The Effect of Antibiotics on Planktonic Cells and Biofilm Formation Ability of Collected Arcobacter-like Strains and Strains Isolated within the Czech Republic
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Susceptibility of Arcobacter-like Strains
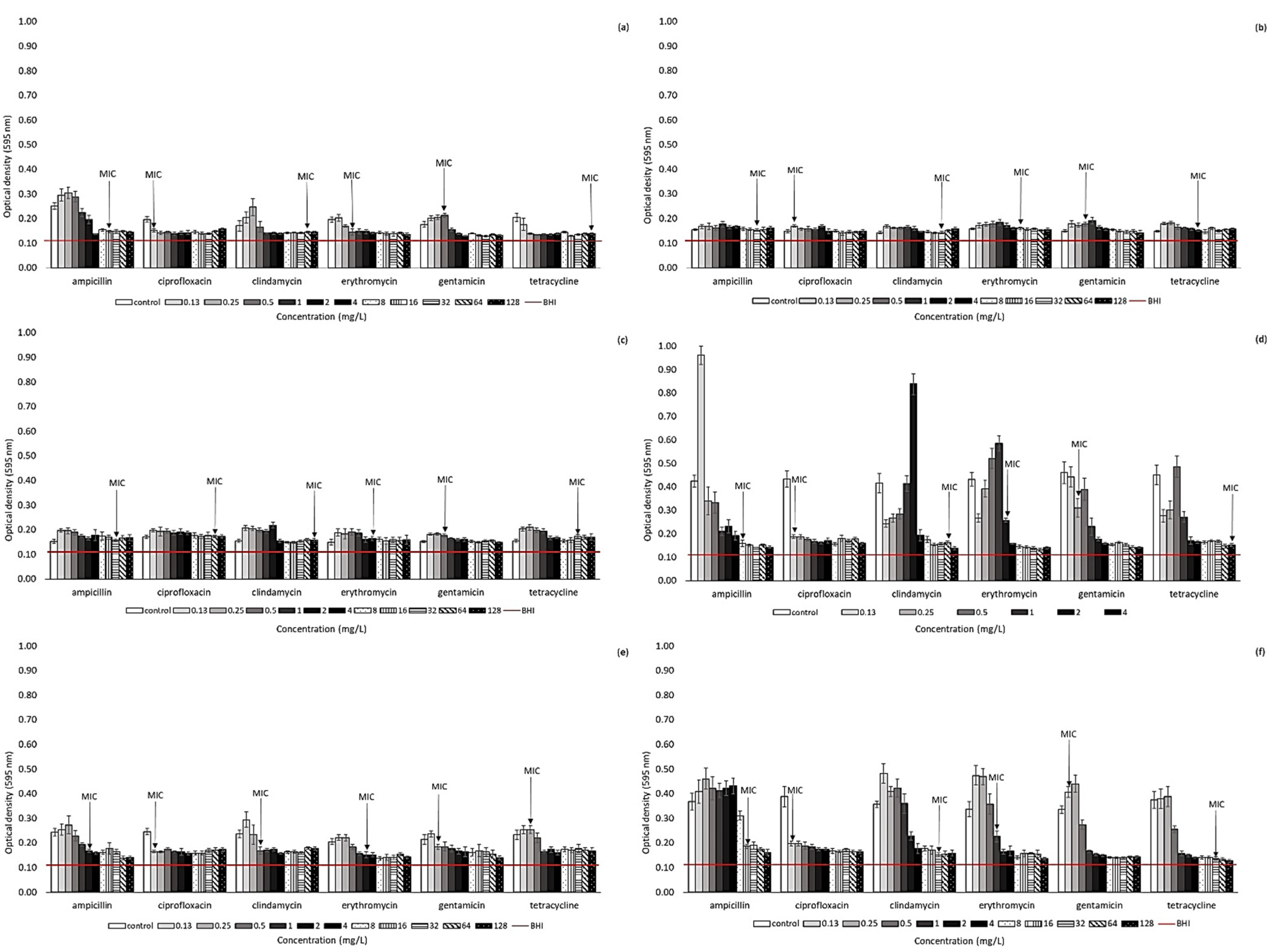
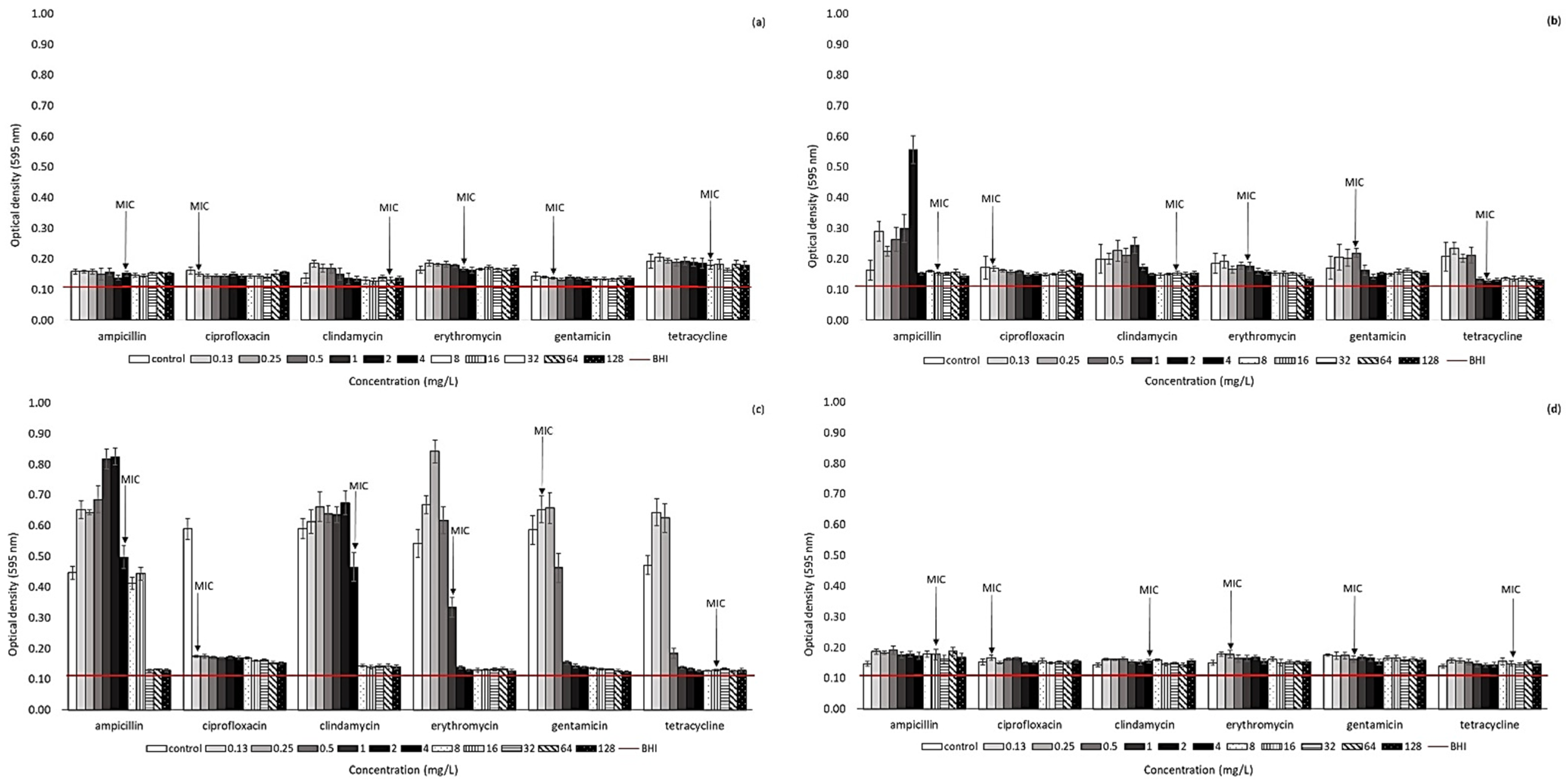
2.2. Effect of Antibiotic Presence on Biofilm Formation Ability of Arcobacter-like Strains
3. Discussion
4. Materials and Methods
4.1. Arcobacter-like Strains
4.2. Antibiotic Susceptibility Testing
4.3. Biofilm Formation of Arcobacter-like Strains in the Presence of Antibiotics
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vandamme, P.; Falsen, E.; Rossau, R.; Hoste, B.; Segers, P.; Tytgat, R.; De Ley, J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: Emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 1991, 41, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, W.; Kroneck, P.M.H.; Pfennig, N. Comparative systematic study on Spirillum 5175, Campylobacter and Wolinella species: Description of Spirillum 5175 as Sulfurospirillum deleyianum gen. nov., spec. nov. Arch. Microbiol. 1992, 158, 287–293. [Google Scholar] [CrossRef]
- Chieffi, D.; Fanelli, F.; Fusco, F. Arcobacter butzleri: Up-to-date taxonomy, ecology, and pathogenicity of an emerging pathogen. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2071–2109. [Google Scholar] [CrossRef]
- Waite, D.W.; Vanwonterghem, I.; Rinke, C.; Parks, D.H.; Zhang, Y.; Takai, K.; Hugenholtz, P. Comparative genomic analysis of the class Epsilonproteobacteria and proposed reclassification to Epsilonbacteraeota (phyl. nov.). Front. Microbiol. 2017, 8, 682. [Google Scholar] [CrossRef]
- Waite, D.W.; Vanwonterghem, I.; Rinke, C.; Parks, D.H.; Zhang, Y.; Takai, K.; Hugenholtz, P. Addendum: Comparative genomic analysis of the class Epsilonproteobacteria and proposed reclassification to Epsilonbacteraeota (phyl. nov.). Front. Microbiol. 2018, 9, 772. [Google Scholar] [CrossRef]
- Ferreira, S.; Oleastro, M.; Domingues, F. Current insights on Arcobacter butzleri in food chain. Curr. Opin. Food Sci. 2019, 26, 9–17. [Google Scholar] [CrossRef]
- Debruyne, L.; Gevers, D.; Vandamme, P. Taxonomy of the Family Campylobacteraceae Campylobacter, 3rd ed.; ASM Press: Washington, DC, USA, 2008; p. 732. [Google Scholar]
- Šilha, D.; Hrušková, L.; Brožková, I.; Moťková, P.; Vytřasová, J. Survival of selected bacteria from the genus Arcobacter on various metallic surfaces. J. Food Nutr. Res. 2014, 53, 217–223. [Google Scholar]
- Fanelli, F.; Pinto Di, A.; Mottola, A.; Mule, G.; Chieffi, D.; Baruzzi, F.; Fusco, V. Genomic characterization of Arcobacter butzleri isolated from shellfish: Novel insight into antibiotic resistance and virulence determinants. Front. Microbiol. 2019, 10, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šilha, D.; Vackova, B.; Šilhova, L. Occurrence of virulence-associated genes in Arcobacter butzleri and Arcobacter cryaerophilus isolates from foodstuff, water, and clinical samples within the Czech Republic. Folia Microbiol. 2019, 64, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Collado, L.; Figueras, M.J. Taxonomy, Epidemiology, and Clinical Relevance of the Genus Arcobacter. Clin. Microbiol. Rev. 2011, 24, 174–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Insights in the pathogenesis and resistance of Arcobacter: A review. Crit. Rev. Microbiol. 2016, 42, 364–383. [Google Scholar]
- Miller, W.G.; Parker, C.T.; Rubenfield, M.; Mendz, G.L.; Wosten, M.M.; Ussery, D.W.; Mandrell, R.E. The complete genome sequence and analysis of the epsilonproteobacterium Arcobacter butzleri. PLoS ONE 2007, 2, e1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girbau, C.; Martinez-Malaxetxebarria, I.; Muruaga, G.; Carmona, S.; Alonso, R.; Fernandez-Astorga, A. Study of biofilm formation ability of foodborne Arcobacter butzleri under different conditions. J. Food Protect. 2017, 80, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Passerini de Rossi, B.; García, C.; Calenda, M.; Vay, C.; Franco, M. Activity of levofloxacin and ciprofloxacin on biofilms and planktonic cells of Stenotrophomonas maltophilia isolates from patients with device-associated infections. Int. J. Antimicrob. Agents 2009, 34, 260–264. [Google Scholar] [CrossRef]
- Šilha, D.; Sirotková, S.; Švarcová, K.; Hofmeisterová, L.; Koryčanová, K.; Šilhová, L. Biofilm Formation Ability of Arcobacter-Like and Campylobacter Strains under Different Conditions and on Food Processing Materials. Microorganisms 2021, 9, 2017. [Google Scholar] [CrossRef]
- Ramees, T.P.; Dhama, K.; Karthik, K.; Rathore, R.S.; Kumar, A.; Saminathan, M.; Tiwari, R.; Malik, Y.S.; Singh, R.K. Arcobacter: An emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control—A comprehensive review. Vet. Q. 2017, 37, 136–161. [Google Scholar] [CrossRef] [Green Version]
- Van den Abeele, V.; Vogelaers, D.; Vanlaere, E.; Houf, K. Antimicrobial susceptibility testing of Arcobacter butzleri and Arcobacter cryaerophilus strains isolated from Belgian patients. J. Antimicrob. Chem. 2016, 71, 1241–1244. [Google Scholar] [CrossRef] [Green Version]
- Šilha, D.; Pejchalova, M.; Šilhova, L. Susceptibility to 18 drugs and multidrug resistance of Arcobacter isolates from different sources within the Czech Republic. J. Glob. Antimicrob. Resist. 2017, 9, 74–77. [Google Scholar] [CrossRef]
- Vicente-Martins, S.; Oleastro, M.; Domingues, F.C.; Ferreira, S. Arcobacter spp. at retail food from Portugal: Prevalence, genotyping and antibiotics resistance. Food Control 2018, 85, 107–112. [Google Scholar] [CrossRef]
- Rathlavath, S.; Kohli, V.; Singh, A.S.; Lekshmi, M.; Tripathi, G.; Kumar, S.; Nayak, B.B. Virulence genotypes and antimicrobial susceptibility patterns of Arcobacter butzleri isolated from seafood and its environment. Int. J. Food Microbiol. 2017, 263, 32–37. [Google Scholar] [CrossRef]
- Riesenberg, A.; Frömke, C.; Stingl, K.; Feßler, A.T.; Gölz, G.; Glocker, E.O.; Kreienbrock, L.; Klarmann, D.; Werckenthin, C.; Schwarz, S. Antimicrobial susceptibility testing of Arcobacter butzleri: Development and application of a new protocol for broth microdilution. J. Antimicrob. Chem. 2017, 72, 2769–2774. [Google Scholar] [CrossRef] [Green Version]
- Zacharow, I.; Bystroń, J.; Wałecka-Zacharska, E.; Podkowik, M.; Bania, J. Prevalence and antimicrobial resistance of Arcobacter butzleri and Arcobacter cryaerophilus isolates from retail meat in Lower Silesia region, Poland. Pol. J. Vet. Sci. 2015, 18, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; ISBN 1-56238-804-5/1-56238-805-3. [Google Scholar]
- Aski, S.; Tabatabaei, H.; Khoshbakht, M.; Raeisi, M. Occurrence and antimicrobial resistance of emergent Arcobacter spp. isolated from cattle and sheep in Iran. Comp. Immunol. Microbiol. Infect. Dis. 2016, 44, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Kayman, T.; Abay, S.; Hizlisoy, H.; Atabay, H.İ.; Diker, K.S.; Aydin, F. Emerging pathogen Arcobacter spp. in acute gastroenteritis: Molecular identification, antibiotic susceptibilities and genotyping of the isolated arcobacters. J. Med. Microbiol. 2012, 10, 1439–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Genotypic and phenotypic features of Arcobacter butzleri pathogenicity. Microb. Pathog. 2014, 76, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, O.; Houf, K.; Douat, N.; Vlaes, L.; Retore, P.; Butzler, J.P.; Dediste, A. An-timicrobial susceptibility of clinical isolates of non-jejuni/coli campylobacters and arcobacters from Belgium. J. Antimicrob. Chem. 2006, 57, 908–913. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.; Fraqueza, M.J.; Queiroz, J.A.; Domingues, F.C.; Oleastro, M. Genetic diversity, antibiotic resistance and biofilm-forming ability of A. butzleri isolated from poultry and environment from a Portuguese slaughterhouse. Int. J. Food Microbiol. 2013, 162, 82–88. [Google Scholar] [CrossRef]
- Houf, K.; Devriese, L.A.; Haesebrouck, F.; Vandenberg, O.; Butzler, J.P.; van Hoof, J.; Vandamme, P. Antimicrobial susceptibility patterns of Arcobacter butzleri and Arcobacter cryaerophilus strains isolated from humans and broilers. Microb. Drug Resist. 2004, 10, 243–247. [Google Scholar] [CrossRef]
- Abay, S.; Kayman, T.; Hizlisoy, H.; Fuat, A. In vitro Antibacterial Susceptibility of Arcobacter butzleri Isolated from Different Sources. J. Vet. Med. Sci. 2011, 74, 613–616. [Google Scholar] [CrossRef] [Green Version]
- Tezel, U.B.; Akcelik, N.; Yuksel, F.N.; Karatuğ, N.; Akcelik, M. Effects of sub-MIC antibiotic concentrations on biofilm production of Salmonella Infantis. Biotechnol. Biotechnolog. Equip. 2016, 30, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Kjeldgaard, J.; Jørgensen, K.; Ingmer, H. Growth and survival at chiller temperatures of Arcobacter butzleri. Int. J. Food Microbiol. 2009, 131, 256–259. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtli, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chem. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Šilhová-Hrušková, L.; Moťková, P.; Šilha, D.; Vytřasová, J. Hodnocení tvorby biofilmu vybraných patogenů vyskytujících se v potravinářském průmyslu. Epidemiol. Mikrobiol. Imunol. 2015, 64, 169–174. [Google Scholar] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. APMIS 2013, 121, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Antibiotic-induced biofilm formation. Int. J. Artif. Org. 2011, 34, 737–751. [Google Scholar] [CrossRef]
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250. [Google Scholar] [CrossRef]
- Wojnicz, D.; Tichaczek-Goska, D. Effect of sub-minimum inhibitory concentrations of ciprofloxacin, amikacin and colistin on biofilm formation and virulence factors of Escherichia coli planktonic and biofilm forms isolated from human urine. Braz. J. Microbiol. 2013, 44, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Rojo-Molinero, E.; Macia, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Šilha, D.; Švarcova, K.; Bajer, T.; Královec, K.; Tesarova, E.; Mouckova, K.; Pejchalová, M.; Bajerova, P. Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms. Molecules 2020, 25, 5654. [Google Scholar] [CrossRef] [PubMed]
| Number of Isolates Susceptible at Given Concentrations and MICs (mg/L) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.13 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | MIC50 | MIC90 | R | |
| Ampicillin | |||||||||||||||||
| A. butzleri strains (50) | 1 | 3 | 2 | 1 | 4 | 5 | 15 | 7 | 6 | 5 | 1 | 8 | 64 | 24% | |||
| A. cryaerophilus strains (3) | 1 | 2 | 8 | 128 | 0% | ||||||||||||
| Ab CCUG 30484 | 1 | ||||||||||||||||
| Ab LMG 10828 | 1 | ||||||||||||||||
| Ac CCM 3933 | 1 | ||||||||||||||||
| Ac CCM 3934 | 1 | ||||||||||||||||
| Ac CCM 7050 | 1 | ||||||||||||||||
| Pd LMG 25694 | 1 | ||||||||||||||||
| As LMG 6621 | 1 | ||||||||||||||||
| Ciprofloxacin | |||||||||||||||||
| A. butzleri strains (50) | 20 | 13 | 12 | 3 | 1 | 1 | 0.06 | 0.13 | 4% | ||||||||
| A. cryaerophilus strains (3) | 1 | 1 | 1 | 0.13 | 64 | 33.3% | |||||||||||
| Ab CCUG 30484 | 1 | ||||||||||||||||
| Ab LMG 10828 | 1 | ||||||||||||||||
| Ac CCM 3933 | 1 | ||||||||||||||||
| Ac CCM 3934 | 1 | ||||||||||||||||
| Ac CCM 7050 | 1 | ||||||||||||||||
| Pd LMG 25694 | 1 | ||||||||||||||||
| As LMG 6621 | 1 | ||||||||||||||||
| Clindamycin | |||||||||||||||||
| A. butzleri strains (50) | 2 | 1 | 3 | 1 | 2 | 2 | 5 | 7 | 15 | 6 | 6 | 32 | 128 | 86% | |||
| A. cryaerophilus strains (3) | 1 | 1 | 1 | 16 | 32 | 100% | |||||||||||
| Ab CCUG 30484 | 1 | ||||||||||||||||
| Ab LMG 10828 | 1 | ||||||||||||||||
| Ac CCM 3933 | 1 | ||||||||||||||||
| Ac CCM 3934 | 1 | ||||||||||||||||
| Ac CCM 7050 | 1 | ||||||||||||||||
| Pd LMG 25694 | 1 | ||||||||||||||||
| As LMG 6621 | 1 | ||||||||||||||||
| Erythromycin | |||||||||||||||||
| A. butzleri strains (50) | 1 | 3 | 4 | 8 | 13 | 17 | 4 | 2 | 4 | 0% | |||||||
| A. cryaerophilus strains (3) | 1 | 1 | 1 | 2 | 4 | 0% | |||||||||||
| Ab CCUG 30484 | 1 | ||||||||||||||||
| Ab LMG 10828 | 1 | ||||||||||||||||
| Ac CCM 3933 | 1 | ||||||||||||||||
| Ac CCM 3934 | 1 | ||||||||||||||||
| Ac CCM 7050 | 1 | ||||||||||||||||
| Pd LMG 25694 | 1 | ||||||||||||||||
| As LMG 6621 | 1 | ||||||||||||||||
| Gentamicin | |||||||||||||||||
| A. butzleri strains (50) | 12 | 22 | 9 | 6 | 1 | 0.25 | 1 | 0% | |||||||||
| A. cryaerophilus strains (3) | 1 | 1 | 1 | 0.5 | 1 | 0% | |||||||||||
| Ab CCUG 30484 | 1 | ||||||||||||||||
| Ab LMG 10828 | 1 | ||||||||||||||||
| Ac CCM 3933 | 1 | ||||||||||||||||
| Ac CCM 3934 | 1 | ||||||||||||||||
| Ac CCM 7050 | 1 | ||||||||||||||||
| Pd LMG 25694 | 1 | ||||||||||||||||
| As LMG 6621 | 1 | ||||||||||||||||
| Tetracycline | |||||||||||||||||
| A. butzleri strains (50) | 3 | 2 | 8 | 10 | 7 | 5 | 7 | 4 | 1 | 3 | 4 | 64 | 54% | ||||
| A. cryaerophilus strains (3) | 1 | 1 | 1 | 16 | 32 | 66.7% | |||||||||||
| Ab CCUG 30484 | 1 | ||||||||||||||||
| Ab LMG 10828 | 1 | ||||||||||||||||
| Ac CCM 3933 | 1 | ||||||||||||||||
| Ac CCM 3934 | 1 | ||||||||||||||||
| Ac CCM 7050 | 1 | ||||||||||||||||
| Pd LMG 25694 | 1 | ||||||||||||||||
| As LMG 6621 | 1 | ||||||||||||||||
| Resistance Patterns | |||||||
|---|---|---|---|---|---|---|---|
| Arcobacter-like Strain | AMP, CLI | AMP, TET | CLI, TET | AMP, CLI, TET | CIP, CLI, TET | AMP, CIP, CLI, TET | AMP, CLI, GEN, TET |
| A. butzleri strains (50) | 3 | 1 | 17 | 6 | 1 | 1 | |
| A. cryaerophilus strains (3) | 1 | 1 | |||||
| Ab CCUG 30484 | 1 | ||||||
| Ab LMG 10828 | 1 | ||||||
| Ac CCM 3933 | 1 | ||||||
| Ac CCM 3934 | 1 | ||||||
| Ac CCM 7050 | 1 | ||||||
| Pd LMG 25694 | 1 | ||||||
| As LMG 6621 | 1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švarcová, K.; Pejchalová, M.; Šilha, D. The Effect of Antibiotics on Planktonic Cells and Biofilm Formation Ability of Collected Arcobacter-like Strains and Strains Isolated within the Czech Republic. Antibiotics 2022, 11, 87. https://doi.org/10.3390/antibiotics11010087
Švarcová K, Pejchalová M, Šilha D. The Effect of Antibiotics on Planktonic Cells and Biofilm Formation Ability of Collected Arcobacter-like Strains and Strains Isolated within the Czech Republic. Antibiotics. 2022; 11(1):87. https://doi.org/10.3390/antibiotics11010087
Chicago/Turabian StyleŠvarcová, Karolína, Marcela Pejchalová, and David Šilha. 2022. "The Effect of Antibiotics on Planktonic Cells and Biofilm Formation Ability of Collected Arcobacter-like Strains and Strains Isolated within the Czech Republic" Antibiotics 11, no. 1: 87. https://doi.org/10.3390/antibiotics11010087






