.2) | 55 (10.6) | 206 (9.8) | 7.8 (4.1) | 8 (8) | |
| Co-amoxiclav–macrolides | 418 (2.4) | 418 (16.3) | 75 (14.4) | 343 (16.3) | 7.9 (4.1) | 7 (8) | |
| III-gen. cephalosporins–co-amoxiclav | 189 (1.1) | 189 (7.4) | 39 (7.5) | 150 (7.1) | 8 (4.4) | 8 (9) | |
| III-gen. cephalosporins–macrolides | 163 (1) | 163 (6.4) | 24 (4.6) | 139 (6.6) | 7.8 (3.8) | 7 (6.5) | |
| Macrolides–co-amoxiclav | 244 (1.4) | 244 (9.5) | 45 (8.6) | 199 (9.5) | 7.7 (4.1) | 7 (8) | |
| Macrolides–III-gen. cephalosporins | 130 (0.8) | 130 (5.1) | 31 (6) | 99 (4.7) | 7.1 (4) | 7 (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, E.; di Chiara, C.; Costenaro, P.; Cantarutti, A.; Giaquinto, C.; Hsia, Y.; Doná, D. Antibiotic Prescribing Patterns in Paediatric Primary Care in Italy: Findings from 2012–2018. Antibiotics 2022, 11, 18. https://doi.org/10.3390/antibiotics11010018
Barbieri E, di Chiara C, Costenaro P, Cantarutti A, Giaquinto C, Hsia Y, Doná D. Antibiotic Prescribing Patterns in Paediatric Primary Care in Italy: Findings from 2012–2018. Antibiotics. 2022; 11(1):18. https://doi.org/10.3390/antibiotics11010018
Chicago/Turabian StyleBarbieri, Elisa, Costanza di Chiara, Paola Costenaro, Anna Cantarutti, Carlo Giaquinto, Yingfen Hsia, and Daniele Doná. 2022. "Antibiotic Prescribing Patterns in Paediatric Primary Care in Italy: Findings from 2012–2018" Antibiotics 11, no. 1: 18. https://doi.org/10.3390/antibiotics11010018








