Quantification of Fosfomycin in Combination with Nine Antibiotics in Human Plasma and Cation-Adjusted Mueller-Hinton II Broth via LCMS
Abstract
:1. Introduction
2. Results
2.1. Assay Validation
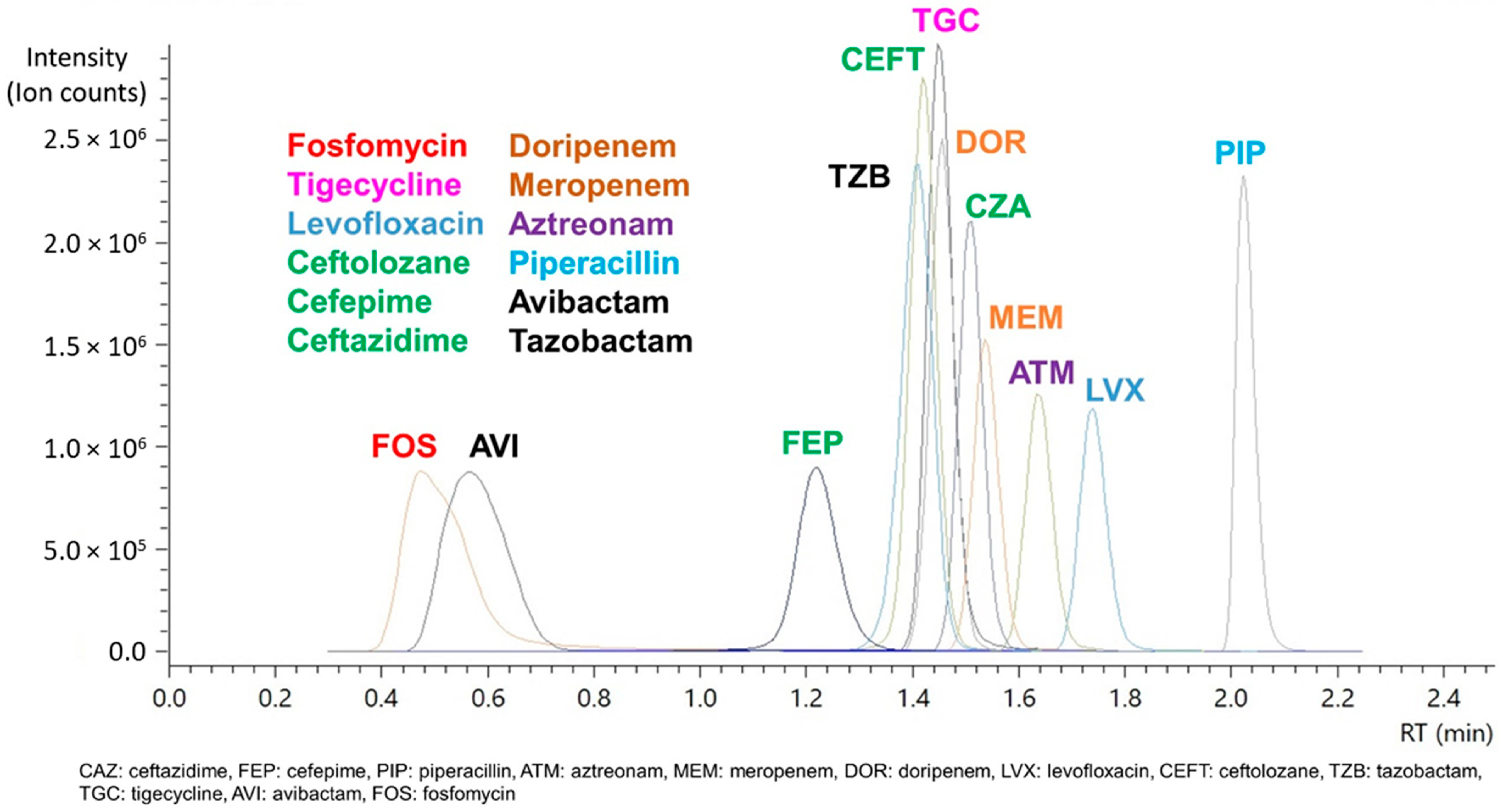
2.1.1. Specificity, Selectivity, and Carryover
2.1.2. Calibration Curve, Accuracy, Precision LLOQ, and Limit of Detection (LOD)
2.1.3. Matrix Factor (MF) and Internal Standard-Normalized Matrix Factor (IS-nMF)
2.2. Stability Studies of Fosfomycin with Other Antibiotics
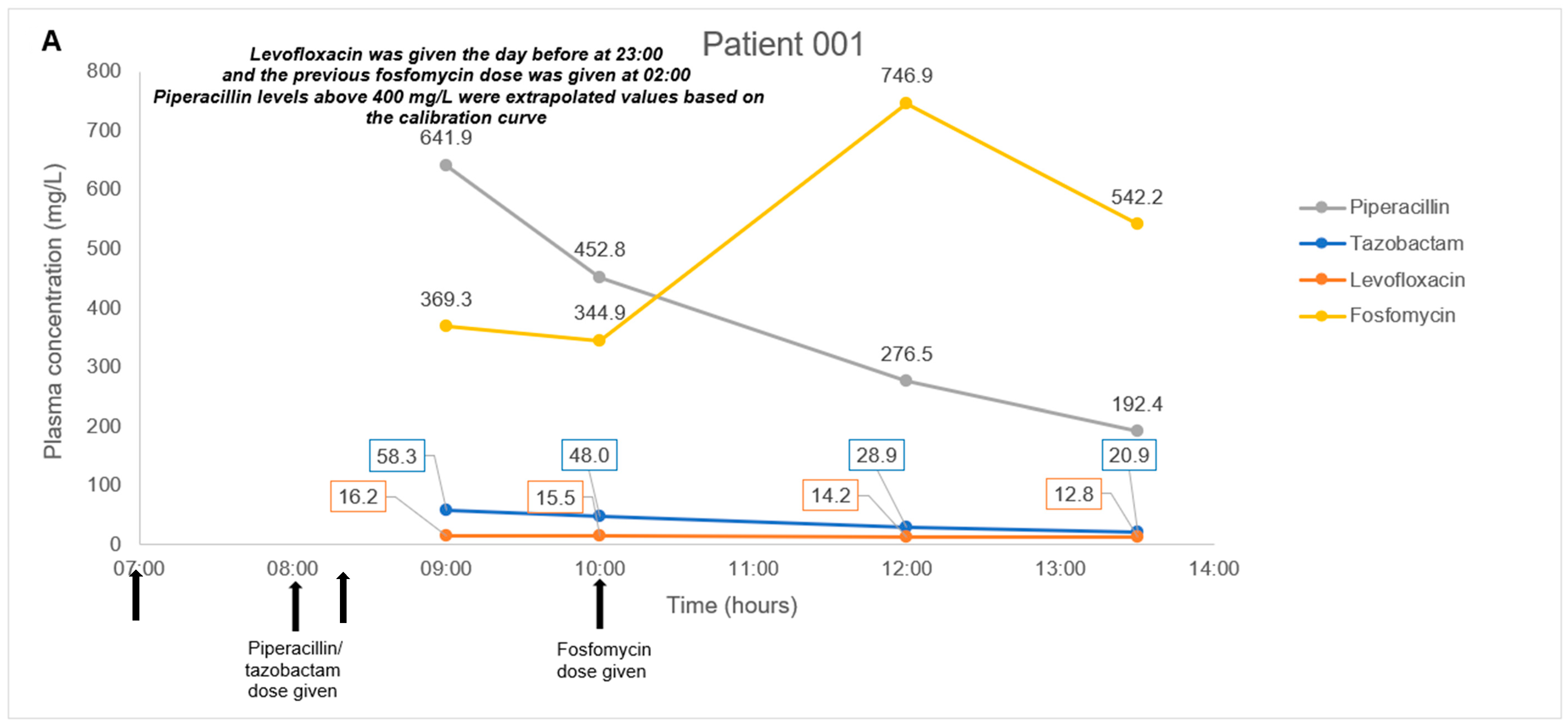
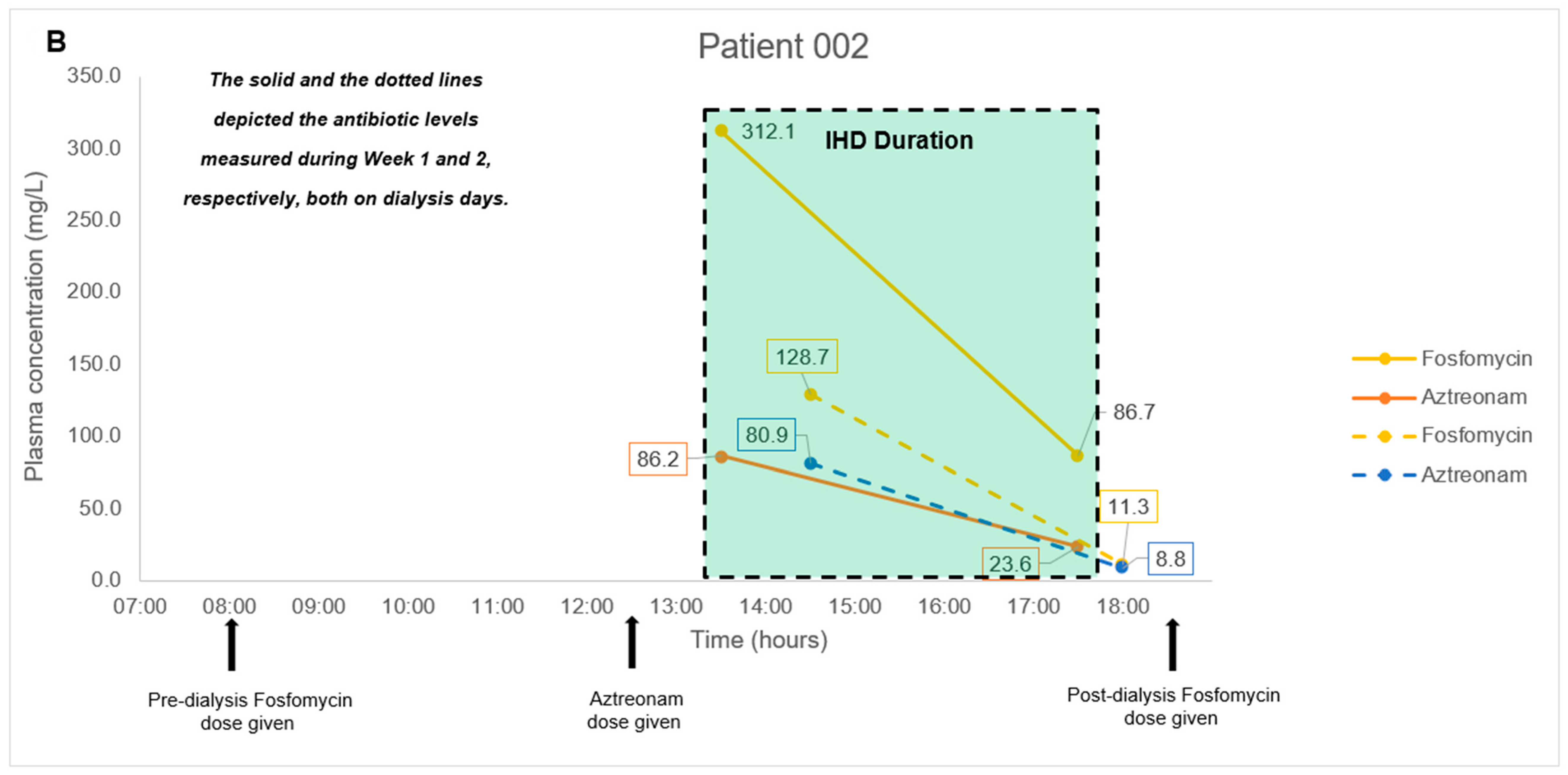
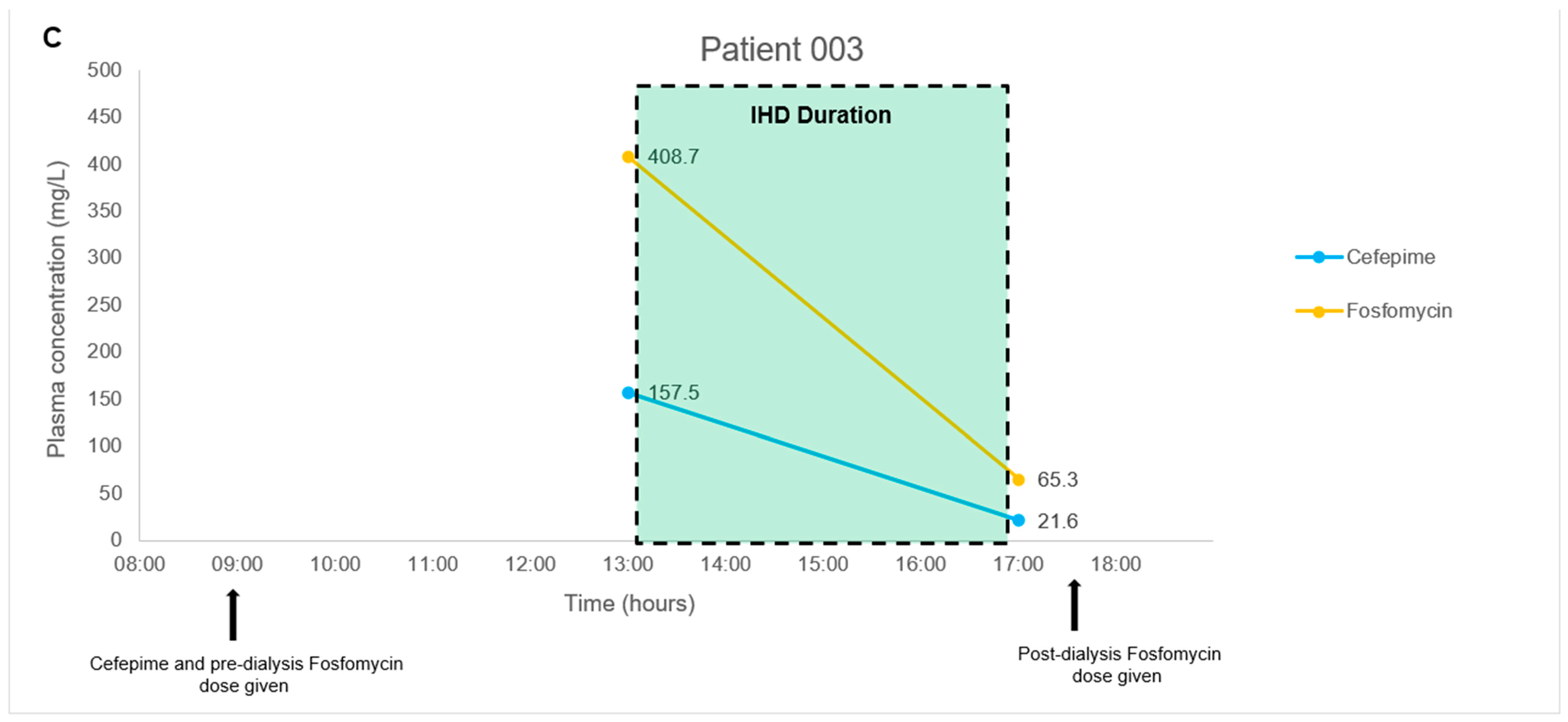
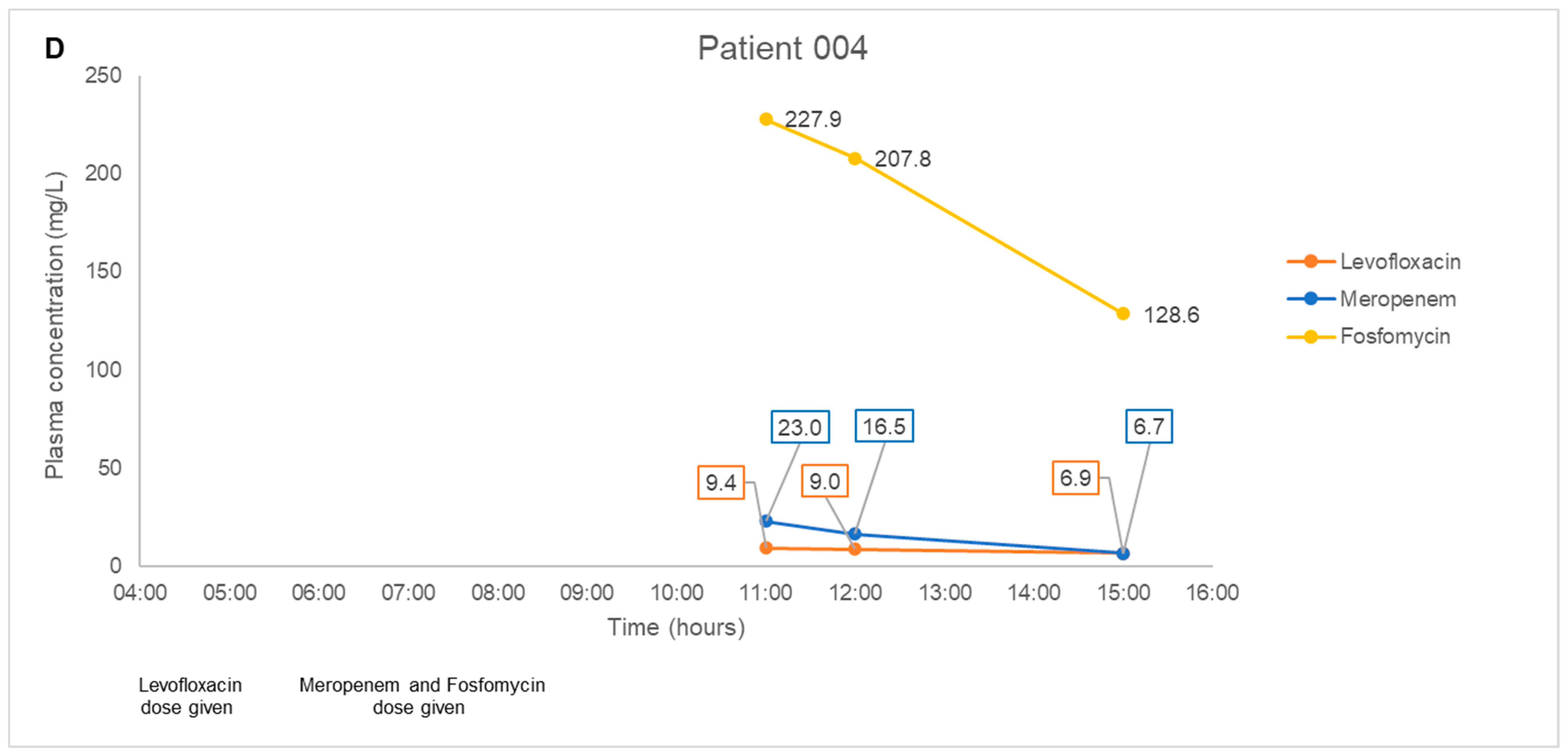
2.3. Application to a Pilot Clinical Feasibility Study and a Case-Series
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation for LCMS/MS Analysis
4.3. LCMS/MS Conditions and Analysis
4.4. Specificity, Selectivity, and Carryover
4.5. Accuracy, Precision, Lower Limit-of-Quantification (LLOQ), and Limit-of-Detection (LOD)
4.6. Matrix Factor (MF) and INTERNAL Standard-Normalized MF (IS-nMF)
4.7. Stability Studies of Fosfomycin with Other Antibiotics
4.8. Application to a Pilot Clinical Feasibility Study and a Case-Series
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial Consumption and Resistance in Adult Hospital Inpatients in 53 Countries: Results of an Internet-Based Global Point Prevalence Survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [Green Version]
- Vardakas, K.Z.; Legakis, N.J.; Triarides, N.; Falagas, M.E. Susceptibility of Contemporary Isolates to Fosfomycin: A Systematic Review of the Literature. Int. J. Antimicrob. Agents 2016, 47, 269–285. [Google Scholar] [CrossRef]
- Falagas, M.E.; Giannopoulou, K.P.; Kokolakis, G.N.; Rafailidis, P.I. Fosfomycin: Use Beyond Urinary Tract and Gastrointestinal Infections. Clin. Infect. Dis. 2008, 46, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.C.; Landersdorfer, C.B.; McIntosh, M.P.; Peleg, A.Y.; Hirsch, E.B.; Kirkpatrick, C.M.; Bergen, P.J. Clinically Relevant Concentrations of Fosfomycin Combined with Polymyxin B, Tobramycin or Ciprofloxacin Enhance Bacterial Killing of Pseudomonas Aeruginosa, but Do Not Suppress the Emergence of Fosfomycin Resistance. J. Antimicrob. Chemother. 2016, 71, 2218–2229. [Google Scholar] [CrossRef] [Green Version]
- VanScoy, B.; McCauley, J.; Bhavnani, S.M.; Ellis-Grosse, E.J.; Ambrose, P.G. Relationship between Fosfomycin Exposure and Amplification of Escherichia Coli Subpopulations with Reduced Susceptibility in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2016, 60, 5141–5145. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-S.; Williamson, P.R.; Zheng, W. Improving Therapy of Severe Infections through Drug Repurposing of Synergistic Combinations. Curr. Opin. Pharmacol. 2019, 48, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Decosterd, L.A.; Widmer, N.; André, P.; Aouri, M.; Buclin, T. The Emerging Role of Multiplex Tandem Mass Spectrometry Analysis for Therapeutic Drug Monitoring and Personalized Medicine. TrAC Trends Anal. Chem. 2016, 84, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. A Validated Method for the Quantification of Fosfomycin in Human Plasma by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2015, 990, 164–168. [Google Scholar] [CrossRef]
- Shopova, T.; Hüppe, T.; Wolf, B.; Sessler, D.I.; Volk, T.; Groesdonk, H.V.; Kreuer, S.; Maurer, F. Quantitative Determination of Fosfomycin in 10 ΜL of Plasma and Dialysate by Hydrophilic Interaction Liquid Chromatography Electrospray Ionization Mass Spectrometry. J. Chromatogr. Sci. 2021, 59, 165–174. [Google Scholar] [CrossRef]
- Wijma, R.A.; Bahmany, S.; Wilms, E.B.; van Gelder, T.; Mouton, J.W.; Koch, B.C.P. A Fast and Sensitive LC–MS/MS Method for the Quantification of Fosfomycin in Human Urine and Plasma Using One Sample Preparation Method and HILIC Chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 263–269. [Google Scholar] [CrossRef]
- Parker, S.L.; Lipman, J.; Dimopoulos, G.; Roberts, J.A.; Wallis, S.C. A Validated Method for the Quantification of Fosfomycin on Dried Plasma Spots by HPLC–MS/MS: Application to a Pilot Pharmacokinetic Study in Humans. J. Pharm. Biomed. Anal. 2015, 115, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, A.; Matta, M.; Garimella, N.; Zere, T.; Weaver, J. Development and Validation of a LC-MS/MS Method for Quantitation of Fosfomycin—Application to In Vitro Antimicrobial Resistance Study Using Hollow-Fiber Infection Model. Biomed. Chromatogr. 2018, 32, e4212. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; Lipman, J.; Roberts, J.A.; Wallis, S.C. A Simple LC–MS/MS Method Using HILIC Chromatography for the Determination of Fosfomycin in Plasma and Urine: Application to a Pilot Pharmacokinetic Study in Humans. J. Pharm. Biomed. Anal. 2015, 105, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Najjar, N.; Jantsch, J.; Gessner, A. A Rapid Liquid Chromatography-Tandem Mass Spectrometry for the Quantification of Fosfomycin in Plasma, Urine, and Aqueous Fluids. J. Chromatogr. B 2017, 1061–1062, 57–64. [Google Scholar] [CrossRef]
- Magréault, S.; Leroux, S.; Touati, J.; Storme, T.; Jacqz-Aigrain, E. UPLC/MS/MS Assay for the Simultaneous Determination of Seven Antibiotics in Human Serum–Application to Pediatric Studies. J. Pharm. Biomed. Anal. 2019, 174, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Mesini, A.; Barbagallo, L.; Maffia, A.; Tripodi, G.; Pea, F.; Saffioti, C.; Castagnola, E.; Cangemi, G. A Liquid Chromatography-Tandem Mass Spectrometry Platform for the Routine Therapeutic Drug Monitoring of 14 Antibiotics: Application to Critically Ill Pediatric Patients. J. Pharm. Biomed. Anal. 2020, 186, 113273. [Google Scholar] [CrossRef] [PubMed]
- Decosterd, L.A.; Mercier, T.; Ternon, B.; Cruchon, S.; Guignard, N.; Lahrichi, S.; Pesse, B.; Rochat, B.; Burger, R.; Lamoth, F.; et al. Validation and Clinical Application of a Multiplex High Performance Liquid Chromatography – Tandem Mass Spectrometry Assay for the Monitoring of Plasma Concentrations of 12 Antibiotics in Patients with Severe Bacterial Infections. J. Chromatogr. B 2020, 1157, 122160. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chua, N.G.; Lim, T.-P.; Teo, J.Q.-M.; Lee, W.; Kurup, A.; Koh, T.-H.; Tan, T.-T.; Kwa, A.L. From Bench-Top to Bedside: A Prospective In Vitro Antibiotic Combination Testing (IACT) Service to Guide the Selection of Rationally Optimized Antimicrobial Combinations against Extensively Drug Resistant (XDR) Gram Negative Bacteria (GNB). PLoS ONE 2016, 11, e0158740. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation; FDA: Silver Spring, MD, USA, 2018.
- Beta-Lactam Therapeutic Drug Monitoring in Singapore (BLAST-2). Available online: https://clinicaltrials.gov/ct2/show/NCT04450680 (accessed on 8 March 2021).
- European Medicines Agency. ICH Guideline M10 on Bioanalytical Method Validation Step 2b; European Medicines Agency: London, UK, 2019. [Google Scholar]
- Rigo-Bonnin, R.; Ribera, A.; Arbiol-Roca, A.; Cobo-Sacristán, S.; Padullés, A.; Murillo, Ò.; Shaw, E.; Granada, R.; Pérez-Fernández, X.L.; Tubau, F.; et al. Development and Validation of a Measurement Procedure Based on Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry for Simultaneous Measurement of β-Lactam Antibiotic Concentration in Human Plasma. Clin. Chim. Acta 2017, 468, 215–224. [Google Scholar] [CrossRef]
- Bruck, F.; Roberts, M.S.; Roberts, J.A.; Robertson, T.A. Simultaneous Determination of Seven-Lactam Antibiotics in Human Plasma for Therapeutic Drug Monitoring and Pharmacokinetic Studies. J. Chromatogr. B 2014, 960, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, S.; van Hateren, K.; Vrubleuskaya, N.; Koster, R.; Touw, D.; Alffenaar, J.-W.C. Determination of Levofloxacin in Human Serum Using Liquid Chromatography Tandem Mass Spectrometry. J. Appl. Bioanal. 2018, 4, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Naser, F.J.; Mahieu, N.G.; Wang, L.; Spalding, J.L.; Johnson, S.L.; Patti, G.J. Two Complementary Reversed-Phase Separations for Comprehensive Coverage of the Semipolar and Nonpolar Metabolome. Anal. Bioanal. Chem. 2018, 410, 1287–1297. [Google Scholar] [CrossRef]
- Forier, K.; Van Heck, V.; Carlier, M.; Van Braeckel, E.; Van Daele, S.; De Baets, F.; Schelstraete, P.; Haerynck, F.; Stove, V.; Van Simaey, L.; et al. Development and Validation of an LC Tandem MS Assay for the Quantification of β-Lactam Antibiotics in the Sputum of Cystic Fibrosis Patients. J. Antimicrob. Chemother. 2018, 73, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Avery, L.M.; Sutherland, C.A.; Nicolau, D.P. In Vitro Investigation of Synergy among Fosfomycin and Parenteral Antimicrobials against Carbapenemase-Producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2019, 95, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Asuphon, O.; Montakantikul, P.; Houngsaitong, J.; Kiratisin, P.; Sonthisombat, P. Optimizing Intravenous Fosfomycin Dosing in Combination with Carbapenems for Treatment of Pseudomonas Aeruginosa Infections in Critically Ill Patients Based on Pharmacokinetic/Pharmacodynamic (PK/PD) Simulation. Int. J. Infect. Dis. 2016, 50, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuba, G.T.; Rocha-Santos, G.; Cayô, R.; Streling, A.P.; Nodari, C.S.; Gales, A.C.; Pignatari, A.C.C.; Nicolau, D.P.; Kiffer, C.R. V In Vitro Synergy of Ceftolozane/Tazobactam in Combination with Fosfomycin or Aztreonam against MDR Pseudomonas Aeruginosa. J. Antimicrob. Chemother. 2020, 75, 1874–1878. [Google Scholar] [CrossRef]
- Perdigão Neto, L.V.; Oliveira, M.S.; Martins, R.C.R.; Marchi, A.P.; Gaudereto, J.J.; Da Costa, L.A.T.J.; De Lima, L.F.A.; Takeda, C.F.V.; Costa, S.F.; Levin, A.S. Fosfomycin in Severe Infections Due to Genetically Distinct Pan-Drug-Resistant Gram-Negative Microorganisms: Synergy with Meropenem. J. Antimicrob. Chemother. 2019, 74, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhail, S.; Singh, N.B.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Castanheira, M.; Rybak, M.J. Evaluation of the Synergy of Ceftazidime-Avibactam in Klebsiella Pneumoniae and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e00779-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, D.E.; et al. Ceftazidime-Avibactam in Combination with Fosfomycin: A Novel Therapeutic Strategy against Multidrug-Resistant Pseudomonas Aeruginosa. J. Infect. Dis. 2020, 221, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, J.J.; Bode-Böger, S.M.; Wilhelmi, M.; Omar, M.; Martens-Lobenhoffer, J.; Welte, T.; Kielstein, J.T. Pharmacokinetics and Total Removal of Fosfomycin in Two Patients Undergoing Intermittent Haemodialysis and Extended Dialysis: Prescription Needs to Avoid under-Dosing. J. Antimicrob. Chemother. 2016, 71, 2673–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Compound | CAZ | FEP | PIP | ATM | MEM | DOR | LVX | CEFT | TZB | TGC | AVI | FOF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | ||||||||||||
| 1 (LLOQ) | 0.6 | 0.3 | 0.6 | 0.6 | 0.6 | 0.3 | 0.06 | 0.6 | 0.15 | 0.15 | 0.15 | 3 |
| 2 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 0.1 | 1 | 0.25 | 0.25 | 0.25 | 5 |
| 3 | 10 | 5 | 10 | 10 | 10 | 5 | 1 | 10 | 2.5 | 2.5 | 2.5 | 50 |
| 4 | 50 | 25 | 50 | 50 | 50 | 25 | 5 | 50 | 12.5 | 12.5 | 12.5 | 250 |
| 5 | 100 | 50 | 100 | 100 | 100 | 50 | 10 | 100 | 25 | 25 | 25 | 500 |
| 6 | 200 | 100 | 200 | 200 | 200 | 100 | 20 | 200 | 50 | 50 | 50 | 1000 |
| 7 | 300 | 150 | 300 | 300 | 300 | 150 | 30 | 300 | 75 | 75 | 75 | 1500 |
| 8 (ULOQ) | 400 | 200 | 400 | 400 | 400 | 200 | 40 | 400 | 100 | 100 | 100 | 2000 |
| LQC | 5 | 2.5 | 5 | 5 | 5 | 2.5 | 0.5 | 5 | 1.25 | 1.25 | 1.25 | 25 |
| MQC | 150 | 75 | 150 | 150 | 150 | 75 | 15 | 150 | 37.5 | 37.5 | 37.5 | 750 |
| HQC | 350 | 175 | 350 | 350 | 350 | 175 | 35 | 350 | 87.5 | 87.5 | 87.5 | 1750 |
| Compound | CAZ | FEP | PIP | ATM | MEM | DOR | LVX | CEFT | TZB | TGC | AVI | FOF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | ||||||||||||
| PLASMA | ||||||||||||
| Within-run | ||||||||||||
| LLOQ | 94.5 (6.1) | 93.4 (4.8) | 108.7 (2.3) | 104.2 (3.7) | 108.4 (10.1) | 105.4 (5.4) | 114.8 (2.2) | 105.1 (12.4) | 106.2 (4.9) | 116.5 (4.2) | 99.6 (2.0) | 106.2 (3.9) |
| ULOQ | 96.8 (2.9) | 98.8 (2.6) | 102.6 (0.5) | 101.1 (4.3) | 101.3 (4.1) | 104.4 (2.5) | 104.0 (1.0) | 103.0 (2.9) | 102.7 (1.5) | 102.4 (2.9) | 101.2 (0.5) | 102.3 (0.8) |
| LQC | 96.2 (3.8) | 101.2 (2.7) | 101.0 (3.2) | 100.7 (5.0) | 96.5 (4.4) | 98.4 (2.8) | 88.9 (0.5) | 94.4 (6.4) | 94.6 (4.4) | 84.0 a (4.8) | 96.8 (1.0) | 95.2 (2.3) |
| MQC | 101.1 (3.6) | 103.0 (3.3) | 97.9 (0.7) | 100.6 (2.6) | 103.5 (4.4) | 97.5 (3.8) | 97.3 (0.3) | 98.1 (4.1) | 100.9 (2.7) | 96.0 (2.5) | 99.7 (0.5) | 98.7 (0.9) |
| HQC | 99.0 (3.9) | 97.7 (0.6) | 103.8 (0.7) | 101.5 (3.0) | 108.7 (3.6) | 100.9 (2.3) | 102.6 (0.8) | 98.0 (2.4) | 104.2 (2.3) | 108.0 (2.4) | 100.5 (0.5) | 102.3 (1.1) |
| r2 value | 0.9988453 | 0.9995205 | 0.9991706 | 0.9998476 | 0.9995018 | 0.998757 | 0.9986312 | 0.9994118 | 0.9994067 | 0.9985256 | 0.9998819 | 0.9996225 |
| gradient | y = 0.79169x | y = 11.0991x | y = 0.21593x | y = 0.398052x | y = 0.45178x | y = 1.29971x | y = 0.22326x | y = 2.03945x | y = 1.04784x | y = 1.93816x | y = 1.17966x | y = 0.82013x |
| y-intercept | −0.000575 | 0.021203 | −0.001160 | −0.001583 | −0.002272 | −0.005883 | −0.001841 | −0.000458 | −0.004811 | −0.037635 | 0.000110 | −0.003660 |
| Between-run | ||||||||||||
| LLOQ | 94.1 (6.0) | 89.2 (4.5) | 113.9 (2.9) | 105.0 (9.2) | 100.8 (8.2) | 102.0 (5.4) | 115.4 (2.1) | 98.3 (7.0) | 106.7 (8.4) | 111.4 (3.1) | 100.4 (2.1) | 109.6 (2.1) |
| ULOQ | 94.4 (2.6) | 97.6 (5.7) | 103.2 (1.7) | 98.9 (1.4) | 98.3 (1.9) | 98.8 (1.2) | 102.3 (1.2) | 100.6 (3.1) | 101.8 (1.0) | 94.7 (3.3) | 101.2 (0.6) | 101.9 (1.1) |
| LQC | 97.6 (3.7) | 101.1 (4.4) | 92.7 (2.4) | 98.0 (3.4) | 98.9 (4.1) | 99.5 (2.9) | 87.2 (1.6) | 93.8 (4.0) | 100.8 (2.5) | 85.3 (2.7) | 99.7 (1.1) | 95.2 (2.7) |
| MQC | 104.8 (2.9) | 103.7 (4.7) | 96.3 (1.6) | 101.0 (2.7) | 102.8 (6.3) | 101.1 (2.8) | 99.6 (0.6) | 97.4 (3.2) | 102.0 (1.4) | 102.2 (3.0) | 98.8 (0.8) | 98.8 (1.7) |
| HQC | 98.2 (3.4) | 98.5 (5.3) | 104.4 (1.7) | 103.4 (1.9) | 99.9 (2.7) | 101.6 (3.4) | 106.8 (0.9) | 98.9 (3.2) | 104.8 (1.0) | 99.8 (2.9) | 101.5 (0.6) | 102.7 (1.5) |
| r2 value | 0.9993882 | 0.9987315 | 0.9987859 | 0.9993404 | 0.9994486 | 0.9990514 | 0.9987863 | 0.9992368 | 0.9994406 | 0.9985435 | 0.9997051 | 0.9989627 |
| gradient | y = 0.58647x | y = 9.58180x | y = 0.17279x | y = 0.38990x | y= 0.46060x | y = 1.17540x | y = 0.22065x | y = 1.64273x | y = 0.88387x | y = 1.51796x | y = 1.18068x | y = 1.14038x |
| y-intercept | 0.001606 | 0.029099 | −0.000765 | −0.001317 | −0.001073 | −0.002664 | −0.001884 | −0.004770 | −0.001693 | −0.035293 | −0.000473 | −0.008392 |
| Compound | CAZ | FEP | PIP | ATM | MEM | DOR | LVX | CEFT | TZB | TGC | AVI | FOF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | ||||||||||||
| CAMHB | ||||||||||||
| Within-run | ||||||||||||
| LLOQ | 96.2 (5.6) | 87.3 (4.0) | 113.7 (0.8) | 113.8 (5.0) | 113.9 (1.0) | 98.9 (7.1) | 112.3 (2.4) | 106.9 (8.4) | 103.0 (9.9) | 115.3 (2.1) | 103.1 (4.2) | 109.9 (2.2) |
| ULOQ | 97.1 (2.2) | 97.7 (3.7) | 101.2 (0.7) | 100.6 (3.7) | 103.2 (5.9) | 100.0 (3.5) | 101.3 (1.4) | 99.9 (1.9) | 100.4 (2.0) | 102.6 (4.1) | 100.7 (0.3) | 102.6 (1.2) |
| LQC | 95.8 (4.7) | 100.1 (4.8) | 90.7 (1.4) | 100.3 (3.0) | 96.7 (7.2) | 98.6 (6.6) | 85.0 (3.7) | 102.2 (5.8) | 100.2 (6.6) | 85.0 (3.7) | 97.1 (1.5) | 94.4 (2.3) |
| MQC | 98.5 (4.1) | 101.9 (5.3) | 97.7 (0.4) | 98.5 (2.4) | 97.0 (6.2) | 100.5 (3.4) | 98.6 (1.8) | 98.4 (4.0) | 99.4 (1.6) | 90.5 (2.7) | 99.3 (0.4) | 97.9 (0.8) |
| HQC | 96.1 (3.5) | 98.3 (6.7) | 102.6 (0.8) | 102.3 (3.8) | 103.8 (3.8) | 101.2 (4.0) | 104.3 (1.0) | 103.5 (2.5) | 100.9 (3.5) | 106.8 (2.3) | 101.2 (0.6) | 102.0 (1.6) |
| r2 value | 0.9989652 | 0.998917 | 0.9989498 | 0.9997173 | 0.9986158 | 0.9997911 | 0.9994429 | 0.9995696 | 0.9998586 | 0.9985048 | 0.9998422 | 0.9992636 |
| gradient | y = 0.86970x | y = 11.1922x | y = 0.22328x | y = 0.43934x | y = 0.48084x | y = 1.19070x | y = 0.24105x | y = 1.98693x | y = 1.11667x | y = 1.99402x | y = 1.18951x | y = 0.81954x |
| y-intercept | −0.000136 | 0.052039 | −0.001203 | −0.001630 | −0.002647 | 0.003514 | −0.001818 | −0.009050 | −0.001200 | −0.045526 | −0.000901 | −0.004389 |
| Between-run | ||||||||||||
| LLOQ | 100.6 (5.1) | 89.1 (4.0) | 105.5 (1.9) | 100.2 (9.1) | 98.2 (7.5) | 101.2 (9.9) | 112.1 (2.9) | 96.6 (6.3) | 107.7 (10.9) | 116.4 (1.3) | 101.6 (3.0) | 108.8 (2.5) |
| ULOQ | 96.8 (1.9) | 99.4 (4.9) | 100.9 (1.3) | 98.6 (2.6) | 99.0 (3.2) | 99.0 (2.3) | 100.9 (0.9) | 96.3 (3.9) | 99.8 (1.7) | 100.6 (1.5) | 100.1 (0.3) | 101.8 (1.3) |
| LQC | 97.9 (4.7) | 105.8 (5.3) | 95.3 (2.1) | 98.3 (2.4) | 96.1 (3.0) | 96.1 (3.5) | 85.5 (1.1) | 97.4 (4.6) | 98.6 (6.8) | 85.6 (5.9) | 97.9 (0.9) | 95.9 (1.9) |
| MQC | 102.6 (3.5) | 104.9 (2.8) | 96.9 (1.3) | 98.9 (1.6) | 101.8 (3.5) | 98.5 (3.9) | 98.6 (0.8) | 101.0 (2.6) | 101.5 (1.3) | 96.6 (4.1) | 99.5 (0.4) | 97.7 (1.4) |
| HQC | 99.8 (5.9) | 104.3 (3.5) | 102.2 (0.3) | 101.7 (2.3) | 101.9 (5.3) | 102.9 (2.4) | 103.0 (0.4) | 99.4 (3.4) | 100.6 (2.2) | 108.5 (4.4) | 101.6 (0.3) | 103.3 (1.0) |
| r2 value | 0.9990416 | 0.9988099 | 0.9995495 | 0.9998553 | 0.9995754 | 0.9998414 | 0.9996996 | 0.9985859 | 0.9998911 | 0.9988101 | 0.999935 | 0.9995843 |
| gradient | y = 0.74695x | y = 10.9393x | y = 0.16590x | y = 0.35829x | y = 0.46184x | y = 1.25148x | y = 0.20729x | y = 1.86809x | y = 0.79040x | y = 2.00391x | y = 1.25686x | y = 1.09679x |
| y-intercept | −0.000178 | 0.091889 | −0.000263 | −0.000033 | 0.000713 | 0.001230 | −0.001247 | 0.008453 | 0.000991 | −0.0390911 | 0.000574 | −0.000973 |
| Patient No. | Age (Years), Gender | Weight, Height | Type Of Carbapenem-Resistant Bacterial Infection | Renal Function Status | Antibiotic Combination Regimen |
|---|---|---|---|---|---|
| 001 | 68, Male | 48 kg, 168 cm | Achromobacter xylosoxidans hospital-associated pneumonia | Normal, not on dialysis | Fosfomycin 8 g every 8 h as a 2-h infusion + Piperacillin/tazobactam 4.5 g every 6 h as a 1-h infusion + Levofloxacin 750 mg every 24 h |
| 002 | 65, Female | 86.4 kg, 154 cm | Acinetobacter baumannii Tenckhoff catheter exit site infection | Impaired, on Intermittent hemodialysis | Fosfomycin 8 g on dialysis days (3 g to be administered at least 4 h before dialysis, and 5 g to be administered at the end of the dialysis session) + Aztreonam 2 g every 12 h as a 2-h infusion |
| 003 | 74, Female | 45.6 kg, 150 cm | Acinetobacter baumannii ventilator-associated pneumonia | Impaired, on Intermittent hemodialysis | Fosfomycin 8 g on dialysis days (3 g to be administered at least 4 h before dialysis, and 5 g to be administered at the end of the dialysis session) + Cefepime 1 g every 12 h as a 4-h infusion |
| 004 | 37, Male | 104 kg, 178 cm | Pseudomonas aeruginosa bilateral gluteal pressure ulcers | Normal, not on dialysis | Fosfomycin 8 g every 8 h as a 3-h infusion + Meropenem 2 g every 8 h as a 3-h infusion + Levofloxacin 500 mg every 12 h |
| Compound | FEP | PIP | ATM | MEM | LVX | TZB | FOF |
|---|---|---|---|---|---|---|---|
| Accuracy (%) | |||||||
| Patient 001 | |||||||
| LLOQ | 117.3 (2.9) | 114.4 (2.0) | 107.3 (5.4) | 101.8 (1.8) | |||
| ULOQ | 101.4 (2.4) | 98.9 (1.1) | 100.6 (2.7) | 99.9 (1.7) | |||
| LQC | 102.7 (1.0) | 99.4 (2.5) | 111.0 (5.6) | 106.9 (1.9) | |||
| MQC | 91.8 (1.8) | 99.7 (1.7) | 98.2 (3.3) | 97.8 (0.5) | |||
| HQC | 100.0 (1.8) | 103.0 (0.4) | 108.1 (2.5) | 103.2 (0.9) | |||
| r2 value | 0.9987362 | 0.9986969 | 0.9994183 | 0.9997751 | |||
| Linearity Equation | y = 0.28770x − 0.167125 | y = 0.90712x − 0.011084 | y = 0.97505x − 0.027416 | y = 1.08956x − 0.005619 | |||
| Patient 002 | |||||||
| LLOQ | 92.7 (1.57) | 104.0 (3.85) | |||||
| ULOQ | 98.3 (2.89) | 103.2 (1.46) | |||||
| LQC | 98.5 (6.18) | 98.7 (3.00) | |||||
| MQC | 105.1 (3.58) | 102.8 (0.42) | |||||
| HQC | 102.5 (4.42) | 102.8 (1.03) | |||||
| r2 value | 0.9989275 | 0.999259 | |||||
| Linearity Equation | y = 0.42009x + 0.002480 | y = 0.74937x + 0.004796 | |||||
| Patient 003 | |||||||
| LLOQ | 81.4 (1.68) | 89.1 (0.49) | |||||
| ULOQ | 99.3 (4.27) | 102.4 (1.45) | |||||
| LQC | 99.5 (7.56) | 104.1 (0.97) | |||||
| MQC | 102.7 (4.37) | 97.9 (1.76) | |||||
| HQC | 101.8 (1.81) | 101.7 (0.60) | |||||
| r2 value | 0.9988506 | 0.9985925 | |||||
| Linearity Equation | y = 8.19150x + 0.060959 | y = 0.81900x + 0.007561 | |||||
| Patient 004 | |||||||
| LLOQ | 99.4 (5.43) | 111.6 (2.90) | 96.5 (2.23) | ||||
| ULOQ | 98.3 (0.60) | 101.2 (0.84) | 99.0 (0.58) | ||||
| LQC | 100.5 (2.11) | 98.5 (1.50) | 99.8 (2.57) | ||||
| MQC | 104.6 (1.67) | 102.7 (0.69) | 100.1 (1.48) | ||||
| HQC | 95.7 (3.95) | 101.8 (0.75) | 96.45 (1.39) | ||||
| r2 value | 0.9995982 | 0.9997402 | 0.9999429 | ||||
| Linearity Equation | y = 0.587167x − 0.000061 | y = 0.297456x − 0.001252 | y = 0.821999x + 0.006490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, K.K.-K.; Toh, W.G.-H.; Hee, D.K.-H.; Ting, E.Z.-W.; Chua, N.G.S.; Zulkifli, F.I.B.; Sin, L.-J.; Tan, T.-T.; Kwa, A.L.-H.; Lim, T.-P. Quantification of Fosfomycin in Combination with Nine Antibiotics in Human Plasma and Cation-Adjusted Mueller-Hinton II Broth via LCMS. Antibiotics 2022, 11, 54. https://doi.org/10.3390/antibiotics11010054
Goh KK-K, Toh WG-H, Hee DK-H, Ting EZ-W, Chua NGS, Zulkifli FIB, Sin L-J, Tan T-T, Kwa AL-H, Lim T-P. Quantification of Fosfomycin in Combination with Nine Antibiotics in Human Plasma and Cation-Adjusted Mueller-Hinton II Broth via LCMS. Antibiotics. 2022; 11(1):54. https://doi.org/10.3390/antibiotics11010054
Chicago/Turabian StyleGoh, Kelvin Kau-Kiat, Wilson Ghim-Hon Toh, Daryl Kim-Hor Hee, Edwin Zhi-Wei Ting, Nathalie Grace Sy Chua, Farah Iffah Binte Zulkifli, Li-Jiao Sin, Thuan-Tong Tan, Andrea Lay-Hoon Kwa, and Tze-Peng Lim. 2022. "Quantification of Fosfomycin in Combination with Nine Antibiotics in Human Plasma and Cation-Adjusted Mueller-Hinton II Broth via LCMS" Antibiotics 11, no. 1: 54. https://doi.org/10.3390/antibiotics11010054






