The Combination of Amoxicillin and 1,8-Cineole Improves the Bioavailability and the Therapeutic Effect of Amoxicillin in a Rabbit Model
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis of the Blood Samples
2.2. Biological Analysis of the Blood Samples
2.3. CSF Samples Biological Analysis
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Animals
4.3. Experimental Design
4.4. Sampling of Blood and Cerebrospinal Fluid (CSF)
4.5. HPLC Analysis
4.6. Pharmacokinetic Analysis
4.7. Blood or CSF Samples and Microbiological Assay
4.8. Ethical Approval
4.9. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lingzhi, L.; Haojie, G.; Dan, G.; Hongmei, M.; Yang, L.; Mengdie, J.; Chengkun, Z.; Xiaohui, Z. The Role of Two-Component Regulatory System in β-Lactam Antibiotics Resistance. Microbiol. Res. 2018, 215, 126–129. [Google Scholar] [CrossRef]
- Fleming, A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to Their Use in the Isolation of B. Influenzae. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar]
- Collignon, P.; Beggs, J.J. Socioeconomic Enablers for Contagion: Factors Impelling the Antimicrobial Resistance Epidemic. Antibiotics 2019, 8, 86. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 619–635. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Figueiredo, A.H.A.; Brouwer, M.C.; van de Beek, D. Acute Community-Acquired Bacterial Meningitis. Neurol. Clin. 2018, 36, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Houri, H.; Pormohammad, A.; Riahi, S.M.; Nasiri, M.J.; Fallah, F.; Dabiri, H.; Pouriran, R. Acute Bacterial Meningitis in Iran: Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169617. [Google Scholar] [CrossRef]
- Ikken, Y.; Charof, R.; Benaouda, A.; Hilali, F.; Akkaoui, S.; Elouennass, M.; Sekhsokh, Y. Epidemiology and Antibiotic Resistance Profile of Bacterial Meningitis in Morocco from 2015 to 2018. Acta Microbiol. Immunol. Hung. 2020, 67, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Gongora, C.; Jessen, L.R.; Kieler, I.N.; Damborg, P.; Bjørnvad, C.R.; Gudeta, D.D.; Pires dos Santos, T.; Sablier-Gallis, F.; Sayah-Jeanne, S.; Corbel, T.; et al. Impact of Oral Amoxicillin and Amoxicillin/Clavulanic Acid Treatment on Bacterial Diversity and β-Lactam Resistance in the Canine Faecal Microbiota. J. Antimicrob. Chemother. 2020, 75, 351–361. [Google Scholar] [CrossRef]
- Veeraraghavan, B.; Bakthavatchalam, Y.D.; Sahni, R.D. Orally Administered Amoxicillin/Clavulanate: Current Role in Outpatient Therapy. Infect. Dis. 2021, 10, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of Antibiotic Resistance in Escherichia Coli Strains Simultaneously Isolated from Humans, Animals, Food, and the Environment: A Systematic Review and Meta-Analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Costerus, J.M.; Brouwer, M.C.; Bijlsma, M.W.; Tanck, M.W.; van der Ende, A.; van de Beek, D. Impact of an Evidence-Based Guideline on the Management of Community-Acquired Bacterial Meningitis: A Prospective Cohort Study. Clin. Microbiol. Infect. 2016, 22, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Padmini, N.; Ajilda, A.A.K.; Sivakumar, N.; Selvakumar, G. Extended Spectrum β-Lactamase Producing Escherichia Coli and Klebsiella Pneumoniae: Critical Tools for Antibiotic Resistance Pattern. J. Basic Microbiol. 2017, 57, 460–470. [Google Scholar] [CrossRef]
- Remmal, A. Pharmaceutical Composition Comprising an Anti-Bacteral Agent and an Active Ingredient Selected from Carveol, Thymol, Eugenol, Borneol and Carvacrol. Available online: https://patents.google.com/patent/WO2006120567A2/en (accessed on 1 June 2022).
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Rosato, A.; Sblano, S.; Salvagno, L.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Anti-Biofilm Inhibitory Synergistic Effects of Combinations of Essential Oils and Antibiotics. Antibiotics 2020, 9, 637. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic Antioxidant and Antimicrobial Activities of Essential Oils of Some Selected Medicinal Plants in Combination and with Synthetic Compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Wani, A.; Mange, H.; Vasudevan, A. Essential Oils: A Novel Approach for Anti-Microbial Therapy. Nat. Prod. J. 2022, 12, e060921196176. [Google Scholar] [CrossRef]
- Akhmouch, A.A.; Hriouech, S.; Mzabi, A.; Tanghort, M.; Chefchaou, H.; Remmal, A.; Chami, N. Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism. Antibiotics 2022, 11, 1002. [Google Scholar] [CrossRef]
- Nau, R.; Sörgel, F.; Eiffert, H. Central Nervous System Infections and Antimicrobial Resistance: An Evolving Challenge. Curr. Opin. Neurol. 2021, 34, 456–467. [Google Scholar] [CrossRef]
- Rinaldi, F.; Oliva, A.; Sabatino, M.; Imbriano, A.; Hanieh, P.N.; Garzoli, S.; Mastroianni, C.M.; De Angelis, M.; Miele, M.C.; Arnaut, M.; et al. Antimicrobial Essential Oil Formulation: Chitosan Coated Nanoemulsions for Nose to Brain Delivery. Pharmaceutics 2020, 12, 678. [Google Scholar] [CrossRef]
- Cabrera-Maqueda, J.M.; Fuentes Rumí, L.; Valero López, G.; Baidez Guerrero, A.E.; García Molina, E.; Díaz Pérez, J.; García-Vázquez, E. Antibiotic diffusion to central nervous system. Rev. Esp. Quim. 2018, 31, 1–12. [Google Scholar]
- Blassmann, U.; Roehr, A.C.; Frey, O.R.; Vetter-Kerkhoff, C.; Thon, N.; Hope, W.; Briegel, J.; Huge, V. Cerebrospinal Fluid Penetration of Meropenem in Neurocritical Care Patients with Proven or Suspected Ventriculitis: A Prospective Observational Study. Crit. Care 2016, 20, 343. [Google Scholar] [CrossRef]
- Kerz, T.; von Loewenich, F.D.; Roberts, J.; Neulen, A.; Ringel, F. Cerebrospinal Fluid Penetration of Very High-Dose Meropenem: A Case Report. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 47. [Google Scholar] [CrossRef]
- MacDougal, C. Penicillins, cephalosporin, and other β-Lactam antibiotics. In The Pharmacological Basis of the Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw Hill: New York, NY, USA, 2018. [Google Scholar]
- El-Hosseiny, L.S.; Alqurashy, N.N.; Sheweita, S.A. Oxidative Stress Alleviation by Sage Essential Oil in Co-Amoxiclav Induced Hepatotoxicity in Rats. Int. J. Biomed. Sci. 2016, 12, 71–78. [Google Scholar]
- Jamshidi, H.R.; Negintaji, S. Effects of Thymol on Co-Amoxiclav-Induced Hepatotoxicity in Rats. Int. J. Med. Lab. 2021, 8, 44–54. [Google Scholar] [CrossRef]
- Papadopoulos, C.J.; Carson, C.F.; Chang, B.J.; Riley, T.V. Role of the MexAB-OprM Efflux Pump of Pseudomonas Aeruginosa in Tolerance to Tea Tree (Melaleuca Alternifolia) Oil and Its Monoterpene Components Terpinen-4-Ol, 1,8-Cineole, and α-Terpineol. Appl. Environ. Microbiol. 2008, 74, 1932–1935. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the Antibiofilm and Antiquorum Sensing Activities of Eucalyptus Globulus Essential Oil and Its Main Component 1,8-Cineole against Methicillin-Resistant Staphylococcus Aureus Strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Magiatis, P.; Skaltsounis, A.-L.; Chinou, I.; Haroutounian, S.A. Chemical Composition and In-Vitro Antimicrobial Activity of the Essential Oils of Three Greek Achillea Species. Z. Naturforsch C J. Biosci. 2002, 57, 287–290. [Google Scholar] [CrossRef]
- Moo, C.-L.; Osman, M.A.; Yang, S.-K.; Yap, W.-S.; Ismail, S.; Lim, S.-H.-E.; Chong, C.-M.; Lai, K.-S. Antimicrobial Activity and Mode of Action of 1,8-Cineol against Carbapenemase-Producing Klebsiella Pneumoniae. Sci. Rep. 2021, 11, 20824. [Google Scholar] [CrossRef]
- Jalilzadeh-Amin, G.; Maham, M. The Application of 1,8-Cineole, a Terpenoid Oxide Present in Medicinal Plants, Inhibits Castor Oil-Induced Diarrhea in Rats. Pharm. Biol. 2015, 53, 594–599. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.; Boyle, R.R.; Brandon, S.; Davies, N.W.; Sorensen, J.S. Pharmacokinetics of 1,8-Cineole, a Dietary Toxin, in the Brushtail Possum (Trichosurus Vulpecula): Significance for Feeding. Xenobiotica 2007, 37, 903–922. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial Efficacy of Eucalyptus Oil and 1,8-Cineole Alone and in Combination with Chlorhexidine Digluconate against Microorganisms Grown in Planktonic and Biofilm Cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Klarić, M.Š. Antimicrobial Potency of Single and Combined Mupirocin and Monoterpenes, Thymol, Menthol and 1,8-Cineole against Staphylococcus Aureus Planktonic and Biofilm Growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Trinh, H.-T.; Lee, I.-A.; Hyun, Y.-J.; Kim, D.-H. Artemisia Princeps Pamp. Essential Oil and Its Constituents Eucalyptol and α -Terpineol Ameliorate Bacterial Vaginosis and Vulvovaginal Candidiasis in Mice by Inhibiting Bacterial Growth and NF- κ B Activation. Planta Med. 2011, 77, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial Activity of Selected Essential Oil Compounds Alone and in Combination with β-Lactam Antibiotics Against MRSA Strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
- Hriouech, S.; Akhmouch, A.A.; Mzabi, A.; Chefchaou, H.; Tanghort, M.; Oumokhtar, B.; Chami, N.; Remmal, A. The Antistaphylococcal Activity of Amoxicillin/Clavulanic Acid, Gentamicin, and 1,8-Cineole Alone or in Combination and Their Efficacy through a Rabbit Model of Methicillin-Resistant Staphylococcus Aureus Osteomyelitis. Evid. Based Complement. Altern. Med. 2020, 2020, 4271017. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, T.; Xiao, H.; Wang, J.; Zhang, S.; Cao, X. The Bioavailability and Pharmacokinetics of an Amoxicillin–Clavulanic Acid Granular Combination after Intravenous and Oral Administration in Swine. J. Vet. Pharm. Ther. 2021, 44, 126–130. [Google Scholar] [CrossRef]
- Ali, B.; Amin, S.; Ahmad, J.; Ali, A.; Mohd, A.; Mir, S.R. Bioavailability Enhancement Studies of Amoxicillin with Nigella. Indian J. Med. Res. 2012, 135, 555–559. [Google Scholar]
- Jiang, Z.; Luo, M.; Ma, W.; Ma, S.; Wang, Y.; Zhang, K. Protective Effects of 1,8-Cineole Microcapsules Against Inflammation and Gut Microbiota Imbalance Associated Weight Loss Induced by Heat Stress in Broiler Chicken. Front. Pharmacol. 2021, 11, 585945. [Google Scholar] [CrossRef]
- Kardos, P.; Khaletskaya, O.; Kropova, O. Efficacy and Safety of Cineole (Soledum®) in the Treatment of Patients with Acute Bronchitis: Results of an Open-Label Randomized Clinical Phase III Study. Clin Phytosci 2021, 7, 83. [Google Scholar] [CrossRef]
- Carceles, C.M.; Escudero, E.; Vicente, M.S.; Serrano, J.M.; Carli, S. Pharmacokinetics of Amoxicillin/Clavulanic Acid Combination after Intravenous and Oral Administration in Goats. Vet. Q. 1995, 17, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, J.; Roy Julie, J.; Messier, S.; Higgins, R.; Besner, J.G.; Matineau, G.P. Metaphylaxis of Streptococcus Suis in Weaned Pigs with Oral Amoxicillin: Results of a Pharmacokineitic and Pharmacodynamic Study. Journ. Rech. Porc. En Fr. 1998, 30, 411–416. [Google Scholar]
- Yang, F.; Yang, F.; Wang, G.; Xi, W.; Zhang, C.; Wang, H. Pharmacokinetics of the Amoxicillin–Clavulanic Acid Combination after Intravenous and Oral Administration in Cats. J. Vet. Pharm. Ther. 2019, 42, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lalanne, S.; Le Vée, M.; Lemaitre, F.; Le Corre, P.; Verdier, M.; Fardel, O. Differential Interactions of the Β-lactam Cloxacillin with Human Renal Organic Anion Transporters (OATs). Fundam. Clin. Pharm. 2020, 34, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sittner, A.; Ben-Shmuel, A.; Glinert, I.; Bar-David, E.; Schlomovitz, J.; Kobiler, D.; Weiss, S.; Levy, H. Using Old Antibiotics to Treat Ancient Bacterium—β-Lactams for Bacillus Anthracis Meningitis. PLoS ONE 2020, 15, e0228917. [Google Scholar] [CrossRef]
- Remmal, A.; Akhmouch, A.A. Pharmaceutical Formulation Comprising Cineole and Amoxicillin. 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US250863563&docAn=16306262 (accessed on 1 June 2022).
- Remmal, A.; Bouchikhi, T.; Rhayour, K.; Ettayebi, M.; Tantaoui-Elaraki, A. Improved Method for the Determination of Antimicrobial Activity of Essential Oils in Agar Medium. J. Essent. Oil Res. 1993, 5, 179–184. [Google Scholar] [CrossRef]
- De Abreu, L.R.P.; Ortiz, R.A.M.; De Castro, S.C.; Pedrazzoli, J. HPLC Determination of Amoxicillin Comparative Bioavailability in Healthy Volunteers after a Single Dose Administration. J. Pharm. Pharm. Sci. 2003, 6, 223–230. [Google Scholar]
- FDA. Bioanalytical Method Validation Guidance for Industry; FDA: Silver Spring, MD, USA, 2018.
- Casey, J.T.; O’Cleirigh, C.; Walsh, P.K.; O’Shea, D.G. Development of a Robust Microtiter Plate-Based Assay Method for Assessment of Bioactivity. J. Microbiol. Methods 2004, 58, 327–334. [Google Scholar] [CrossRef]
- Patton, T.; Barrett, J.; Brennan, J.; Moran, N. Use of a Spectrophotometric Bioassay for Determination of Microbial Sensitivity to Manuka Honey. J. Microbiol. Methods 2006, 64, 84–95. [Google Scholar] [CrossRef]


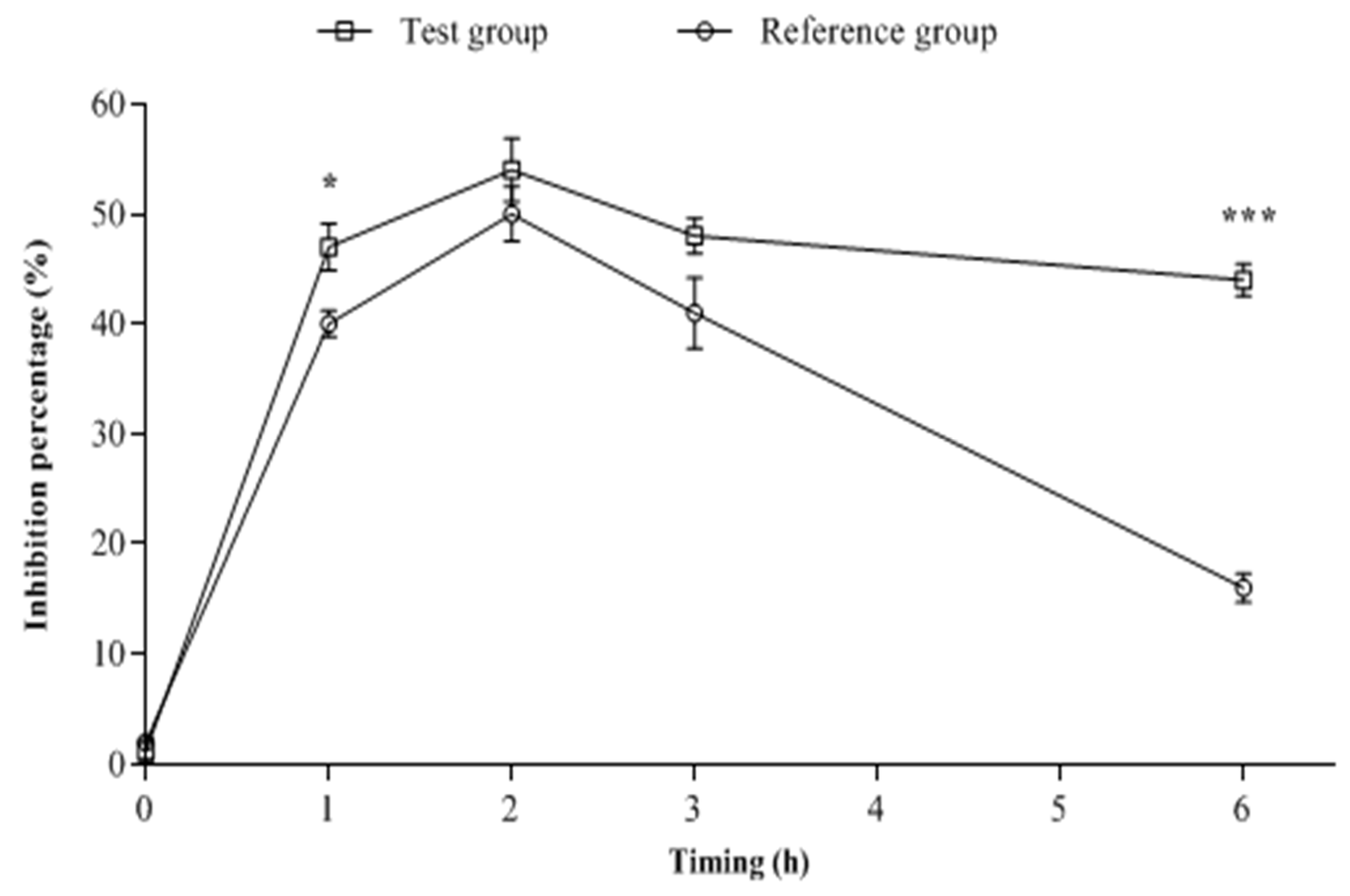
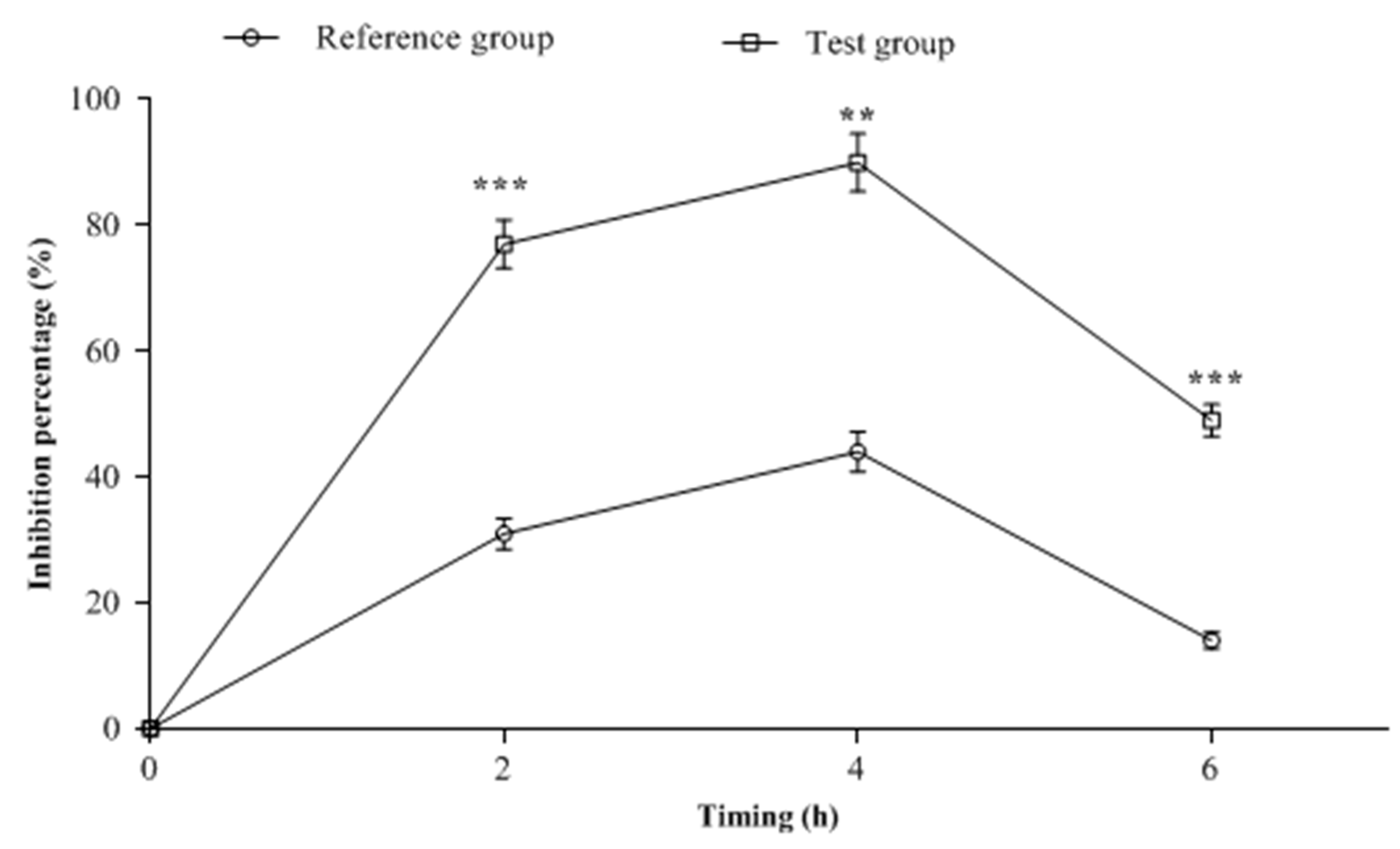
| Rabbit Groups | AUC0–6h (µg.h/mL) | Cmax (µg/mL) | Tmax (h) | Ke | T1/2 (h) |
|---|---|---|---|---|---|
| Reference group | 14.74 ± 0.9 | 3.49 ± 0.2 | 1.55 ± 0.1 | 0.32 ± 0.04 | 2.21 ± 0.3 |
| Test group | 22.30 ± 0.4 ** | 5.79 ± 0.2 ** | 1.18 ± 0.1 | 0.24 ± 0.01 | 2.94 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmouch, A.A.; Hriouech, S.; Chefchaou, H.; Tanghort, M.; Mzabi, A.; Chami, N.; Remmal, A. The Combination of Amoxicillin and 1,8-Cineole Improves the Bioavailability and the Therapeutic Effect of Amoxicillin in a Rabbit Model. Antibiotics 2022, 11, 1294. https://doi.org/10.3390/antibiotics11101294
Akhmouch AA, Hriouech S, Chefchaou H, Tanghort M, Mzabi A, Chami N, Remmal A. The Combination of Amoxicillin and 1,8-Cineole Improves the Bioavailability and the Therapeutic Effect of Amoxicillin in a Rabbit Model. Antibiotics. 2022; 11(10):1294. https://doi.org/10.3390/antibiotics11101294
Chicago/Turabian StyleAkhmouch, Ahmed Amin, Soukayna Hriouech, Hanane Chefchaou, Mariam Tanghort, Aouatef Mzabi, Najat Chami, and Adnane Remmal. 2022. "The Combination of Amoxicillin and 1,8-Cineole Improves the Bioavailability and the Therapeutic Effect of Amoxicillin in a Rabbit Model" Antibiotics 11, no. 10: 1294. https://doi.org/10.3390/antibiotics11101294
APA StyleAkhmouch, A. A., Hriouech, S., Chefchaou, H., Tanghort, M., Mzabi, A., Chami, N., & Remmal, A. (2022). The Combination of Amoxicillin and 1,8-Cineole Improves the Bioavailability and the Therapeutic Effect of Amoxicillin in a Rabbit Model. Antibiotics, 11(10), 1294. https://doi.org/10.3390/antibiotics11101294






