Local Treatment of Driveline Infection with Bacteriophages
Abstract
:1. Introduction
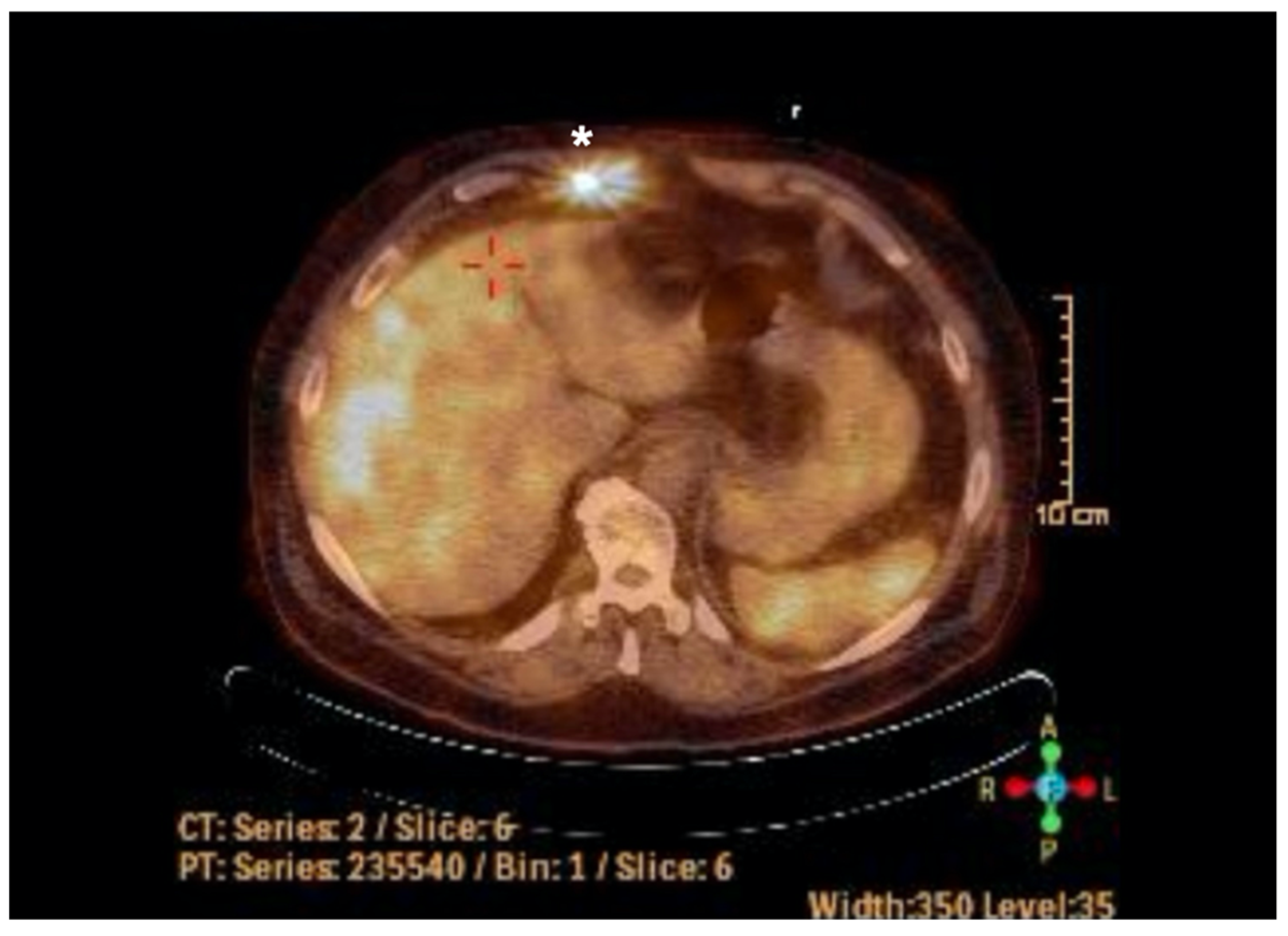
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gordon, R.J.; Quagliarello, B.; Lowy, F.D. Ventricular assist device-related infections. Lancet Infect. Dis. 2006, 6, 426–437. [Google Scholar] [CrossRef]
- Pereda, D.; Conte, J.V. Left Ventricular Assist Device Driveline Infections. Cardiol. Clin. 2011, 29, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Leuck, A.-M. Left ventricular assist device driveline infections: Recent advances and future goals. J. Thorac. Dis. 2015, 7, 2151–2157. [Google Scholar] [CrossRef]
- Nienaber, J.J.C.; Kusne, S.; Riaz, T.; Walker, R.C.; Baddour, L.M.; Wright, A.J.; Park, S.J.; Vikram, H.R.; Keating, M.R.; Arabia, F.A.; et al. Clinical Manifestations and Management of Left Ventricular Assist Device-Associated Infections. Clin. Infect. Dis. 2013, 57, 1438–1448. [Google Scholar] [CrossRef]
- Macia, M.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef]
- Levy, D.; Guo, Y.; Simkins, J.; Puius, Y.; Muggia, V.; Goldstein, D.; D’Alessandro, D.; Minamoto, G. Left ventricular assist device exchange for persistent infection: A case series and review of the literature. Transpl. Infect. Dis. 2014, 16, 453–460. [Google Scholar] [CrossRef]
- Comeau, A.M.; Hatfull, G.F.; Krisch, H.M.; Lindell, D.; Mann, N.H.; Prangishvili, D. Exploring the prokaryotic virosphere. Res. Microbiol. 2008, 159, 306–313. [Google Scholar] [CrossRef]
- Hesse, S.; Adhya, S. Phage Therapy in the Twenty-First Century: Facing the Decline of the Antibiotic Era; Is It Finally Time for the Age of the Phage? Annu. Rev. Microbiol. 2019, 73, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef]
- Greene, W.; Chan, B.; Bromage, E.; Grose, J.H.; Walsh, C.; Kortright, K.; Forrest, S.; Perry, G.; Byrd, L.; Stamper, M.A. The Use of Bacteriophages and Immunological Monitoring for the Treatment of a Case of Chronic Septicemic Cutaneous Ulcerative Disease in a Loggerhead Sea Turtle Caretta caretta. J. Aquat. Anim. Health 2021, 33, 139–154. [Google Scholar] [CrossRef]
- Herten, M.; Idelevich, E.A.; Sielker, S.; Becker, K.; Scherzinger, A.S.; Osada, N.; Torsello, G.B.; Bisdas, T. Vascular Graft Impregnation with Antibiotics: The Influence of High Concentrations of Rifampin, Vancomycin, Daptomycin, and Bacteriophage Endolysin HY-133 on Viability of Vascular Cells. Med Sci. Monit. Basic Res. 2017, 23, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage Therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Alisky, J.; Iczkowski, K.; Rapoport, A.; Troitsky, N. Bacteriophages show promise as antimicrobial agents. J. Infect. 1998, 36, 5–15. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.-C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Aslam, S.; Pretorius, V.; Lehman, S.M.; Morales, S.; Schooley, R.T. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J. Hear. Lung Transplant. 2019, 38, 475–476. [Google Scholar] [CrossRef]
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.-P.; Costechareyre, D.; Molle, V. Bacteriophage Therapy for Staphylococcus Aureus Infections: A Review of Animal Models, Treatments, and Clinical Trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314. [Google Scholar] [CrossRef]
- Hughes, K.A.; Sutherland, I.W.; Jones, M.V. Biofilm susceptibility to bacteriophage attack: The role of phage-borne polysaccharide depolymerase. Microbiology 1998, 144, 3039–3047. [Google Scholar] [CrossRef]
- Spellberg, B.; Gilbert, D.N. The Future of Antibiotics and Resistance: A Tribute to a Career of Leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. 2), S71–S75. [Google Scholar] [CrossRef]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef]
- Seed, K.D. Battling Phages: How Bacteria Defend against Viral Attack. PLOS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef]
- Yilmaz, C.; Colak, M.; Yilmaz, B.C.; Ersoz, G.; Kutateladze, M.; Gozlugol, M. Bacteriophage Therapy in Implant-Related Infections. J. Bone Jt. Surg. 2013, 95, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, W.N.; Concepción-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Antibiotics | Proteus mirabilis | Staphylococcus aureus |
|---|---|---|
| Oxacillin | R | S |
| Ampicillin | S | S |
| Ampicillin/Sulbactam | S | S |
| Piperacillin/Tazobactam | S | S |
| Cefuroxime | I | S |
| Cefotaxime | S | S |
| Ceftazidime | S | S |
| Imipenem | I | S |
| Meropenem | S | S |
| Gentamicin | S | S |
| Tetracycline | R | S |
| Cotimoxazole | S | S |
| Erythromycin | R | S |
| Clindamycin | R | S |
| Vancomycin | R | S |
| Fosfomycin | S | S |
| Fusidic acid | R | S |
| Rifampin | R | S |
| Linezolid | R | S |
| Daptomycin | R | S |
| Tigecycline | R | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Püschel, A.; Skusa, R.; Bollensdorf, A.; Gross, J. Local Treatment of Driveline Infection with Bacteriophages. Antibiotics 2022, 11, 1310. https://doi.org/10.3390/antibiotics11101310
Püschel A, Skusa R, Bollensdorf A, Gross J. Local Treatment of Driveline Infection with Bacteriophages. Antibiotics. 2022; 11(10):1310. https://doi.org/10.3390/antibiotics11101310
Chicago/Turabian StylePüschel, Anja, Romy Skusa, Antonia Bollensdorf, and Justus Gross. 2022. "Local Treatment of Driveline Infection with Bacteriophages" Antibiotics 11, no. 10: 1310. https://doi.org/10.3390/antibiotics11101310
APA StylePüschel, A., Skusa, R., Bollensdorf, A., & Gross, J. (2022). Local Treatment of Driveline Infection with Bacteriophages. Antibiotics, 11(10), 1310. https://doi.org/10.3390/antibiotics11101310






