Farm Animal Veterinarians’ Knowledge and Attitudes toward Antimicrobial Resistance and Antimicrobial Use in the Republic of Serbia
Abstract
:1. Introduction
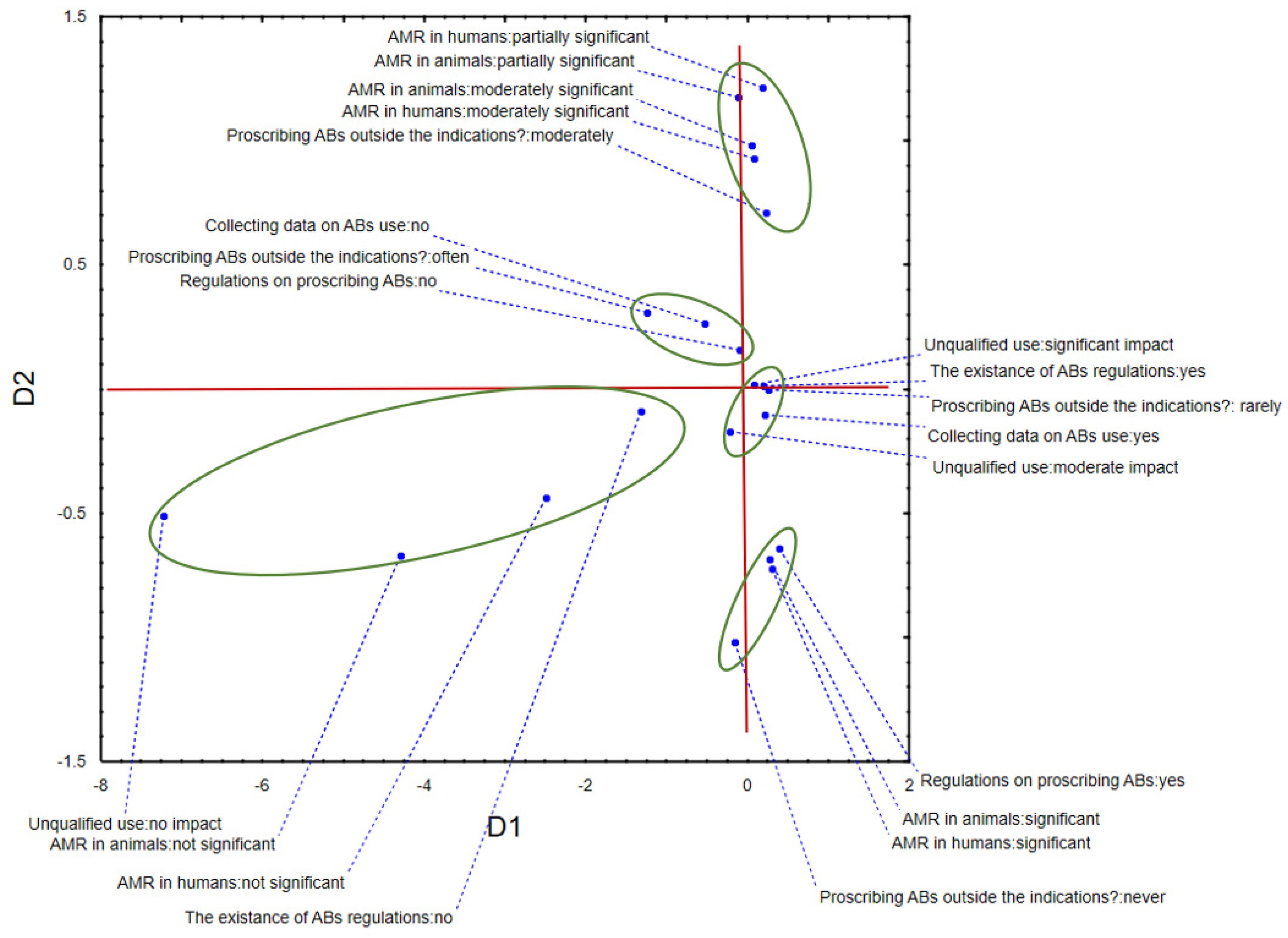
2. Results
2.1. Sociodemographic Data
2.2. Significance of Bacterial Resistance to Antibiotics
2.3. Veterinarians’ Prescribing Habits
2.4. Attitudes toward AMR
2.5. Cow Mastitis Therapy
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Ethical Approval
4.2. Study Population and Sample Size
4.3. Study Design—The Questionnaire
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collignon, P.C.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A.; tHe World Health Organization Advisory Group, Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR); Agerso, Y.; Andremont, A.; Collignon, P.; Conly, J.; et al. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin. Infect. Dis. 2016, 63, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreten, V.; Kadlec, K.; Schwarz, S.; Grönlund Andersson, U.; Finn, M.; Greko, C.; Moodley, A.; Kania, S.A.; Frank, L.A.; Bemis, D.A.; et al. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: An international multicentre study. J. Antimicrob. Chemother. 2010, 65, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, S.; Wong, H.S.; Turnidge, J.; Johnson, J.R.; Trott, D.J. Carbapenemase-Producing bacteria in companion animals: A public health concern on the horizon. J. Antimicrob. Chemother. 2014, 69, 1155–1157. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.; Christley, R.M.; McKane, S.A.; Roberts, V.L.H.; Clegg, P.D.; Williams, N.J. Antimicrobial resistance changes in enteric Escherichia coli of horses during hospitalisation: Resistance profiling of isolates. Vet. J. 2013, 195, 121–126. [Google Scholar] [CrossRef]
- Rubin, J.E.; Pitout, J.D. Extended-Spectrum β-lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet. Microbiol. 2014, 170, 10–18. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2013, 69, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, A.; McCarthy, A.; Lloyd, D.H.; Musilová, E.; Pfeiffer, D.U.; Lindsay, J.A. Whole-Genome comparison of meticillin-resistant Staphylococcus aureus CC22 SCCmecIV from people and their in-contact pets. Vet. Dermatol. 2013, 24, 538-e128. [Google Scholar] [CrossRef]
- Lloyd, D.H.; Page, S.W.; Aarestrup, F.M.; Schwarz, S.; Shen, J.; Cavaco, L. Antimicrobial stewardship in veterinary medicine. Microbiol. Spectr. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in understanding antimicrobial resistance across domesticated animal, human, and environmental systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.J.; McEwen, S.A. One health-its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Cantas, L.; Suer, K. The important bacterial zoonoses in “one health” concept. Front. Public Health 2014, 2, 144. [Google Scholar] [CrossRef] [Green Version]
- van den Bogaard, A.E.; Stobberingh, E.E. Epidemiology of resistance to antibiotics. Links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Locke, H.; Meldrum, H. Use and misuse of antimicrobials. Vet. Rec. 2011, 169, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboob, A.; Altaf, I.U.K. Misuse of antimicrobials. Pak. J. Chest Med. 2018, 24, 131–132. [Google Scholar]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 1 November 2021).
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef] [Green Version]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Borck Høg, B.; Dalhoff Andersen, V.; Chauvin, C.; et al. Monitoring of farm-level antimicrobial use to guide stewardship: Overview of existing systems and analysis of key components and processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef]
- Krömker, V.; Leimbach, S. Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 2017, 52, 21–29. [Google Scholar] [CrossRef] [Green Version]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Vet. J. 2020, 73, 2. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.P.; Staerk, K. Antimicrobial resistance and antimicrobial use animal monitoring policies in Europe: Where are we? J. Public Health Policy 2017, 38, 185–202. [Google Scholar] [CrossRef] [Green Version]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [Green Version]
- Babra, C.; Tiwari, J.G.; Pier, G.; Thein, T.H.; Sunagar, R.; Sundareshan, S.; Isloor, S.; Hegde, N.R.; de Wet, S.; Deighton, M.; et al. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiol. 2013, 58, 469–474. [Google Scholar] [CrossRef]
- Magouras, I.; Carmo, L.P.; Stärk, K.D.C.; Schüpbach-Regula, G. Antimicrobial usage and -resistance in livestock: Where should we focus? Front. Vet. Sci. 2017, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Tomas, A.; Pavlović, N.; Stilinović, N.; Horvat, O.; Paut-Kusturica, M.; Dugandžija, T.; Tomić, Z.; Sabo, A. Increase and change in the pattern of antibiotic use in Serbia (2010–2019). Antibiotics 2021, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Iwamoto, K.; Hoxha, I.; Ghazaryan, L.; Abilova, V.; Cvijanovic, A.; Pyshnik, H.; Darakhvelidze, M.; Makalkina, L.; Jakupi, A.; et al. Antimicrobial medicines consumption in eastern Europeand central Asia—An updated cross-national study and assessment of QuantitativeMetrics for policy action. Front. Pharmacol. 2019, 9, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sl. glasnik RS 30/2010. Law on Medicines and Medical Devices of Serbia. Available online: https://www.paragraf.rs/propisi/zakon_o_lekovima_i_medicinskim_sredstvima.html (accessed on 20 December 2021).
- Medicines and Medical Devices Agency of Serbia (ALIMS). PROMET VETERINARSKIH LEKOVA 2017–2018. Available online: https://www.alims.gov.rs/ciril/files/2021/12/PROMETVETERINARSKIHLEKOVA2017–2018.pdf (accessed on 20 December 2021).
- Medicines and Medical Devices Agency of Serbia (ALIMS). Marketing and Consumption of Medicinal Products. Available online: https://www.alims.gov.rs/latin/veterinarski-lekovi/promet-i-potrosnja-veterinarskih-lekova/ (accessed on 20 December 2021).
- European Union (EU). Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria and Repealing Implementing Decision 2013/652/EU (Notified under Document C(2020) 7894) (Only the English Version Is Authentic). Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32020D1729 (accessed on 20 December 2021).
- Kovacevic, Z.; Blagojevic, B.; Suran, J.; Horvat, O. Mapping knowledge and comprehension of antimicrobial stewardship and biosecurity among veterinary students. PLoS ONE 2020, 15, e0235866. [Google Scholar] [CrossRef] [PubMed]
- Wangmo, K.; Dorji, T.; Pokhrel, N.; Dorji, T.; Dorji, J.; Tenzin, T. Knowledge, attitude, and practice on antibiotic use and antibiotic resistance among the veterinarians and para-veterinarians in Bhutan. PLoS ONE 2021, 16, e0251327. [Google Scholar] [CrossRef]
- Taylor, D.D.; Martin, J.N.; Morley, P.S.; Belk, K.E.; White, A.E.; Scallan Walter, E.J. Survey of production animal veterinarians’ prescription practices, factors influencing antimicrobial drug use, and perceptions of and attitudes toward antimicrobial resistance. J. Am. Vet. Med. Assoc. 2020, 257, 87–96. [Google Scholar] [CrossRef]
- Weese, J.S. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital: 1995–2004. J. Am. Vet. Med. Assoc. 2006, 228, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Raasch, S.; Collineau, L.; Postma, M.; Backhans, A.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stärk, K.; Dewulf, J.; et al. Effectiveness of alternative measures to reduce antimicrobial usage in pig production in four European countries. Porc. Health Manag. 2020, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Adekanye, U.O.; Ekiri, A.B.; Galipó, E.; Muhammad, A.B.; Mateus, A.; La Ragione, R.M.; Wakawa, A.; Armson, B.; Mijten, E.; Alafiatayo, R.; et al. Knowledge, attitudes and practices of veterinarians towards antimicrobial resistance and stewardship in Nigeria. Antibiotics 2020, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Madubuike Umunna, A.; Oluwatosin Ajoke, K. Veterinarians’ perception, knowledge and practices of antibiotic stewardship in enugu state southeast, Nigeria. Not. Sci. Biol. 2017, 9, 321–331. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 7 October 2021).
- Ministry of Health. National Antimicrobial Resistance Control Program for 2019–2023. Available online: http://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/prilozi/1.html&doctype=reg&abc=cba&eli=true&eliActId=427789®actid=427789 (accessed on 7 October 2021).
- Hardefeldt, L.Y.; Gilkerson, J.R.; Billman-Jacobe, H.; Stevenson, M.A.; Thursky, K.; Bailey, K.E.; Browning, G.F. Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J. Vet. Intern. Med. 2018, 32, 1092–1099. [Google Scholar] [CrossRef]
- Espinosa-Gongora, C.; Jessen, L.R.; Dyar, O.J.; Bousquet-Melou, A.; González-Zorn, B.; Pulcini, C.; Re, G.; Schwarz, S.; Timofte, D.; Toutain, P.-L.; et al. Towards a better and harmonized education in antimicrobial stewardship in European veterinary curricula. Antibiotics 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Llanos-Soto, S.G.; Vezeau, N.; Wemette, M.; Bulut, E.; Greiner Safi, A.; Moroni, P.; Shapiro, M.A.; Ivanek, R. Survey of perceptions and attitudes of an international group of veterinarians regarding antibiotic use and resistance on dairy cattle farms. Prev. Vet. Med. 2021, 188, 105253. [Google Scholar] [CrossRef]
- Ekakoro, J.E. Antimicrobial use practices of veterinary clinicians at a veterinary teaching hospital in the United States. Vet. Anim. Sci. 2018, 7, 100038. [Google Scholar] [CrossRef]
- Wayne, A.; McCarthy, R.; Lindenmayer, J. Therapeutic antibiotic use patterns in dogs: Observations from a veterinary teaching hospital. J. Small Anim. Pract. 2011, 52, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Gozdzielewska, L.; King, C.; Flowers, P.; Mellor, D.; Dunlop, P.; Price, L. Scoping review of approaches for improving antimicrobial stewardship in livestock farmers and veterinarians. Prev. Vet. Med. 2020, 180, 105025. [Google Scholar] [CrossRef] [PubMed]
- Scherpenzeel, C.G.M.; Santman-Berends, I.M.G.A.; Lam, T.J.G.M. Veterinarians’ attitudes toward antimicrobial use and selective dry cow treatment in the Netherlands. J. Dairy Sci. 2018, 101, 6336–6345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.W.; Moore, J.E.; Rao, J.R. Antimicrobial resistance (AMR): Significance to food quality and safety. Food Qual. Saf. 2019, 3, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Aubry-Damon, H.; Grenet, K.; Sall-Ndiaye, P.; Che, D.; Cordeiro, E.; Bougnoux, M.-E.; Rigaud, E.; Le Strat, Y.; Lemanissier, V.; Armand-Lefèvre, L.; et al. Antimicrobial resistance in commensal flora of pig farmers. Emerg. Infect. Dis. J. 2004, 10, 873. [Google Scholar] [CrossRef] [PubMed]
- Damborg, P.; Olsen, K.E.; Møller Nielsen, E.; Guardabassi, L. Occurrence of Campylobacter jejuni in pets living with human patients infected with C. jejuni. J. Clin. Microbiol. 2004, 42, 1363–1364. [Google Scholar] [CrossRef] [Green Version]
- Pozza, G.; Pinto, A.; Crovato, S.; Mascarello, G.; Bano, L.; Dacasto, M.; Battisti, A.; Bartoli, B.; Ravarotto, L.; Marangon, S. Antimicrobial use and antimicrobial resistance: Standpoint and prescribing behaviour of Italian cattle and pig veterinarians. Ital. J. Anim. Sci. 2020, 19, 905–916. [Google Scholar] [CrossRef]
- Busani, L.; Graziani, C.; Binkin, N.; Franco, A.; Di Egidio, A.; Battisti, A. Survey of the knowledge, attitudes and practice of Italian beef and dairy cattle veterinarians concerning the use of antibiotics. Vet. Rec. 2004, 155, 733–738. [Google Scholar] [PubMed]
- World Health Organization (WHO). No Time to Wait: Securing the Future from Drug-Resistant Infections. Available online: https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdfsfvrsn=5b424d7_6 (accessed on 7 October 2021).
- Singh, S.B.; Young, K.; Silver, L.L. What is an “ideal” antibiotic? Discovery challenges and path forward. Biochem. Pharmacol. 2017, 133, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief overview of approaches and challenges in new antibiotic development: A focus on drug repurposing. Front. Cell. Infect. Microbiol. 2021, 11, 442. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; JP, D.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic non-antibiotic therapies to combat antibiotic resistance: A review. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Sirichokchatchawan, W.; Apiwatsiri, P.; Pupa, P.; Saenkankam, I.; Khine, N.O.; Lekagul, A.; Lugsomya, K.; Hampson, D.J.; Prapasarakul, N. Reducing the risk of transmission of critical antimicrobial resistance determinants from contaminated pork products to humans in south-east Asia. Front. Microbiol. 2021, 12, 689015. [Google Scholar] [CrossRef]
- Servia-Dopazo, M.; Taracido-Trunk, M.; Figueiras, A. Non-Clinical factors determining the prescription of antibiotics by veterinarians: A systematic review. Antibiotics 2021, 10, 133. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P.; Price, S. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet. Rec. 2013, 173, 475. [Google Scholar] [CrossRef] [Green Version]
- Chipangura, J.K.; Eagar, H.; Kgoete, M.; Abernethy, D.; Naidoo, V. An investigation of antimicrobial usage patterns by small animal veterinarians in South Africa. Prev. Vet. Med. 2017, 136, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gupta, J.; Meena, H. Assessment of awareness about antibiotic resistance and practices followed by veterinarians for judicious prescription of antibiotics: An exploratory study in eastern haryana region of India. Trop. Anim. Health Prod. 2019, 51, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Bourély, C.; Fortané, N.; Calavas, D.; Leblond, A.; Gay, É. Why do veterinarians ask for antimicrobial susceptibility testing? A qualitative study exploring determinants and evaluating the impact of antibiotic reduction policy. Prev. Vet. Med. 2018, 159, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Hardy, B. The issue of antibiotic use in the livestock industry: What have we learned? Anim. Biotechnol. 2002, 13, 129–147. [Google Scholar] [CrossRef]
- Ungemach, F.R.; Müller-Bahrdt, D.; Abraham, G. Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int. J. Med. Microbiol. 2006, 296, 33–38. [Google Scholar] [CrossRef]
- Jorritsma, R.; Van der Heide, A.; Van Geijlswijk, I.M. Survey of veterinarians in the Netherlands on antimicrobial use for surgical prophylaxis in dairy practice. J. Dairy Sci. 2021, 104, 9106–9114. [Google Scholar] [CrossRef]
- Firouzabadi, D.; Mahmoudi, L. Knowledge, attitude, and practice of health care workers towards antibiotic resistance and antimicrobial stewardship programmes: A cross-sectional study. J. Eval. Clin. Pract. 2020, 26, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, A.; Labbate, M.; Norris, J.M.; Gilbert, G.L.; Ward, M.P.; Bajorek, B.V.; Degeling, C.; Rowbotham, S.J.; Dawson, A.; Nguyen, K.A.; et al. Opportunities and challenges to improving antibiotic prescribing practices through a One Health approach: Results of a comparative survey of doctors, dentists and veterinarians in Australia. BMJ Open 2018, 8, e020439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odoi, A.; Samuels, R.; Carter, C.N.; Smith, J. Antibiotic prescription practices and opinions regarding antimicrobial resistance among veterinarians in Kentucky, USA. PLoS ONE 2021, 16, e0249653. [Google Scholar] [CrossRef]
- Speksnijder, D.C.; Jaarsma, A.D.C.; van der Gugten, A.C.; Verheij, T.J.M.; Wagenaar, J.A. Determinants associated with veterinary antimicrobial prescribing in farm animals in the Netherlands: A qualitative study. Zoonoses Public Health 2015, 62, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, A.A.; Wilson, R.; Doohan, D.; LeJeune, J.T. Bovine veterinarians’ knowledge, beliefs, and practices regarding antibiotic resistance on Ohio dairy farms. J. Dairy Sci. 2009, 92, 3494–3502. [Google Scholar] [CrossRef]
- McDougall, S.; Compton, C.; Botha, N. Factors influencing antimicrobial prescribing by veterinarians and usage by dairy farmers in New Zealand. N. Z. Vet. J. 2017, 65, 84–92. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervinkova, D.; Vlkova, H.; Borodacova, I.; Makovcova, J.; Babak, V.; Lorencova, A.; Vrtkova, I.; Marosevic, D.; Jaglic, Z. Prevalence of mastitis pathogens in milk from clinically healthy cows. Vet. Med. 2013, 58, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Oliveira, L.; Ruegg, P.L. Treatments of clinical mastitis occurring in cows on 51 large dairy herds in Wisconsin. J. Dairy Sci. 2014, 97, 5426–5436. [Google Scholar] [CrossRef] [Green Version]
- Vakanjac, S.; Pavlović, V.; Magaš, V.; Pavlović, M.; Đurić, M.; Maletić, M.; Nedić, S.; Sočo, I. Investigations of efficacy of intramammary applied antimicrobials and glucocorticosteroides in the treatment of subclinical and clinical mastitis in cows. Vet. Glas. 2013, 67, 15–27. [Google Scholar] [CrossRef]
- Anđelković, J.; Radonjić, V. Usage of intramammary antimicrobial veterinary medicinal products in the republic of Serbia from 2011 to 2014. Serb. J. Exp. Clin. Res. 2017, 18, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural agents against bovine mastitis pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New perspective of Origanum vulgare L. and Satureja montana L. essential oils as bovine mastitis treatment alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Rafa, N. Common barriers, attitudes, and practices of veterinary practitioners regarding antimicrobial resistance and stewardship in Chattogram, Bangladesh. Open Vet. Sci. 2021, 2, 72–80. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Critically Important Antimicrobials for Human Medicine. Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 2 November 2021).
- OIE. List of Antimicrobial Agents of Veterinary Importance. Available online: https://www.oie.int/app/uploads/2021/03/a-oie-list-antimicrobials-june2019.pdf (accessed on 25 October 2021).
- Acharya, K.R.; Brankston, G.; Soucy, J.-P.R.; Cohen, A.; Hulth, A.; Löfmark, S.; Davidovitch, N.; Ellen, M.; Fisman, D.N.; Moran-Gilad, J.; et al. Evaluation of an OPEN stewardship generated feedback intervention to improve antibiotic prescribing among primary care veterinarians in Ontario, Canada and Israel: Protocol for evaluating usability and an interrupted time-series analysis. BMJ Open 2021, 11, e039760. [Google Scholar] [CrossRef]
- Postma, M.; Speksnijder, D.C.; Jaarsma, A.D.; Verheij, T.J.; Wagenaar, J.A.; Dewulf, J. Opinions of veterinarians on antimicrobial use in farm animals in Flanders and the Netherlands. Vet. Rec 2016, 179, 68. [Google Scholar] [CrossRef]
- Fan, W.; Yan, Z. Factors affecting response rates of the web survey: A systematic review. Comput. Hum. Behav. 2010, 26, 132–139. [Google Scholar] [CrossRef]

| Variable | Response | Frequency (n = 110) | Percentage (%) |
|---|---|---|---|
| Gender | Male Female | 92 18 | 83.6 16.4 |
| Age group | 25–34 years old | 28 | 25.5 |
| 35–44 years old 45–54 years old 55–64 years old >65 years old | 47 19 15 1 | 42.7 17.3 13.6 0.9 | |
| Level of education | Doctor of veterinary medicine | 76 | 69.1 |
| Master of veterinary medicine | 6 | 5.5 | |
| Doctor of medical sciences—veterinary medicine | 14 | 12.7 | |
| Doctor of veterinary medicine—specialist | 14 | 12.7 | |
| Type of employment | Private institution State institution | 84 26 | 76.4 23.6 |
| Number of years working in practice | 0–5 | 17 | 15.5 |
| 6–15 | 56 | 50.9 | |
| >15 | 37 | 33.6 |
| Variable | Response | Frequency (n = 110) | Percentage (%) |
|---|---|---|---|
| Received any education on the rational use of antimicrobials or AMR in the last 3 years | Yes No Do not remember | 80 29 1 | 72.7 26.4 0.9 |
| Used domestic or foreign guidelines when prescribing antibiotic therapy | Often Moderately Rarely Never There are no good guidelines | 32 39 25 6 8 | 29.1 35.5 22.7 5.5 7.2 |
| Thought there is a need for more local guidelines for AMU | Yes No Do not know | 97 7 6 | 88.2 6.4 5.4 |
| Encountered ineffective antibiotic therapy for bacterial infections | Daily Weekly Monthly Rarely | 5 28 31 44 2 | 4.5 25.5 28.2 40.0 1.8 |
| Never | |||
| Heard of antimicrobial stewardship | Yes No | 34 76 | 30.9 69.1 |
| Had protocols for prescribing antibiotics in their practice | Yes No | 22 88 | 20.0 80.0 |
| Did not have protocols for AMU but thought they should have them | Yes No | 96 14 | 87.3 12.7 |
| Kept records of AMU in their practice | Yes No | 79 31 | 71.8 28.2 |
| Prescribed antibiotics outside of indications for their usage | Often Moderately Rarely Never | 12 28 47 23 | 10.9 25.5 42.7 20.9 |
| To which extent does the use of antibiotics by unqualified people negatively impact AMR | There is no impact There is a moderate impact There is a significant impact | 1 8 101 | 0.9 7.3 91.8 |
| Conducted antibiograms (AST tests) routinely | Yes No | 54 56 | 49.1 50.9 |
| Variable | Frequency (n = 110) | Percentage (%) |
|---|---|---|
| Increase in responsible prescribing of antibiotics | 67 | 35.6 |
| Reduction in resistant bacteria in humans | 49 | 26.1 |
| Reduction in resistant bacteria in animals | 51 | 27.1 |
| The situation would not significantly change | 21 | 11.2 |
| Variable | Frequency (n = 110) | Percentage (%) |
|---|---|---|
| Animal products | 68 | 38.9 |
| All of the above | 35 | 20.0 |
| Contact with animals | 33 | 18.9 |
| Contact with other people | 17 | 9.7 |
| Environment | 12 | 6.8 |
| Plants | 10 | 5.7 |
| Item | Farm Hygiene | Rational AB Prescribing | Application of AB Therapy by Animal Owners |
|---|---|---|---|
| Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | |
| Great impact | 47 42.7 | 77 70.0 | 79 71.8 |
| Medium impact | 32 29.1 | 25 22.7 | 18 16.4 |
| Small impact | 26 23.6 | 5 4.6 | 9 8.2 |
| No impact | 5 4.6 | 3 2.7 | 4 3.6 |
| Variable | Frequency (n = 110) | Percentage (%) |
|---|---|---|
| Probiotics | 87 | 20.5 |
| Vaccines | 80 | 18.9 |
| Prebiotics | 62 | 14.6 |
| Feed enzymes | 46 | 10.9 |
| Immunostimulants | 44 | 10.4 |
| Antimicrobial peptides | 24 | 5.7 |
| Synbiotics | 24 | 5.7 |
| Bacteriocins | 21 | 5.0 |
| Phytocomponents | 20 | 4.7 |
| Phage therapy | 9 | 2.1 |
| Nanoparticles | 7 | 1.7 |
| Item | Great Impact | Medium Impact | Small Impact |
|---|---|---|---|
| Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | |
| Excessive use of AB | 103 93.6 | 5 4.6 | 2 1.8 |
| AB use without clear indications (antibiograms) | 81 73.6 | 21 19.1 | 8 7.3 |
| Wrong therapy length | 74 67.3 | 30 27.3 | 6 5.4 |
| Low therapy dosage of AB | 69 62.7 | 32 29.1 | 9 8.2 |
| Variable | Frequency (n = 110) | Percentage (%) |
|---|---|---|
| Animal owners’ financial situation/cost of laboratory tests | 67 | 23.8 |
| The lack of quick diagnostic tests | 61 | 21.6 |
| Pressure from animal owners | 47 | 16.7 |
| Prescribing habits | 38 | 13.5 |
| The lack of clear guidelines for certain diseases | 35 | 12.4 |
| Insufficient education of veterinarians | 34 | 12.1 |
| Item | Very Important | Moderately Important | Slightly Important | Not Important |
|---|---|---|---|---|
| Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | |
| Clinical symptoms | 78 70.9 | 23 20.9 | 6 5.4 | 3 2.7 |
| Antibiograms | 72 65.4 | 21 19.1 | 9 8.2 | 8 7.3 |
| Milk withholding period for drugs | 69 62.7 | 24 21.8 | 12 10.9 | 5 4.5 |
| Concern over AMR spread among animals | 62 56.4 | 34 30.9 | 10 9.1 | 4 3.6 |
| Anamnesis | 62 56.4 | 29 26.4 | 13 11.8 | 6 5.4 |
| Concern over AMR spread among people | 57 51.8 | 29 26.4 | 15 13.6 | 9 8.2 |
| Therapy cost | 55 50.0 | 36 32.7 | 8 7.3 | 11 10.0 |
| AB availability | 52 47.3 | 35 31.8 | 16 14.5 | 7 6.4 |
| Good practice guidelines | 47 42.7 | 39 35.4 | 16 14.5 | 8 7.3 |
| Expectations from animal owners | 46 41.8 | 27 24.5 | 19 17.3 | 18 16.4 |
| Expectations from colleagues | 20 18.2 | 31 28.2 | 28 25.4 | 31 28.2 |
| Variable | Frequency (n = 110) | Percentage (%) |
|---|---|---|
| Exclusively for therapy | 106 | 77.4 |
| For prophylaxis | 16 | 11.7 |
| For metaphylaxis | 15 | 10.9 |
| Item | Completely Agree | Partially Agree | Neither Agree nor Disagree | Slightly Disagree | Disagree |
|---|---|---|---|---|---|
| Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | Frequency (n = 110) Percentage (%) | |
| AMR is an important problem in both human and veterinary medicine | 102 92.7 | 4 3.7 | 2 1.8 | 2 1.8 | 0 0 |
| AMR will become much worse in the near future if we do not do something about it now | 99 90.0 | 9 8.2 | 1 0.9 | 1 0.9 | 0 0 |
| Over-the-counter antibiotics should be prohibited | 87 79.1 | 12 10.9 | 5 4.5 | 3 2.7 | 3 2.7 |
| I am open to using alternatives to antibiotics if they are proven to be successful in practice | 85 77.3 | 18 16.4 | 3 2.7 | 3 2.7 | 1 0.9 |
| Uncontrolled use of antibiotics in farm animals is an important cause of resistance to bacterial infections in humans | 78 70.9 | 23 20.9 | 7 6.4 | 2 1.8 | 0 0 |
| The antibiotics I prescribe contribute to the problem of antimicrobial resistance | 51 46.4 | 32 29.1 | 21 19.1 | 4 3.6 | 2 1.8 |
| There is insufficient information on the direct effect of antibiotic use in animals with the development of antimicrobial resistance in humans | 51 46.4 | 37 33.6 | 12 10.9 | 4 3.6 | 6 5.4 |
| AMR is mainly a problem in hospital settings | 35 31.8 | 32 29.1 | 18 16.4 | 6 5.4 | 19 17.3 |
| Variable | Frequency (n = 110) | Percentage (%) |
|---|---|---|
| Enrofloxacin | 56 | 17.4 |
| Amoxicillin | 48 | 14.9 |
| Amoxicillin + clavulanic acid | 48 | 14.9 |
| Penicillin | 33 | 10.3 |
| Ceftriaxone | 30 | 9.3 |
| Tetracycline | 27 | 8.4 |
| Gentamicin | 23 | 7.2 |
| Trimethoprim + sulfamethoxazole | 14 | 4.4 |
| Cloxacillin | 11 | 3.4 |
| Neomycin | 9 | 2.8 |
| Streptomycin | 8 | 2.5 |
| Ampicillin | 5 | 1.6 |
| Erythromycin | 4 | 1.2 |
| Lincomycin | 3 | 0.9 |
| Novobiocin | 2 | 0.6 |
| Variable | Frequency (n = 110) | Percentage (%) |
|---|---|---|
| Experience and knowledge of clinical symptoms | 91 | 60.6 |
| Exclusively diagnostic tests (antibiograms) | 25 | 16.7 |
| Milk withholding period for antimicrobials | 31 | 20.7 |
| Guidelines (foreign and domestic) | 3 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidović, J.; Stojanović, D.; Cagnardi, P.; Kladar, N.; Horvat, O.; Ćirković, I.; Bijelić, K.; Stojanac, N.; Kovačević, Z. Farm Animal Veterinarians’ Knowledge and Attitudes toward Antimicrobial Resistance and Antimicrobial Use in the Republic of Serbia. Antibiotics 2022, 11, 64. https://doi.org/10.3390/antibiotics11010064
Vidović J, Stojanović D, Cagnardi P, Kladar N, Horvat O, Ćirković I, Bijelić K, Stojanac N, Kovačević Z. Farm Animal Veterinarians’ Knowledge and Attitudes toward Antimicrobial Resistance and Antimicrobial Use in the Republic of Serbia. Antibiotics. 2022; 11(1):64. https://doi.org/10.3390/antibiotics11010064
Chicago/Turabian StyleVidović, Jovana, Dragica Stojanović, Petra Cagnardi, Nebojša Kladar, Olga Horvat, Ivana Ćirković, Katarina Bijelić, Nenad Stojanac, and Zorana Kovačević. 2022. "Farm Animal Veterinarians’ Knowledge and Attitudes toward Antimicrobial Resistance and Antimicrobial Use in the Republic of Serbia" Antibiotics 11, no. 1: 64. https://doi.org/10.3390/antibiotics11010064
APA StyleVidović, J., Stojanović, D., Cagnardi, P., Kladar, N., Horvat, O., Ćirković, I., Bijelić, K., Stojanac, N., & Kovačević, Z. (2022). Farm Animal Veterinarians’ Knowledge and Attitudes toward Antimicrobial Resistance and Antimicrobial Use in the Republic of Serbia. Antibiotics, 11(1), 64. https://doi.org/10.3390/antibiotics11010064







