An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas aeruginosa and Acinetobacter baumannii in Critically Ill Adult Patients
Abstract
1. Introduction
2. Results
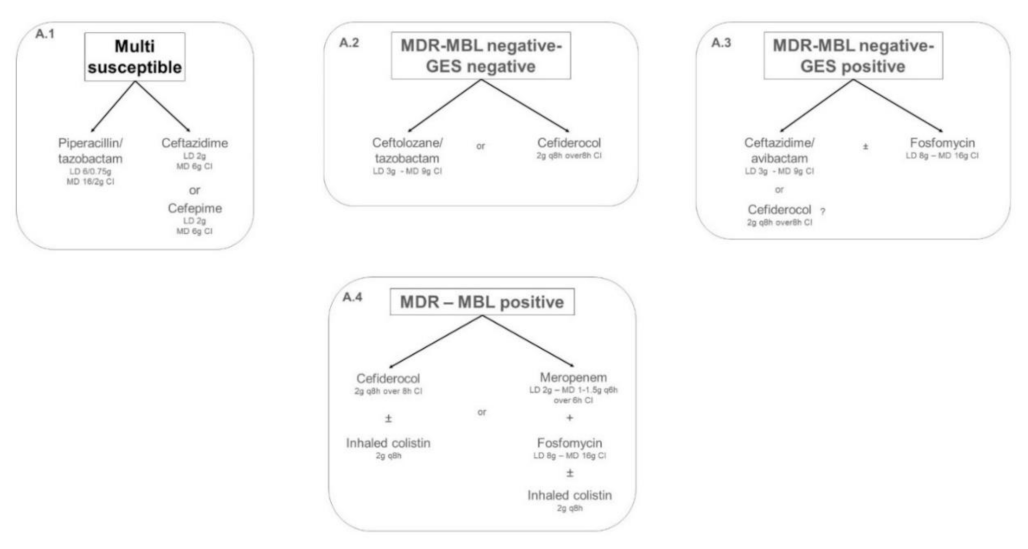
2.1. Targeted Treatment of IVACs Caused by Pseudomonas aeruginosa in Critically Ill Adult Patients
2.1.1. Multi-Susceptible Pseudomonas aeruginosa
2.1.2. Multidrug-Resistant (MDR) Metallo-Beta-Lactamase (MBL)-Negative Pseudomonas aeruginosa
2.1.3. Multidrug-Resistant (MDR) Metallo-Beta-Lactamase (MBL)-Positive Pseudomonas aeruginosa
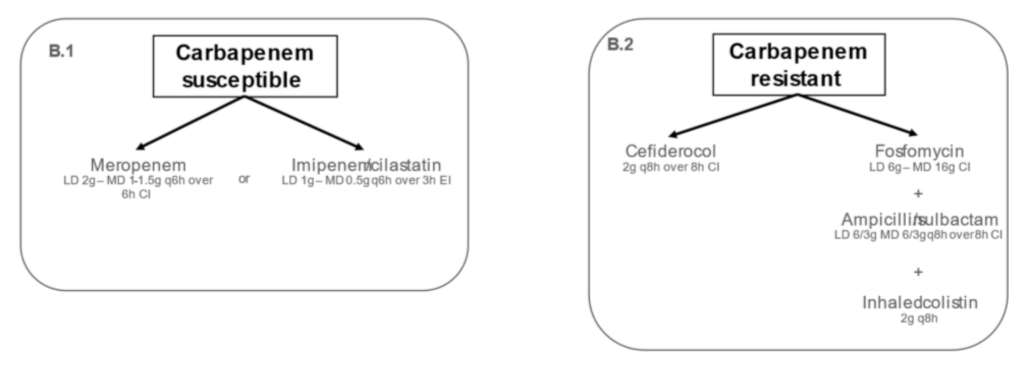
2.2. Targeted Treatment of IVACs Caused by Acinetobacter baumannii in Critically Ill Adult Patients
2.2.1. Carbapenem-Susceptible Acinetobacter baumannii
2.2.2. Carbapenem-Resistant Acinetobacter baumannii
3. Overview of Recommendations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, C.; Brun-Buisson, C.; Burchardi, H.; Martin, C.; Goodman, S.; Artigas, A.; Sicignano, A.; Palazzo, M.; Moreno, R.; Boulmé, R.; et al. Epidemiology of Sepsis and Infection in ICU Patients from an International Multicentre Cohort Study. Intensive Care Med. 2002, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Vena, A.; Graziano, E.; Russo, A.; Peghin, M. Risk Stratification and Treatment of ICU-Acquired Pneumonia Caused by Multidrug- Resistant/Extensively Drug-Resistant/Pandrug-Resistant Bacteria. Curr. Opin. Crit. Care 2018, 24, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.H.; Bergmans, D.C.J.J.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable Mortality of Ventilator-Associated Pneumonia: A Meta-Analysis of Individual Patient Data from Randomised Prevention Studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Bekaert, M.; Timsit, J.-F.; Vansteelandt, S.; Depuydt, P.; Vésin, A.; Garrouste-Orgeas, M.; Decruyenaere, J.; Clec’h, C.; Azoulay, E.; Benoit, D.; et al. Attributable Mortality of Ventilator-Associated Pneumonia: A Reappraisal Using Causal Analysis. Am. J. Respir. Crit. Care Med. 2011, 184, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Kidd, J.M.; Kuti, J.L.; Nicolau, D.P. Novel Pharmacotherapy for the Treatment of Hospital-Acquired and Ventilator-Associated Pneumonia Caused by Resistant Gram-Negative Bacteria. Expert Opin. Pharmacother. 2018, 19, 397–408. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Timsit, J.-F. Managing Acinetobacter Baumannii Infections. Curr. Opin. Infect. Dis. 2019, 32, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Villar, R.; Garnacho-Montero, J. How Should We Treat Acinetobacter Pneumonia? Curr. Opin. Crit. Care 2019, 25, 465–472. [Google Scholar] [CrossRef]
- Giannella, M.; Bartoletti, M.; Gatti, M.; Viale, P. Advances in the Therapy of Bacterial Bloodstream Infections. Clin. Microbiol Infect. 2020, 26, 158–167. [Google Scholar] [CrossRef]
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control: Solna, Sweden, 2020. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Poole, S.; Clark, T.W. Rapid Syndromic Molecular Testing in Pneumonia: The Current Landscape and Future Potential. J. Infect. 2020, 80, 1–7. [Google Scholar] [CrossRef]
- Crémet, L.; Gaborit, B.; Bouras, M.; Drumel, T.; Guillotin, F.; Poulain, C.; Persyn, E.; Lakhal, K.; Rozec, B.; Vibet, M.-A.; et al. Evaluation of the FilmArray® Pneumonia Plus Panel for Rapid Diagnosis of Hospital-Acquired Pneumonia in Intensive Care Unit Patients. Front. Microbiol. 2020, 11, 2080. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.-F.; Bassetti, M.; Cremer, O.; Daikos, G.; de Waele, J.; Kallil, A.; Kipnis, E.; Kollef, M.; Laupland, K.; Paiva, J.-A.; et al. Rationalizing Antimicrobial Therapy in the ICU: A Narrative Review. Intensive Care Med. 2019, 45, 172–189. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Jaccard, C.; Troillet, N.; Harbarth, S.; Zanetti, G.; Aymon, D.; Schneider, R.; Chiolero, R.; Ricou, B.; Romand, J.; Huber, O.; et al. Prospective Randomized Comparison of Imipenem-Cilastatin and Piperacillin-Tazobactam in Nosocomial Pneumonia or Peritonitis. Antimicrob. Agents Chemother. 1998, 42, 2966–2972. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Bernstein, J.; Solomkin, J.; Wester, B.A.; Kuye, O. Piperacillin/Tazobactam plus Tobramycin versus Ceftazidime plus Tobramycin for the Treatment of Patients with Nosocomial Lower Respiratory Tract Infection. Piperacillin/Tazobactam Nosocomial Pneumonia Study Group. J. Antimicrob. Chemother. 1999, 43, 389–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Abdel Fattah, M.; Haquin, J.; et al. Ceftazidime, Carbapenems, or Piperacillin-Tazobactam as Single Definitive Therapy for Pseudomonas Aeruginosa Bloodstream Infection: A Multisite Retrospective Study. Clin. Infect. Dis. 2020, 70, 2270–2280. [Google Scholar] [CrossRef]
- Su, T.-Y.; Ye, J.-J.; Yang, C.-C.; Huang, C.-T.; Chia, J.-H.; Lee, M.-H. Influence of Borderline Cefepime MIC on the Outcome of Cefepime-Susceptible Pseudomonas aeruginosa Bacteremia Treated with a Maximal Cefepime Dose: A Hospital-Based Retrospective Study. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 52. [Google Scholar] [CrossRef]
- Ratliff, A.R.; Gentry, C.A.; Williams, R.J. A Propensity Score-Matched Analysis of the Impact of Minimum Inhibitory Concentration on Mortality in Patients with Pseudomonas Aeruginosa Bacteremia Treated with Cefepime or Ceftazidime. Diagn. Microbiol. Infect. Dis 2017, 87, 376–381. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus Short-Term Intravenous Infusion of Antipseudomonal β-Lactams for Patients with Sepsis: A Systematic Review and Meta-Analysis of Randomised Trials. Lancet Infect. Dis 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas Aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.-F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane-Tazobactam versus Meropenem for Treatment of Nosocomial Pneumonia (ASPECT-NP): A Randomised, Controlled, Double-Blind, Phase 3, Non-Inferiority Trial. Lancet Infect. Dis. 2019, 19, 1299–1311. [Google Scholar] [CrossRef]
- Pogue, J.M.; Kaye, K.S.; Veve, M.P.; Patel, T.S.; Gerlach, A.T.; Davis, S.L.; Puzniak, L.A.; File, T.M.; Olson, S.; Dhar, S.; et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-Based Regimens for the Treatment of Drug-Resistant Pseudomonas Aeruginosa. Clin. Infect. Dis 2020, 71, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Castaldo, N.; Cattelan, A.; Mussini, C.; Righi, E.; Tascini, C.; Menichetti, F.; Mastroianni, C.M.; Tumbarello, M.; Grossi, P.; et al. Ceftolozane/Tazobactam for the Treatment of Serious Pseudomonas aeruginosa Infections: A Multicentre Nationwide Clinical Experience. Int. J. Antimicrob. Agents 2019, 53, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Balandin, B.; Ballesteros, D.; Ruiz de Luna, R.; López-Vergara, L.; Pintado, V.; Sancho-González, M.; Soriano-Cuesta, C.; Pérez-Pedrero, M.J.; Asensio-Martín, M.J.; Fernández-Simón, I.; et al. Multicenter Study of Ceftolozane/Tazobactam for Treatment of Pseudomonas Aeruginosa Infections in Critically Ill Patients. Int. J. Antimicrob. Agents 2021, 57, 106270. [Google Scholar] [CrossRef]
- Fernández-Cruz, A.; Alba, N.; Semiglia-Chong, M.A.; Padilla, B.; Rodríguez-Macías, G.; Kwon, M.; Cercenado, E.; Chamorro-de-Vega, E.; Machado, M.; Pérez-Lago, L.; et al. A Case-Control Study of Real-Life Experience with Ceftolozane-Tazobactam in Patients with Hematologic Malignancy and Pseudomonas Aeruginosa Infection. Antimicrob. Agents Chemother. 2019, 63, e02340-18. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Satlin, M.J.; Elabor, A.; Saraiya, N.; McCreary, E.K.; Molnar, E.; El-Beyrouty, C.; Jones, B.M.; Dixit, D.; Heil, E.L.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: A Multicenter Study. Open Forum Infect. Dis. 2018, 5, ofy280. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, O.; Periañez-Parraga, L.; Oliver, A.; Munita, J.M.; Boté, A.; Gasch, O.; Nuvials, X.; Dinh, A.; Shaw, R.; Lomas, J.M.; et al. Higher MICs (>2 Mg/L) Predict 30-Day Mortality in Patients With Lower Respiratory Tract Infections Caused by Multidrug- and Extensively Drug-Resistant Pseudomonas aeruginosa Treated With Ceftolozane/Tazobactam. Open Forum Infect. Dis. 2019, 6, ofz416. [Google Scholar] [CrossRef]
- Munita, J.M.; Aitken, S.L.; Miller, W.R.; Perez, F.; Rosa, R.; Shimose, L.A.; Lichtenberger, P.N.; Abbo, L.M.; Jain, R.; Nigo, M.; et al. Multicenter Evaluation of Ceftolozane/Tazobactam for Serious Infections Caused by Carbapenem-Resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2017, 65, 158–161. [Google Scholar] [CrossRef]
- Haidar, G.; Philips, N.J.; Shields, R.K.; Snyder, D.; Cheng, S.; Potoski, B.A.; Doi, Y.; Hao, B.; Press, E.G.; Cooper, V.S.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin. Infect. Dis. 2017, 65, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Bosaeed, M.; Ahmad, A.; Alali, A.; Mahmoud, E.; Alswidan, L.; Alsaedy, A.; Aljuhani, S.; Alalwan, B.; Alshamrani, M.; Alothman, A. Experience With Ceftolozane-Tazobactam for the Treatment of Serious Pseudomonas aeruginosa Infections in Saudi Tertiary Care Center. Infect. Dis. 2020, 13, 1178633720905977. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cañestro, M.; Periañez, L.; Mulet, X.; Martin-Pena, M.L.; Fraile-Ribot, P.A.; Ayestarán, I.; Colomar, A.; Nuñez, B.; Maciá, M.; Novo, A.; et al. Ceftolozane/Tazobactam for the Treatment of Multidrug Resistant Pseudomonas aeruginosa: Experience from the Balearic Islands. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Escolà-Vergé, L.; Pigrau, C.; Los-Arcos, I.; Arévalo, Á.; Viñado, B.; Campany, D.; Larrosa, N.; Nuvials, X.; Ferrer, R.; Len, O.; et al. Ceftolozane/Tazobactam for the Treatment of XDR Pseudomonas aeruginosa Infections. Infection 2018, 46, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Xipell, M.; Paredes, S.; Fresco, L.; Bodro, M.; Marco, F.; Martínez, J.A.; Soriano, A. Clinical Experience with Ceftolozane/Tazobactam in Patients with Serious Infections Due to Resistant Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2018, 13, 165–170. [Google Scholar] [CrossRef]
- Castón, J.J.; De la Torre, Á.; Ruiz-Camps, I.; Sorlí, M.L.; Torres, V.; Torre-Cisneros, J. Salvage Therapy with Ceftolozane-Tazobactam for Multidrug-Resistant Pseudomonas aeruginosa Infections. Antimicrob. Agents Chemother. 2017, 61, e02136-16. [Google Scholar] [CrossRef]
- Dinh, A.; Wyplosz, B.; Kernéis, S.; Lebeaux, D.; Bouchand, F.; Duran, C.; Béraud, G.; Lazaro, P.; Davido, B.; Hénard, S.; et al. Use of Ceftolozane/Tazobactam as Salvage Therapy for Infections Due to Extensively Drug-Resistant Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2017, 49, 782–783. [Google Scholar] [CrossRef]
- Gelfand, M.S.; Cleveland, K.O. Ceftolozane/Tazobactam Therapy of Respiratory Infections Due to Multidrug-Resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2015, 61, 853–855. [Google Scholar] [CrossRef]
- Hakki, M.; Lewis, J.S. Ceftolozane-Tazobactam Therapy for Multidrug-Resistant Pseudomonas aeruginosa Infections in Patients with Hematologic Malignancies and Hematopoietic-Cell Transplant Recipients. Infection 2018, 46, 431–434. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and Safety of Cefiderocol or Best Available Therapy for the Treatment of Serious Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria (CREDIBLE-CR): A Randomised, Open-Label, Multicentre, Pathogen-Focused, Descriptive, Phase 3 Trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus High-Dose, Extended-Infusion Meropenem for the Treatment of Gram-Negative Nosocomial Pneumonia (APEKS-NP): A Randomised, Double-Blind, Phase 3, Non-Inferiority Trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Delgado-Valverde, M.; Conejo, M.D.C.; Serrano, L.; Fernández-Cuenca, F.; Pascual, Á. Activity of Cefiderocol against High-Risk Clones of Multidrug-Resistant Enterobacterales, Acinetobacter Baumannii, Pseudomonas aeruginosa and Stenotrophomonas Maltophilia. J. Antimicrob. Chemother. 2020, 75, 1840–1849. [Google Scholar] [CrossRef]
- Mushtaq, S.; Sadouki, Z.; Vickers, A.; Livermore, D.M.; Woodford, N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Tsuji, M.; Wise, M.G.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against a Recent Collection of Clinically Relevant Carbapenem-Non-Susceptible Gram-Negative Bacilli, Including Serine Carbapenemase- and Metallo-β-Lactamase-Producing Isolates (SIDERO-WT-2014 Study). Int. J Antimicrob. Agents 2019, 53, 177–184. [Google Scholar] [CrossRef]
- Pilmis, B.; Petitjean, G.; Lesprit, P.; Lafaurie, M.; El Helali, N.; Le Monnier, A.; on behalf the ATB PK/PD Study Group. Continuous Infusion of Ceftolozane/Tazobactam Is Associated with a Higher Probability of Target Attainment in Patients Infected with Pseudomonas Aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Beisken, S.; Bergman, Y.; Posch, A.E.; Avdic, E.; Sharara, S.L.; Cosgrove, S.E.; Simner, P.J. Modifiable Risk Factors for the Emergence of Ceftolozane-Tazobactam Resistance. Clin. Infect. Dis. 2020, 73, e4599–e4606. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.-F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-Avibactam versus Meropenem in Nosocomial Pneumonia, Including Ventilator-Associated Pneumonia (REPROVE): A Randomised, Double-Blind, Phase 3 Non-Inferiority Trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Trinh, T.D.; Zasowski, E.J.; Lagnf, A.M.; Bhatia, S.; Melvin, S.M.; Steed, M.E.; Simon, S.P.; Estrada, S.J.; Morrisette, T.; et al. Real-World Experience with Ceftazidime-Avibactam for Multidrug-Resistant Gram-Negative Bacterial Infections. Open Forum Infect. Dis. 2019, 6, ofz522. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; Rosa, F.G.D.; Del Puente, F.; Mastroianni, C.M.; Cascio, A.; et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections Due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics 2020, 9, E71. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, O.; Ripa, M.; Morata, L.; de la Calle, C.; Cardozo, C.; Fehér, C.; Pellicé, M.; Valcárcel, A.; Puerta-Alcalde, P.; Marco, F.; et al. Evaluation of Ceftazidime/Avibactam for Serious Infections Due to Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas Aeruginosa. J. Glob. Antimicrob. Resist. 2018, 15, 136–139. [Google Scholar] [CrossRef]
- Santevecchi, B.A.; Smith, T.T.; MacVane, S.H. Clinical Experience with Ceftazidime/Avibactam for Treatment of Antibiotic-Resistant Organisms Other than Klebsiella Pneumoniae. Int. J. Antimicrob. Agents 2018, 51, 629–635. [Google Scholar] [CrossRef]
- Xipell, M.; Bodro, M.; Marco, F.; Losno, R.A.; Cardozo, C.; Soriano, A. Clinical Experience with Ceftazidime/Avibactam in Patients with Severe Infections, Including Meningitis and Lung Abscesses, Caused by Extensively Drug-Resistant Pseudomonas Aeruginosa. Int. J. Antimicrob. Agents 2017, 49, 266–268. [Google Scholar] [CrossRef]
- Recio, R.; Villa, J.; Viedma, E.; Orellana, M.Á.; Lora-Tamayo, J.; Chaves, F. Bacteraemia Due to Extensively Drug-Resistant Pseudomonas Aeruginosa Sequence Type 235 High-Risk Clone: Facing the Perfect Storm. Int J. Antimicrob. Agents 2018, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, D.E.; et al. Ceftazidime-Avibactam in Combination With Fosfomycin: A Novel Therapeutic Strategy Against Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2019, 220, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Avery, L.M.; Sutherland, C.A.; Nicolau, D.P. In Vitro Investigation of Synergy among Fosfomycin and Parenteral Antimicrobials against Carbapenemase-Producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2019, 95, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, S.; Singh, N.B.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Castanheira, M.; Rybak, M.J. Evaluation of the Synergy of Ceftazidime-Avibactam in Combination with Meropenem, Amikacin, Aztreonam, Colistin, or Fosfomycin against Well-Characterized Multidrug-Resistant Klebsiella Pneumoniae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-Avibactam Use for KPC-Kp Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef]
- Gatti, M.; Bartoletti, M.; Cojutti, P.G.; Gaibani, P.; Conti, M.; Giannella, M.; Viale, P.; Pea, F. A Descriptive Case Series of PK/PD Target Attainment and Microbiological Outcome in Critically Ill Patients with Documented Severe XDR Acinetobacter Baumannii BSI and/or VAP Treated with Cefiderocol. J. Glob. Antimicrob. Resist. 2021, 27, 294–298. [Google Scholar] [CrossRef]
- Nicolau, D.P.; Siew, L.; Armstrong, J.; Li, J.; Edeki, T.; Learoyd, M.; Das, S. Phase 1 Study Assessing the Steady-State Concentration of Ceftazidime and Avibactam in Plasma and Epithelial Lining Fluid Following Two Dosing Regimens. J. Antimicrob. Chemother. 2015, 70, 2862–2869. [Google Scholar] [CrossRef]
- Katsube, T.; Nicolau, D.P.; Rodvold, K.A.; Wunderink, R.G.; Echols, R.; Matsunaga, Y.; Menon, A.; Portsmouth, S.; Wajima, T. Intrapulmonary Pharmacokinetic Profile of Cefiderocol in Mechanically Ventilated Patients with Pneumonia. J. Antimicrob. Chemother. 2021, 76, 2902–2905. [Google Scholar] [CrossRef]
- Katsube, T.; Saisho, Y.; Shimada, J.; Furuie, H. Intrapulmonary Pharmacokinetics of Cefiderocol, a Novel Siderophore Cephalosporin, in Healthy Adult Subjects. J. Antimicrob. Chemother. 2019, 74, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.R.; Abdelhamed, A.M.; Good, C.E.; Rhoads, D.D.; Hujer, K.M.; Hujer, A.M.; Domitrovic, T.N.; Rudin, S.D.; Richter, S.S.; van Duin, D.; et al. ARGONAUT-I: Activity of Cefiderocol (S-649266), a Siderophore Cephalosporin, against Gram-Negative Bacteria, Including Carbapenem-Resistant Nonfermenters and Enterobacteriaceae with Defined Extended-Spectrum β-Lactamases and Carbapenemases. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Albiero, J.; Mazucheli, J.; Barros, J.P.D.R.; Szczerepa, M.M.D.A.; Nishiyama, S.A.B.; Carrara-Marroni, F.E.; Sy, S.; Fidler, M.; Sy, S.K.B.; Tognim, M.C.B. Pharmacodynamic Attainment of the Synergism of Meropenem and Fosfomycin Combination against Pseudomonas aeruginosa Producing Metallo-β-Lactamase. Antimicrob. Agents Chemother. 2019, 63, e00126-19. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Samonis, G.; Falagas, M.E. Inhaled Colistin Monotherapy for Respiratory Tract Infections in Adults without Cystic Fibrosis: A Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents 2018, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alamarat, Z.I.; Babic, J.; Tran, T.T.; Wootton, S.H.; Dinh, A.Q.; Miller, W.R.; Hanson, B.; Wanger, A.; Gary, J.L.; Arias, C.A.; et al. Long-Term Compassionate Use of Cefiderocol To Treat Chronic Osteomyelitis Caused by Extensively Drug-Resistant Pseudomonas aeruginosa and Extended-Spectrum-β-Lactamase-Producing Klebsiella Pneumoniae in a Pediatric Patient. Antimicrob. Agents Chemother. 2020, 64, e01872-19. [Google Scholar] [CrossRef]
- Zingg, S.; Nicoletti, G.J.; Kuster, S.; Junker, M.; Widmer, A.; Egli, A.; Hinic, V.; Sendi, P.; Battegay, M.; Bättig, V.; et al. Cefiderocol for Extensively Drug-Resistant Gram-Negative Bacterial Infections: Real-World Experience From a Case Series and Review of the Literature. Open Forum Infect. Dis. 2020, 7, ofaa185. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Gatti, M.; Rinaldi, M.; Tonetti, T.; Laici, C.; Mega, C.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Impact of Maximizing Css/MIC Ratio on Efficacy of Continuous Infusion Meropenem Against Documented Gram-Negative Infections in Critically Ill Patients and Population Pharmacokinetic/Pharmacodynamic Analysis to Support Treatment Optimization. Front. Pharmacol. 2021, 12, 781892. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Ortiz-Leyba, C.; Jiménez-Jiménez, F.J.; Barrero-Almodóvar, A.E.; García-Garmendia, J.L.; Bernabeu-WittelI, M.; Gallego-Lara, S.L.; Madrazo-Osuna, J. Treatment of Multidrug-Resistant Acinetobacter Baumannii Ventilator-Associated Pneumonia (VAP) with Intravenous Colistin: A Comparison with Imipenem-Susceptible VAP. Clin. Infect. Dis. 2003, 36, 1111–1118. [Google Scholar] [CrossRef]
- Kallel, H.; Hergafi, L.; Bahloul, M.; Hakim, A.; Dammak, H.; Chelly, H.; Hamida, C.B.; Chaari, A.; Rekik, N.; Bouaziz, M. Safety and Efficacy of Colistin Compared with Imipenem in the Treatment of Ventilator-Associated Pneumonia: A Matched Case-Control Study. Intensive Care Med. 2007, 33, 1162–1167. [Google Scholar] [CrossRef]
- Wang, D. Experience with Extended-Infusion Meropenem in the Management of Ventilator-Associated Pneumonia Due to Multidrug-Resistant Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2009, 33, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, A.; Pournaras, S.; Maniatis, A.N.; Legakis, N.J.; Tsakris, A. Discordance of Meropenem versus Imipenem Activity against Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2006, 28, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Mezzatesta, M.L.; Trovato, G.; Gona, F.; Nicolosi, V.M.; Nicolosi, D.; Carattoli, A.; Fadda, G.; Nicoletti, G.; Stefani, S. In Vitro Activity of Tigecycline and Comparators against Carbapenem-Susceptible and Resistant Acinetobacter Baumannii Clinical Isolates in Italy. Ann. Clin. Microbiol. Antimicrob. 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Guzek, A.; Korzeniewski, K.; Nitsch-Osuch, A.; Rybicki, Z.; Prokop, E. In Vitro Sensitivity of Acinetobacter Baumannii and Pseudomonas aeruginosa to Carbapenems among Intensive Care Unit Patients. Adv. Exp. Med. Biol. 2013, 788, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Sader, H.S.; Fritsche, T.R. Comparative Activity of Doripenem and Three Other Carbapenems Tested against Gram-Negative Bacilli with Various Beta-Lactamase Resistance Mechanisms. Diagn. Microbiol. Infect. Dis. 2005, 52, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Belati, A.; Diella, L.; Stufano, M.; Romanelli, F.; Scalone, L.; Stolfa, S.; Ronga, L.; Maurmo, L.; Dell’Aera, M.; et al. Cefiderocol-Based Combination Therapy for “Difficult-to-Treat” Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics 2021, 10, 652. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Nicastro, M.; Leonildi, A.; Vecchione, A.; Casella, C.; Forfori, F.; Malacarne, P.; Guarracino, F.; Barnini, S.; et al. Cefiderocol as Rescue Therapy for Acinetobacter Baumannii and Other Carbapenem-Resistant Gram-Negative Infections in ICU Patients. Clin. Infect. Dis 2020, 72, 2021–2024. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Quirino, A.; Scaglione, V.; Longhini, F.; Garofalo, E.; Bruni, A.; Biamonte, E.; Lionello, R.; Serapide, F.; Mazzitelli, M.; et al. Successful Treatment with Cefiderocol for Compassionate Use in a Critically Ill Patient with XDR Acinetobacter Baumannii and KPC-Producing Klebsiella Pneumoniae: A Case Report. J. Antimicrob. Chemother. 2019, 74, 3399–3401. [Google Scholar] [CrossRef]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study). Antimicrob. Agents Chemother. 2017, 61, e00093-17. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Frantzeskaki, F.; Xanthaki, A.; Georgiadis, G. High-Dose Ampicillin-Sulbactam as an Alternative Treatment of Late-Onset VAP from Multidrug-Resistant Acinetobacter baumannii. Scand. J. Infect. Dis. 2007, 39, 38–43. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Frantzeskaki, F.; Xanthaki, A.; Douzinas, E.E. Efficacy and Safety of High-Dose Ampicillin/Sulbactam vs. Colistin as Monotherapy for the Treatment of Multidrug Resistant Acinetobacter Baumannii Ventilator-Associated Pneumonia. J. Infect. 2008, 56, 432–436. [Google Scholar] [CrossRef]
- Mellon, G.; Clec’h, C.; Picard, B.; Cohen, Y.; Jauréguy, F. Postsurgical Meningitis Due to Multiresistant Acinetobacter baumannii Successfully Treated with High Doses of Ampicillin/Sulbactam Combined with Rifampicin and Fosfomycin. J. Infect. Chemother. 2012, 18, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sazlly Lim, S.; Heffernan, A.J.; Roberts, J.A.; Sime, F.B. Semi-Mechanistic PK/PD Modelling of Fosfomycin and Sulbactam Combination against Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e02472-20. [Google Scholar] [CrossRef]
- Kuo, S.-C.; Lee, Y.-T.; Yang, S.-P.; Chen, C.-P.; Chen, T.-L.; Hsieh, S.-L.; Siu, L.-K.; Fung, C.-P. Eradication of Multidrug-Resistant Acinetobacter baumannii from the Respiratory Tract with Inhaled Colistin Methanesulfonate: A Matched Case-Control Study. Clin. Microbiol. Infect. 2012, 18, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to Manage Pseudomonas Aeruginosa Infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- Caro, L.; Nicolau, D.P.; De Waele, J.J.; Kuti, J.L.; Larson, K.B.; Gadzicki, E.; Yu, B.; Zeng, Z.; Adedoyin, A.; Rhee, E.G. Lung Penetration, Bronchopulmonary Pharmacokinetic/Pharmacodynamic Profile and Safety of 3 g of Ceftolozane/Tazobactam Administered to Ventilated, Critically Ill Patients with Pneumonia. J. Antimicrob. Chemother. 2020, 75, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Pea, F. Continuous versus Intermittent Infusion of Antibiotics in Gram-Negative Multidrug-Resistant Infections. Curr. Opin. Infect. Dis. 2021, 34, 737–747. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.; Drusano, G.L. Piperacillin-Tazobactam for Pseudomonas Aeruginosa Infection: Clinical Implications of an Extended-Infusion Dosing Strategy. Clin. Infect. Dis 2007, 44, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Boisson, M.; Jacobs, M.; Grégoire, N.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Comparison of Intrapulmonary and Systemic Pharmacokinetics of Colistin Methanesulfonate (CMS) and Colistin after Aerosol Delivery and Intravenous Administration of CMS in Critically Ill Patients. Antimicrob. Agents Chemother. 2014, 58, 7331–7339. [Google Scholar] [CrossRef]
- Benítez-Cano, A.; de Antonio-Cuscó, M.; Luque, S.; Sorlí, L.; Carazo, J.; Ramos, I.; Bermejo, S.; Campillo, N.; Horcajada, J.P.; Samsó, E.; et al. Systemic Pharmacokinetics and Safety of High Doses of Nebulized Colistimethate Sodium in Critically Ill Patients with Hospital-Acquired and Ventilator-Associated Pneumonia. J. Antimicrob. Chemother. 2019, 74, 3268–3273. [Google Scholar] [CrossRef]
- Biagi, M.; Butler, D.; Tan, X.; Qasmieh, S.; Wenzler, E. A Breath of Fresh Air in the Fog of Antimicrobial Resistance: Inhaled Polymyxins for Gram-Negative Pneumonia. Antibiotics 2019, 8, E27. [Google Scholar] [CrossRef]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2019, 63, e01110-18. [Google Scholar] [CrossRef] [PubMed]
- Heil, E.L.; Tamma, P.D. Cefiderocol: The Trojan Horse Has Arrived but Will Troy Fall? Lancet Infect. Dis. 2021, 21, 153–155. [Google Scholar] [CrossRef]
- Matzi, V.; Lindenmann, J.; Porubsky, C.; Kugler, S.A.; Maier, A.; Dittrich, P.; Smolle-Jüttner, F.M.; Joukhadar, C. Extracellular Concentrations of Fosfomycin in Lung Tissue of Septic Patients. J. Antimicrob. Chemother. 2010, 65, 995–998. [Google Scholar] [CrossRef]
- Russo, A.; Bassetti, M.; Bellelli, V.; Bianchi, L.; Marincola Cattaneo, F.; Mazzocchetti, S.; Paciacconi, E.; Cottini, F.; Schiattarella, A.; Tufaro, G.; et al. Efficacy of a Fosfomycin-Containing Regimen for Treatment of Severe Pneumonia Caused by Multidrug-Resistant Acinetobacter Baumannii: A Prospective, Observational Study. Infect. Dis. Ther. 2021, 10, 187–200. [Google Scholar] [CrossRef]
- Thabit, A.K.; Hobbs, A.L.V.; Guzman, O.E.; Shea, K.M. The Pharmacodynamics of Prolonged Infusion β-Lactams for the Treatment of Pseudomonas aeruginosa Infections: A Systematic Review. Clin. Ther. 2019, 41, 2397–2415. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Pharmacokinetic/Pharmacodynamic Target Attainment in Critically Ill Renal Patients on Antimicrobial Usage: Focus on Novel Beta-Lactams and Beta Lactams/Beta-Lactamase Inhibitors. Expert Rev. Clin. Pharmacol. 2021, 14, 583–599. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharm. 2019, 58, 1407–1443. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P. Bench-to-Bedside Review: Appropriate Antibiotic Therapy in Severe Sepsis and Septic Shock-Does the Dose Matter? Crit. Care 2009, 13, 214. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Antimicrobial Dose Reduction in Continuous Renal Replacement Therapy: Myth or Real Need? A Practical Approach for Guiding Dose Optimization of Novel Antibiotics. Clin. Pharm. 2021, 60, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.G.L.; van Hest, R.M.; Lipman, J.; Taccone, F.S.; Roberts, J.A. Therapeutic Drug Monitoring of Anti-Infective Agents in Critically Ill Patients. Expert Rev. Clin. Pharmacol. 2016, 9, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Cotta, M.O.; Roberts, J.A. Pharmacokinetics/Pharmacodynamics of β-Lactams and Therapeutic Drug Monitoring: From Theory to Practical Issues in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2019, 40, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Sandaradura, I.; Alffenaar, J.-W.; Cotta, M.O.; Daveson, K.; Day, R.O.; Van Hal, S.; Lau, C.; Marriott, D.J.E.; Penm, J.; Roberts, J.A.; et al. Emerging Therapeutic Drug Monitoring of Anti-Infective Agents in Australian Hospitals: Availability, Performance and Barriers to Implementation. Br. J. Clin. Pharmacol. 2021; Early View. [Google Scholar] [CrossRef]
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Marotta, C.; Amicone, M.; Bavaro, D.F.; Bernaudo, F.; Frisicale, E.M.; Kurotschka, P.K.; Mazzari, A.; Veronese, N.; Murri, R.; et al. Italian Young Doctors’ Knowledge, Attitudes and Practices on Antibiotic Use and Resistance: A National Cross-Sectional Survey. J. Glob. Antimicrob. Resist. 2020, 23, 167–173. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-Lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas Aeruginosa with Difficult-to-Treat Resistance (DTR-P. Aeruginosa). Clin. Infect. Dis. 2021, 72, 1109–1116. [Google Scholar] [CrossRef]
- Hawkey, P.M.; Warren, R.E.; Livermore, D.M.; McNulty, C.A.M.; Enoch, D.A.; Otter, J.A.; Wilson, A.P.R. Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacteria: Report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J. Antimicrob. Chemother. 2018, 73, iii2–iii78. [Google Scholar] [CrossRef]
- Pouch, S.M.; Patel, G. AST Infectious Diseases Community of Practice Multidrug-Resistant Gram-Negative Bacterial Infections in Solid Organ Transplant Recipients-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13594. [Google Scholar] [CrossRef]
- Peña-López, Y.; Ramirez-Estrada, S.; Eshwara, V.K.; Rello, J. Limiting Ventilator-Associated Complications in ICU Intubated Subjects: Strategies to Prevent Ventilator-Associated Events and Improve Outcomes. Expert Rev. Respir. Med. 2018, 12, 1037–1050. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New Evidence Pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [PubMed]
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Rate of IVACs | Isolates | Severity | Clinical Outcomes | Relapse Rate— Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Piperacillin–tazobactam | |||||||||
| Kalil et al., 2016 [15] | Guidelines | Piperacillin–tazobactam at dosage of 4.5 g q6h (preferring EI or CI) for empiric or definitive treatment of HAP/VAP caused by Pseudomonas aeruginosa according to antimicrobial susceptibility test (Strong recommendation; low-quality evidence) | |||||||
| Jaccard et al., 1998 [16] | RCT, multicentre | 371 (IMI vs. PIT) | IMI 500 mg q6h vs. PIT 4.5 g q8h | 49.2% HAP | 28% P. aeruginosa | Mechanical ventilation 47% APACHE II score: 14.6 ± 6.8 | Clinical failure rate: 17% (PIT) vs. 29% (IMI) p = 0.09 Mortality rate for infection: 8% (PIT) vs. 9% (IMI) p = 0.78 Clinical failure rate in P. aeruginosa HAP: 10% (PIT) vs. 50% (IMI) p = 0.004 | Resistance development 25.0% IMI vs. 4.8% PIT | PIT monotherapy is at least as effective and safe as IMI monotherapy in the treatment of HAP. In P. aeruginosa HAP, PIT achieved a better clinical efficacy than IMI, due to reduced development of microbiological resistance. |
| Joshi et al., 1999 [17] | RCT, multicentre | 300 (155 PIT + tobramycin vs. 145 CTZ + tobramycin) | PIT 3.375 g q4h + tobramycin 5 mg/kg/day vs. CTZ 2 g q8h + tobramycin 5 mg/kg/day | 87% HAP | 7.7% P. aeruginosa | Severe infection 21% | Clinical cure rate: 74.2% (PIT) vs. 57.9% (CTZ) p = 0.004 Clinical cure rate in P. aeruginosa HAP: 67% (PIT) vs. 30% (CTZ) p = NS | NA | PIT plus tobramycin was shown to be more effective and as safe as CTZ plus tobramycin in the treatment of patients with HAP. A trend to higher microbiological eradication was found P. aeruginosa subgroup with PIT. |
| Babich et al., 2020 [18] | Retrospective, multicentre, propensity score adjusted analysis | 767 (213 CTZ vs. 210 MER/IMI vs. 344 PIT) | All monotherapy 83.3% Intermittent infusion | All BSI 14.7% HAP/VAP | 100% P. aeruginosa 7.6% MDR | ICU admission 16.6% Mechanical ventilation 12.1% SOFA score 4 (2–6) | Mortality rate: 17.4% (CTZ) vs. 20% (MER- IMI) vs. 16% (PIT) p = NS | Resistance development: 17.5% (MER-IMI) vs. 12.4% (CTZ) vs. 8.4% (PIT) p = 0.007 | No significant difference in mortality, clinical, and microbiological outcomes or adverse events was demonstrated between CTZ, carbapenems, and PIT as definitive treatment of P. aeruginosa bacteraemia. Higher rates of resistance development were found in patients treated with carbapenems. |
| Third/fourth-generation cephalosporins (Ceftazidime–Cefepime) | |||||||||
| Kalil et al., 2016 [15] | Guidelines | Both ceftazidime and cefepime at dosage of 2 g q8h (preferring EI or CI) for empiric or definitive treatment of HAP/VAP caused by Pseudomonas aeruginosa according to antimicrobial susceptibility test (strong recommendation; low-quality evidence) | |||||||
| Babich et al., 2020 [18] | Retrospective, multicentre, propensity score adjusted analysis | 767 (213 CTZ vs. 210 MER/IMI vs. 344 PIT) | All monotherapy 83.3% Intermittent infusion | All BSI 14.7% HAP/VAP | 100% P. aeruginosa 7.6% MDR | ICU admission 16.6% Mechanical ventilation 12.1% SOFA score 4 (2–6) | Mortality rate: 17.4% (CTZ) vs. 20% (MER- IMI) vs. 16% (PIT) p = NS | Resistance development: 17.5% (MER- IMI) vs. 12.4% (CTZ) vs. 8.4% (PIP-TZB) p = 0.007 | No difference in mortality rate between ceftazidime and carbapenems at propensity score analysis (OR 1.14; CI 0.52–2.46). Significant higher occurrence of new resistance development in P. aeruginosa isolates in patients treated with carbapenems compared to ceftazidime (17.5% vs. 12.4%; p = 0.007). |
| Su et al., 2017 [19] | Retrospective | 90 | Cefepime 2 g q8h II | All BSIs 30% HAP/VAP | All P. aeruginosa cefepime-susceptible | ICU admission 32.2% Mechanical ventilation 25.6% Severe sepsis/septic shock 23.3% Mean APACHE II score: 22.07 Neutropenia 20% | Overall 30-day mortality rate: 36.7% Overall 30-day mortality rate in HAP/VAP subgroup: 59.3% | NA | A cefepime MIC of 4 mg/L may predict an unfavourable outcome among patients with serious infections caused by P. aeruginosa. |
| Ratliff et al., 2017 [20] | Retrospective, propensity score matched analysis | 58 (29 MIC ≤ 2 mg/L vs. 29 MIC > 2 mg/L) | Ceftazidime 2 g q8h or Cefepime 2 g q12h | All BSIs 22.4% HAP/VAP | All P. aeruginosa | NA | 30-day mortality rate: 17.2% vs. 27.6% (p = 0.34) | NA | No subgroup analysis was performed according to site of infection. |
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Rate of IVACs | Isolates | Severity | Clinical Outcomes | Relapse Rate— Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Ceftolozane–tazobactam | |||||||||
| Kollef et al., 2019 [24] | phase III RCT, multicentre (ASPECT-NP) | 726 (362 CTT vs. 364 MER) | CTT 3 g q8h vs. MER 1 g q8h | All nosocomial pneumonia 71% VAP 6% secondary BSI | 17.4% P. aeruginosa 38.1% MDR-PA 15.9% XDR-PA | ICU admission 92% APACHE II score ≥20 33% Median SOFA score 6 Median CPIS 10 Median duration of mechanical ventilation: 5 days | 28-day mortality rate in P. aeruginosa subgroup: 25.4% vs. 18.5% (p = NS) Clinical cure rate 57.1% vs. 60% (p = NS) | NA | High-dose CTT is an efficacious and well tolerated treatment for Gram-negative HAP/VAP No difference in mortality and clinical cure rate for P. aeruginosa between CTT and MER, including MDR (54.2% vs. 54.5%) and XDR isolates (40% vs. 40%) |
| Pogue et al., 2019 [25] | Retrospective observational comparative, multicentre | 200 (100 LOZ-TAZ vs. 100 polymyxin- or aminoglycoside-combination therapy) | CTT 1.5–3 g q8h vs. COL/polymyxin B or gentamycin— amikacin—tobramycin | 52% VAP 12% HAP | 100% P. aeruginosa | ICU admission 69% Mechanical ventilation 63% Severe sepsis/septic shock 42% Immunosuppression 14% | Clinical cure rate: 81% vs. 61% (p = 0.002) Overall AKI rate: 6% vs. 34% (p < 0.001) In-hospital mortality rate: 20% vs. 25% (p = 0.40) | Relapse 14% vs. 16% (p = NS) | CTT was independently associated with clinical cure (OR 2.63; 95% CI 1.31–5.30) and protective against AKI (OR 0.08; 95% CI 0.03–0.22) Preferential use CTT over polymyxins or aminoglycosides for MDR-PA infections. |
| Bassetti et al., 2019 [26] | Retrospective observational, multicentre (CEFTABUSE) | 101 | CTT 1.5–3 g q8h (CI/EI 18.8%) 38.6% first-line therapy | 31.7% HAP/VAP | 100% P. aeruginosa 17.8% MDR-PA 50.5% XDR-PA 2% PDR-PA | ICU admission 23.8% Mechanical ventilation 18.8% Septic shock 11.9% Solid organ transplant recipients 10.9% Haematological malignancy 12.9% Neutropenia 10.9% | Clinical cure rate: 83.2% | Relapse 7% Resistance 3% | Lower clinical success in patients with sepsis or requiring CRRT. Higher clinical failure (25.0) in pneumonia subgroup compared to other types of infection |
| Balandin et al., 2020 [27] | Retrospective observational, multicentre | 95 | CTT 1.5–3 g q8h | 56.2% HAP/VAP 8.4% VAT | 100% P. aeruginosa 48.4% XDR-PA 36.8% MDR-PA | ICU admission 100% Mechanical ventilation 80% Septic shock 45.3% RRT 27.4% Mean SOFA 6.9 Solid organ transplant recipients 6.2% | Microbiological eradication: 42.1% ICU mortality: 36.5% | Relapse 22.9% | Mortality rate in pneumonia subgroup: 34% |
| Fernandez-Cruz et al., 2019 [28] | Retrospective case-control | 57 (19 CTT vs. 38 other agents) | CTT 3 g q8h (HAP/VAP or BSI) 84.6% targeted therapy | 26.3% HAP/VAP | 100% P. aeruginosa 52.6% MDR-PA 47.4% XDR-PA 100% ST-175 clone | ICU admission 26.3% Haematological malignancy 100% Neutropenia 63.2% Sepsis 15.8% Mean SOFA 5.42 | 14-day clinical cure rate: 89.5% vs. 71.1% (p = 0.18) 30-day mortality rate: 5.4% vs. 28.9% (p = 0.045) | Relapse 15.8% | CTT showed lower mortality compared to traditional therapy in severe PA infections in haematological patients. No subgroup analysis in patients with HAP/VAP was performed. |
| Gallagher et al., 2018 [29] | Retrospective observational, multicentre | 205 | CTT 1.5–3 g q8h Dose adjustment according to renal function | 59% HAP/VAP | 100% P. aeruginosa | ICU admission 51.2% Median APACHE II score 19 Solid organ transplant recipients 17.1% | Overall mortality rate: 19% Clinical cure rate: 73.7% | NA | Mortality rate was higher in VAP subgroup (37.9% vs. 19%) Clinical success was lower in VAP subgroup (50% vs. 73.7%) Pneumonia was associated with significant lower microbiological cure (OR 0.12; 95% CI 0.05–0.30) |
| Rodriguez-Nunez et al., 2019 [30] | Retrospective observational, multicentre | 90 | CTT 60% 3 g q8h | 70% HAP/VAP 30% VAT | 76.7% XDR-PA 23.3% MDR-PA Median MIC 2 mg/L | Septic shock 34.4% RRT 12.2% Solid organ transplant recipients 8.9% | 30-day mortality rate: 27.8% | NA | MIC > 2 mg/L was an independent predictor of mortality at multivariate analysis |
| Munita et al., 2017 [31] | Retrospective observational | 35 | CTT 1.5–3 g q8h Dose adjustment according to renal function | 51% HAP/VAP | 100% CR-PA | NA | Overall clinical cure rate: 74% | NA | 38.9% clinical failure rate in HAP/VAP subgroup |
| Haidar et al., 2017 [32] | Retrospective observational | 21 | CTT 1.5–3 g q8h | 85.7% HAP/VAP | 100% MDR-PA | Mechanical ventilation 38% Immunosuppression 43% | 30-day mortality rate: 10% Clinical failure rate: 29% | Relapse 29% Resistance 14% | 33.3% clinical failure rate in HAP/VAP subgroup |
| Bosaeed et al., 2020 [33] | Retrospective observational | 19 | CTT 1.5–3 g q8h | 16% HAP 16% VAP | 100% CR-PA | ICU admission 63% Haematological malignancy 26% | 30-day mortality rate: 21% Microbiological eradication: 74% | NA | Microbiological failure in 50% of HAP/VAP cases |
| Diaz -Canestro et al., 2018 [34] | Prospective observational | 58 | CTT 1.5–3 g q8h 91.4% targeted therapy | 60.3% HAP/VAP | 86.2% XDR-PA 10.3% MDR-PA 50% ST-175 clone | ICU admission 27.6% Mechanical ventilation 32.8% Immunosuppression 12.1% Median SOFA 3 | Clinical cure rate: 63.8% 30-day mortality rate: 27.6% | Resistance 13.8% | Clinical failure was documented in 42.9% of HAP/VAP ST-175 clone associated with higher risk of clinical failure at multivariate analysis |
| Escola-Verge et al., 2018 [35] | Retrospective observational | 38 | CTT 1.5–3 g q8h | 36.8% HAP/VAP | 100% XDR-PA Median CTT MIC: 2 mg/L | ICU admission 31.6% Solid organ transplant recipients 28.9% Neutropenia 15.8% | 90-day clinical cure: 68.4% 90-day mortality rate: 13.2% | Relapse 21.1% | Clinical failure in HAP/VAP subgroup: 25% |
| Xipell et al., 2018 [36] | Retrospective observational | 23 | CTT | 17.4% HAP 17.4% VAT | 79% XDR-PA 17% MDR-PA 4% PDR-PA | NA | Clinical cure rate: 87.5% 6-weeks mortality rate: 21.7% | NA | Higher mortality rate in respiratory tract infections (37%). Significant higher mortality rate in patients with HAP/VAP treated with low-dosage (1.5 g q8h) vs. high-dose (3 g q8h) CTT (60% vs. 0%) |
| Caston et al., 2017 [37] | Case series | 12 | CTT 100% targeted therapy | 50% HAP/VAP | 100% MDR-PA | Septic shock 83.3% | Overall mortality rate: 25% Microbiological eradication: 83.3% | Resistance: 16.6% | Mortality rate in HAP/VAP subgroup: 33.3% |
| Dinh et al., 2017 [38] | Case series | 15 | CTT Median daily dose 6 g/day | 46.7% nosocomial pneumonia (85.7% VAP) | 100% XDR-PA | ICU admission 53.3% Mean SOFA score 7.6 Immunosuppression 66.7% | Clinical failure: 33.3% In-hospital mortality rate: 27% | Relapse 11.1% | Clinical failure in nosocomial pneumonia subgroup: 40% |
| Gelfand et al., 2015 [39] | Case series | 3 | CTT 3 g q8h | 100% VAP | 100% MDR-PA | ICU admission 100% Mechanical ventilation 100% | Clinical cure: 100% | NA | |
| Hakki et al., 2018 [40] | Case series | 6 | CTT 3 g q8h | 50% pneumonia | 100% MDR-PA | Haematopoietic-cell transplant recipients 100% | Clinical cure rate: 66.7% | Relapse 28.6% | 33.3% clinical failure rate in patients with nosocomial pneumonia |
| Cefiderocol | |||||||||
| Bassetti et al., 2020 [41] | Phase 3, randomized, prospective, multicentre, open-label (CREDIBLE-CR) | 150 (101 cefiderocol vs. 49 BAT) | Cefiderocol 2 g q8h (3h-infusion) 100% target therapy Dose adjustment according to renal function | 44.6% HAP/VAP | 15% P. aeruginosa | ICU admission 56% Septic shock 19% Mechanical ventilation 50% Immunocompromised 27% Mean SOFA score 5.1 | Mortality rate in PA subgroup: 35% vs. 17% (p = NS) Clinical cure at the end of treatment (HAP/VAP subgroup): 60% vs. 63% | NA | A numerically higher proportion of patients with CRE infections achieved a clinical cure in the cefiderocol group than in the BAT group (66% vs. 45%). |
| Wunderink et al., 2020 [42] | Phase 3, randomized, prospective, multicentre, open-label (APEKS-NP) | 300 (148 cefiderocol vs. 152 meropenem) | Cefiderocol 2 g q8h (3 h infusion) vs. MER 2 g q8h (3 h infusion) | 123 VAP 119 HAP 50 HCAP | 16.4% P. aeruginosa 8% carbapenemase-producers | ICU admission: 68% Mechanical ventilation: 60% Mean SOFA score 4.8 APACHE II score ≥ 16: 49% | Mortality rate at 14-day in PA subgroup: 8% vs. 13% (p = NS) Clinical cure rate in PA subgroup: 67% vs. 71% (p = NS) | NA | Cefiderocol was non-inferior to high-dose, extended-infusion MER in terms of all-cause mortality on day 14 in patients with Gram-negative nosocomial pneumonia |
| Delgado-Valverde et al., 2020 [43] | In vitro study | 6 | 5 ST-175; 1 IMP+. Cefiderocol MIC range: 0.125–0.5 (100% susceptibility) | ||||||
| Mushtaq et al., 2020 [44] | In vitro study | 111 | 30 VIM+; 25 IMP+; 20 GES+; 15 PER+; 11 NDM+; 10 VEB+. Overall resistance rate (MIC > 2): 18.9%. Susceptibility: VIM 93.3%; GES 90.0%; VEB 90.0%; IMP 80.0%; PER 66.7%; NDM 45.5%. | ||||||
| Kazmierczak et al., 2019 [45] | In vitro study | 353 | 321 carbapenemase-negative meropenem non-susceptible (MIC range 0.002–8; MIC50 0.12; MIC90 1); 26 VIM+ (MIC range 0.008–2; MIC50 0.25; MIC90 2); 4 IMP+ (MIC range 1–2); 4 GES+ (MIC range 0.12–0.25) | ||||||
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Rate of IVACs | Isolates | Severity | Clinical Outcomes | Relapse Rate—Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime–avibactam | |||||||||
| Torres et al., 2018 [48] | Phase III RCT, multicentre (REPROVE) | 808 (405 CTV vs. 403 MER) | CTV 2.5 g q8h vs. MER 1 g q8h | 67% HAP 33% VAP | 30% P. aeruginosa CTV MIC90: 8 mg/L | Mechanical ventilation 43% | Overall clinical cure: 68.8% vs. 73% (p = NS) Clinical cure in PA subgroup: 64.3% vs. 77.1% (p = NS) | NA | CTV potential alternative to carbapenems in the management of nosocomial pneumonia, also caused by PA |
| Jorgensen et al., 2019 [49] | Retrospective observational, multicentre | 63 | CTV 2.5 g q8h Dose adjustment according to renal function | 60.3% HAP/VAP | 100% P. aeruginosa CTV MIC50: 2 mg/L CTV MIC90: 6 mg/L | ICU admission 55.6% Median SOFA score 5 Immunocompromised 6.3% | Clinical failure: 30.2% 30-day mortality rate: 17.5% | Relapse 6.3% Resistance 0% | CTV could be an effective therapy for MDR-PA as well as CRE infections. No difference in mortality rate between PA and CRE treated with CTV (17.5% vs. 16.2%; p = NS) |
| Vena et al., 2020 [50] | Retrospective observational, multicentre | 41 | CTV 2.5 g q8h (36.6% CI/EI) 80.5% targeted therapy Dose adjustment according to renal function | 48.8% nosocomial pneumonia (65% VAP—35% HAP) | 80.5% P. aeruginosa | ICU admission 41.5% Mechanical ventilation 34.1% Septic shock 17.1% CRRT 12.2% Solid organ transplant recipients 19.5% Haematological malignancies 9.8% Neutropenia 12.2% | Clinical success in HAP/VAP: 90% Clinical cure rate in PA subgroup: 87.8% | NA | CTV as value option for XDR-PA infection, including HAP/VAP |
| Rodriguez-Nunez, 2018 [51] | Case series | 8 | CTV 2.5 g q8h Dose adjustment according to renal function | 62.5% HAP/VAP | MDR/XDR PA | NA | Clinical cure rate: 50% (40% in HAP/VAP subgroup) 30-day mortality rate: 37.5% (60% in HAP/VAP subgroup) | Relapse 20% | |
| Santevecchi et al., 2018 [52] | Case series | 3 | CTV 2.5 q8h Dose adjustment according to renal function | 100% VAP | 2 MDR-PA 1 XDR-PA | ICU admission 100% Mechanical ventilation 100% | Clinical cure rate: 66.7% | None | |
| Xipell et al., 2017 [53] | Case report | 1 | CTV 2.5 g q8h | HAP | XDR-PA | NA | Clinical cure 100% | None | |
| Recio et al., 2018 [54] | In vitro analysis of a retrospective study | 24 | CTV | 33.3% HAP/VAP | All XDR-PA 45.8% GES-5-positive ST235 clone 41.1% VIM-2 ST175 clone 13.1% non-carbapenemase producers | Overall susceptibility rate to CTV in GES-5-positive strains:100%; MIC90 6 mg/L CTV demonstrated in vitro high activity against GES-positive strains | |||
| Ceftazidime-Avibactam + Fosfomycin | |||||||||
| Papp -Wallace et al., 2019 [55] | Preclinical study— murine model infection | The association between CTV and FOS significantly reduced the P. aeruginosa (CFUs), by approximately 2 and 5 logs, compared with stasis and in the vehicle-treated control, respectively. Administration of ceftazidime–avibactam and fosfomycin separately significantly increased CFUs, by approximately 3 logs and 1 log, respectively, compared with the number at stasis, and only reduced CFUs by approximately 1 log and 2 logs, respectively, compared with the number in the vehicle-treated control. The combination of CTV + FOS was superior to either drug alone and has the potential to offer infected patients with high bacterial burdens a valid therapeutic choice against infection with MDR-PA that lack metallo-beta-lactamases. | |||||||
| Avery et al., 2019 [56] | In vitro study | 53 | CR-PA: CTV baseline susceptibility 89.5%. Synergism with FOS in 25% of isolates (FICI ≤ 0.5) | ||||||
| Mikhail et al., 2019 [57] | In vitro study | 21 | MDR-PA. CTV MIC reduction in 13/21 (61.9%) of isolates in combination with FOS. Combination between CTV and FOS was indifferent at time-kill analysis. | ||||||
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Rate of IVACs | Isolates | Severity | Clinical Outcomes | Relapse Rate—Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Cefiderocol | |||||||||
| Mushtaq et al., 2020 [44] | In vitro study | 66 | 30 VIM+; 25 IMP+; 11 NDM+. Susceptibility: VIM 93.3%; IMP 80.0%; NDM 45.5%. | ||||||
| Kazmierczak et al., 2019 [45] | In vitro study | 30 | 26 VIM+ (MIC range 0.008–2; MIC50 0.25; MIC90 2); 4 IMP+ (MIC range 1–2). | ||||||
| Jacobs et al., 2019 [63] | In vitro study | 27 | VIM+ (number of isolates not reported); MIC range 0.03–1; MIC50 0.25; MIC90 0.5 | ||||||
| Meropenem + Fosfomycin + inhaled colistin | |||||||||
| Albiero et al., 2019 [64] | In vitro study | 19 | 10 MBL+. Synergism was found in 100% of isolates with a FICI ≤ 0.5. Median reduction in MIC50 and MIC90 by 8-fold. PK/PD simulation showed that 6–8 g q8h FOS achieved the probability of target attainment of ≥90% at an MIC of 32 mg/L. 1.5 g q6h MER in 3 h EI achieved the probability of target attainment of ≥90% at an MIC of 16 mg/L. Combination therapy significantly increase the cumulative fraction rate against MBL-PA compared to monotherapy with MER (32% vs. 68%) or FOS (0% vs. 74%). | ||||||
| Inhaled colistin | |||||||||
| Vardakas et al., 2018 [65] | Systematic review and meta-analysis | 12 studies including 373 patients (8 VAP–2 HAP–2 VAT) | MDR-PA and MDR-AB mainly investigated. Pooled all-cause mortality: 33.8%; clinical success 70.4%; eradication of Gram-negative pathogens 71.3% of cases. | ||||||
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Rate of IVACs | Isolates | Severity | Clinical Outcomes | Relapse Rate—Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Carbapenems (Meropenem–Imipenem) | |||||||||
| Garnacho -Montero et al., 2003 [69] | Prospective observational | 35 (21 colistin vs. 14 imipenem) | Colistin 2.5–5 mg/kg/day in three doses vs. Imipenem 2–3 g/day in three/four doses | 100% VAP | 21 carbapenem -resistant AB 14 carbapenem- susceptible AB | ICU admission 100% Septic shock 57.1% APACHE II score: 20.5 ± 7 SOFA score: 11.7 ± 6.6 | Clinical cure rate: 57% vs. 57% (p = NS) Mortality rate: 61.9% vs. 64.2% (p = NS) VAP-related mortality rate: 38.0% vs. 35.7% (p=NS) | NA | No difference in efficacy and safety between carbapenem and intravenous colistin in the management of VAP caused by MDR-AB |
| Kallel et al., 2007 [70] | Retrospective matched case-control | 120 (60 colistin vs. 60 imipenem) | Colistin 2 MU q8h vs. Imipenem 500 mg q6h | 100% VAP | 61.7% carbapenem- susceptible AB 38.3% carbapenem- susceptible P. aeruginosa (in patients receiving imipenem) | ICU admission 100% SAPS-II 33.2 ± 10.8 Septic shock 23.3% | Clinical cure rate: 75% vs. 71.7% (p = 0.68) Mortality rate: 41.7% vs. 35% (p = 0.45) | Relapse: 8.3% Resistance development: 0.0% | No difference in efficacy and safety between carbapenem and intravenous colistin in the management of VAP caused by MDR-AB |
| Wang, 2009 [71] | Retrospective, observational monocentric | 30 | MER 1 g q8h 1 h infusion vs. MER 500 mg q6h 3 h infusion | 100% HAP | 100% MDR cabapenem- susceptible AB | ICU admission 100% Mechanical ventilation 100% | Clinical cure rate at day 7: 100.0% vs. 100.0% (p = NS) | Relapse rate: 3.3% Resistance development: 0.0% | EI treatment with MER is a cost-effective approach for the management of HAP due to MDR-AB, being equally clinically effective to II |
| Ikonomidis et al., 2006 [72] | In vitro study | 320 | 40.6% resistance to meropenem (MIC50 4 mg/L; MIC90 8 mg/L); 67.8% resistance to imipenem (MIC50 8 mg/L MIC90 64 mg/L) | ||||||
| Mezzatesta et al., 2008 [73] | In vitro study | 107 | 88.8% MDR-AB. 59% resistance to meropenem (MIC90 64 mg/L); 50% resistance to imipenem (MIC90 32 mg/L) | ||||||
| Guzek et al., 2013 [74] | In vitro study | 54 | 22.2% resistance to doripenem; 22.2% resistance to imipenem; 42.6% resistance to meropenem | ||||||
| Jones et al., 2005 [75] | In vitro study | 33 | 100% wild-type Acinetobacter spp isolates. 75.8% susceptibility to meropenem (MIC90 > 8 mg/L); 75.8% susceptibility to imipenem (MIC90 > 8 mg/L); 75.8% susceptibility to doripenem (MIC90 16 mg/L) | ||||||
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Rate of IVACs | Isolates | Severity | Clinical Outcomes | Relapse Rate—Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Cefiderocol | |||||||||
| Bassetti et al., 2020 [41] | Phase 3, randomized, prospective, multicentre, open-label (CREDIBLE-CR) | 150 (101 cefiderocol vs. 49 BAT) | Cefiderocol 2 g q8h (3h-infusion) 100% target therapy Dose adjustment according to renal function | 44.6% HAP/VAP | 65% A. baumannii | ICU admission 56% Septic shock 19% Mechanical ventilation 50% Immunocompromised 27% Mean SOFA score 5.1 | Mortality rate in AB subgroup: 49% vs. 18% (p = 0.04) Clinical cure at the end of treatment (HAP/VAP subgroup) :60% vs. 63% | NA | A significant higher mortality rate in patients affected by AB infections was found with cefiderocol compared to BAT |
| Gatti et al., 2021 [59] | Case series | 13 | Cefiderocol 1.5–2 g q8h | 84.6% | 100% XDR-AB | 100% ICU admission 100% mechanical ventilation | 30-day mortality rate: 30.8% Microbiological failure: 54% | NA | Microbiological failure occurred in 80% of patients with suboptimal fCmin/MIC compared to 29% of those achieving optimal or quasi-optimal fCmin/MIC ratio. |
| Bavaro et al., 2021 [76] | Case series | 13 | Cefiderocol 2 g q8h (3h-infusion) Dose adjustment according to renal function | 7.7% | 76.9% Carbapenem-resistant-AB 15.4% XDR-PA 7.7% KPC | 38.5% ICU admission | 30-day mortality rate: 23.1% Microbiological eradication: 100.0% | NA | Combination therapy with fosfomycin was successfully implemented in 9 cases, including VAP due to carbapenem-resistant AB |
| Falcone et al., 2020 [77] | Case series | 10 | Cefiderocol 1.5–2 g q6-8h | 2 VAP | 2 XDR Acinetobacter baumannii | ICU admission 100% Mean SOFA score 10 CRRT 50% | Clinical failure rate in AB VAP: 50% 30-day mortality rate in AB VAP: 50% | No relapse in AB VAP | Cefiderocol suggests that it may be useful to treat unresponsive ICU-acquired infections due to MDR AB |
| Trecarichi et al., 2019 [78] | Case report | 1 | Cefiderocol 2 g q8h (3h-infusion) target therapy | VAP/BSI | XDR–Acinetobacter baumannii | ICU admission Mechanical ventilation Septic shock | Clinical cure | NA | |
| Hackel et al., 2017 [79] | In vitro study | 173 MER-non susceptible (US) 595 MER-non susceptible (EU) | MIC range 0.002–8; MIC50 0.25; MIC90 1 MIC range 0.004-64; MIC50 0.12; MIC90 1 | ||||||
| Mushtaq et al., 2020 [44] | In vitro study | 99 | 41 OXA-23; 20 NDM; 19 OXA-51; 10 OXA-58; 9 OXA-24/40. Susceptibility: 94.7% OXA-51; 90% OXA-58; 88.9% OXA-24/40; 85.4% OXA-23; 50% NDM | ||||||
| Kazmierczak et al., 2019 [45] | In vitro study | 768 | 543 OXA-23; 124 OXA-24; 86 carbapenemase-negative/MER non-susceptible; 14 OXA-58; 7 GES; 2 NDM. MIC range 0.002-64; MIC50 0.12; MIC90 1 | ||||||
| Jacobs et al., 2019 [63] | In vitro study | 101 | Carbapenem non-susceptible isolates: MIC range 0.03–>64; MIC50 0.25; MIC90 1 (96.0% susceptibility) | ||||||
| Fosfomycin + Ampicillin/Sulbactam | |||||||||
| Betrosian et al., 2007 [80] | RCT | 27 | AMS 6 g/3 g q8h vs. AMS 8 g/4 g q8h | 100% VAP | MDR Acinetobacter baumannii | ICU admission 100% Mechanical ventilation 100% Mean APACHE II score 15 | Clinical cure rate: 64.3% vs. 69.2% (p = 0.79) 30-day mortality rate: 42.9% vs. 53.8% (p = NS) | NA | The use of high-dose AMS regimens is effective for the treatment of VAP caused by MDR-AB. |
| Betrosian et al., 2008 [81] | Prospective observational | 28 (15 COL vs. 13 AMS) | AMS 6 g/3 g q8h vs. COL 3 MU q8h | 100% VAP | MDR Acinetobacter baumannii | ICU admission 100% Mechanical ventilation 100% Mean APACHE II score 14 | Clinical cure rate: 61.5% vs. 60% (p = NS) 28-day mortality rate: 30% vs. 33% (p = NS) | NA | COL and high-dose AMS were comparably safe and effective treatments for critically ill patients with MDR A. baumannii VAP. |
| Mellon et al., 2012 [82] | Case report | 1 | AMS 3 g/1.5 g q4h + FOS 4 g q6h | Meningitis | MDR Acinetobacter baumannii MIC AMS 32 mg/L | ICU admission | Clinical cure | NA | The only case reporting the clinical efficacy of combination therapy between fosfomycin and high-dose sulbactam for the management of deep-seated AB infection. |
| Mohd Sazlly Lim et al., 2021 [83] | In vitro study | 50 | Fosfomycin in combination with sulbactam showed synergism in 74% of AB isolates, resulting in a median MIC50 and MIC90 reduction respectively of 4–8-fold. | ||||||
| Inhaled colistin | |||||||||
| Vardakas et al., 2018 [65] | Systematic review and meta-analysis | 12 studies including 373 patients (8 VAP—2 HAP—2 VAT) | MDR-PA and MDR-AB mainly investigated. Pooled all-cause mortality: 33.8%; clinical success 70.4%; eradication of Gram-negative pathogens 71.3% of cases. | ||||||
| Kuo et al., 2012 [84] | Retrospective, case-control | 78 (39 inhaled colistin + other antibiotics with activity against AB vs. 39 other antibiotics with activity against AB) | Inhaled COL | 41% HAP/VAP 59% respiratory colonization | 100% MDR-AB | ICU admission 71.8% Mechanical ventilation 38.5% RRT 7.7% APACHE II score 20.0 ± 6.2 | Microbiological eradication at 14-day: 84.6% vs. 10.3% (p < 0.001) 28-day mortality rate: 12.8% vs. 10.3% (p = 0.72) | Relapse rate: 21.2% COL MIC increase: 28.6% | The use of inhaled COL was the only independent factor associated with the eradication of MDR-AB within 14 days after the index day (OR 266.33; 95% CI 11.26–6302.18, p < 0.001), and shortened the duration of MDR-AB recovery from the respiratory tract by 13.3 ± 1.45 days. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas aeruginosa and Acinetobacter baumannii in Critically Ill Adult Patients. Antibiotics 2022, 11, 33. https://doi.org/10.3390/antibiotics11010033
Gatti M, Viaggi B, Rossolini GM, Pea F, Viale P. An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas aeruginosa and Acinetobacter baumannii in Critically Ill Adult Patients. Antibiotics. 2022; 11(1):33. https://doi.org/10.3390/antibiotics11010033
Chicago/Turabian StyleGatti, Milo, Bruno Viaggi, Gian Maria Rossolini, Federico Pea, and Pierluigi Viale. 2022. "An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas aeruginosa and Acinetobacter baumannii in Critically Ill Adult Patients" Antibiotics 11, no. 1: 33. https://doi.org/10.3390/antibiotics11010033
APA StyleGatti, M., Viaggi, B., Rossolini, G. M., Pea, F., & Viale, P. (2022). An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas aeruginosa and Acinetobacter baumannii in Critically Ill Adult Patients. Antibiotics, 11(1), 33. https://doi.org/10.3390/antibiotics11010033








