In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus faecium
Abstract
1. Introduction
2. Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Synergy Testing by Checkerboard Assay
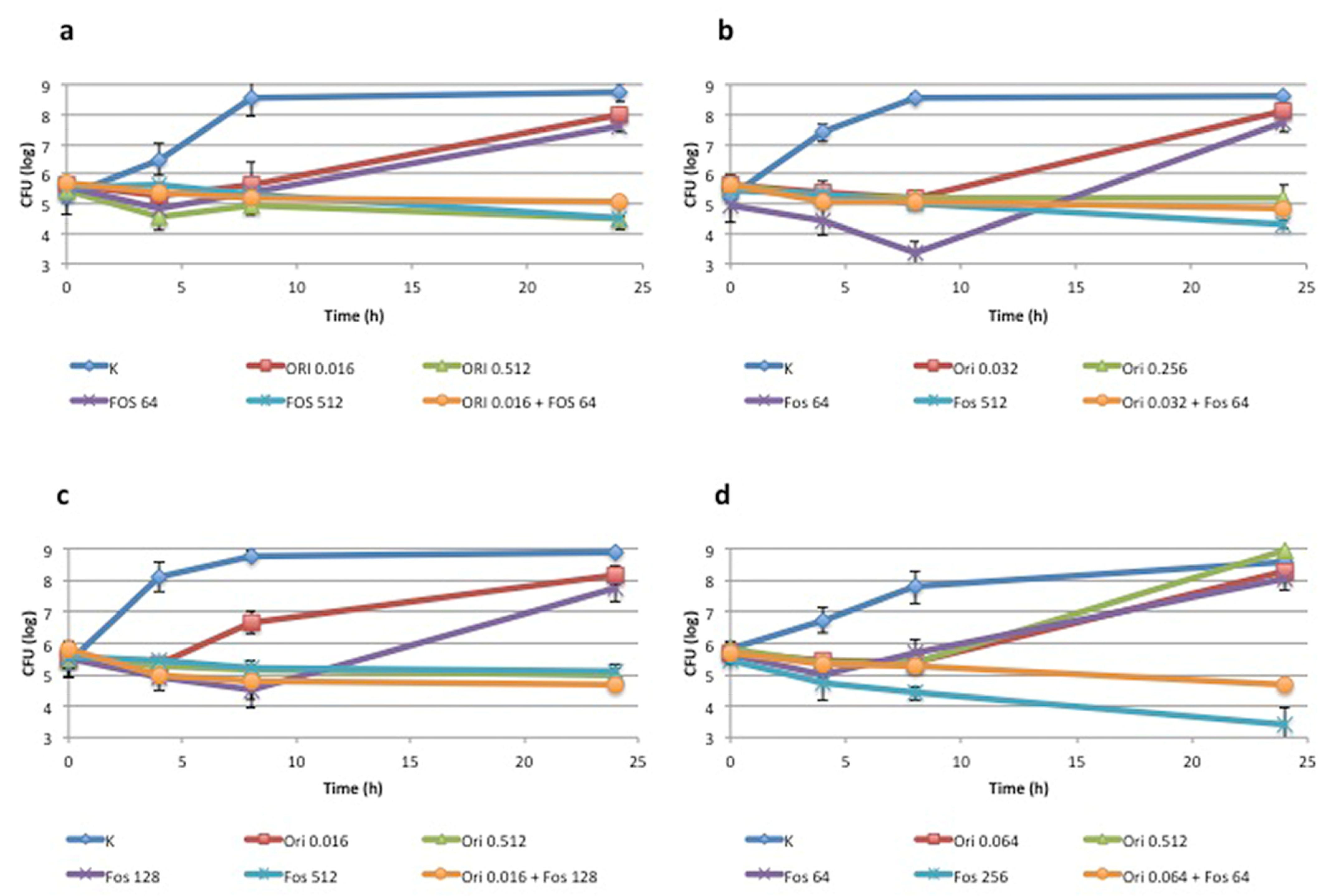
2.3. Time-Kill Assay
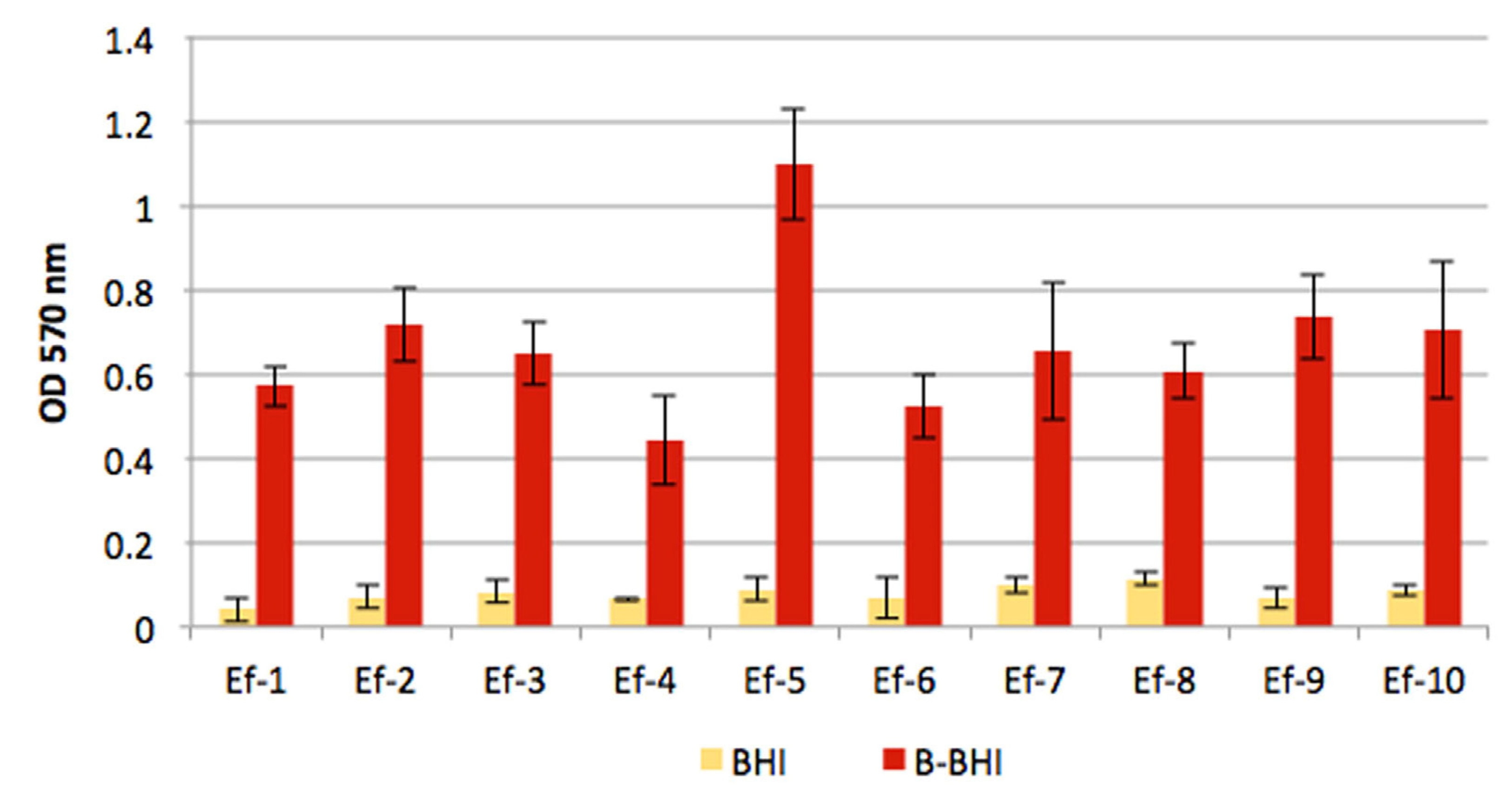
2.4. Biofilm Assays
2.5. In Vivo Toxicity in Galleria mellonella
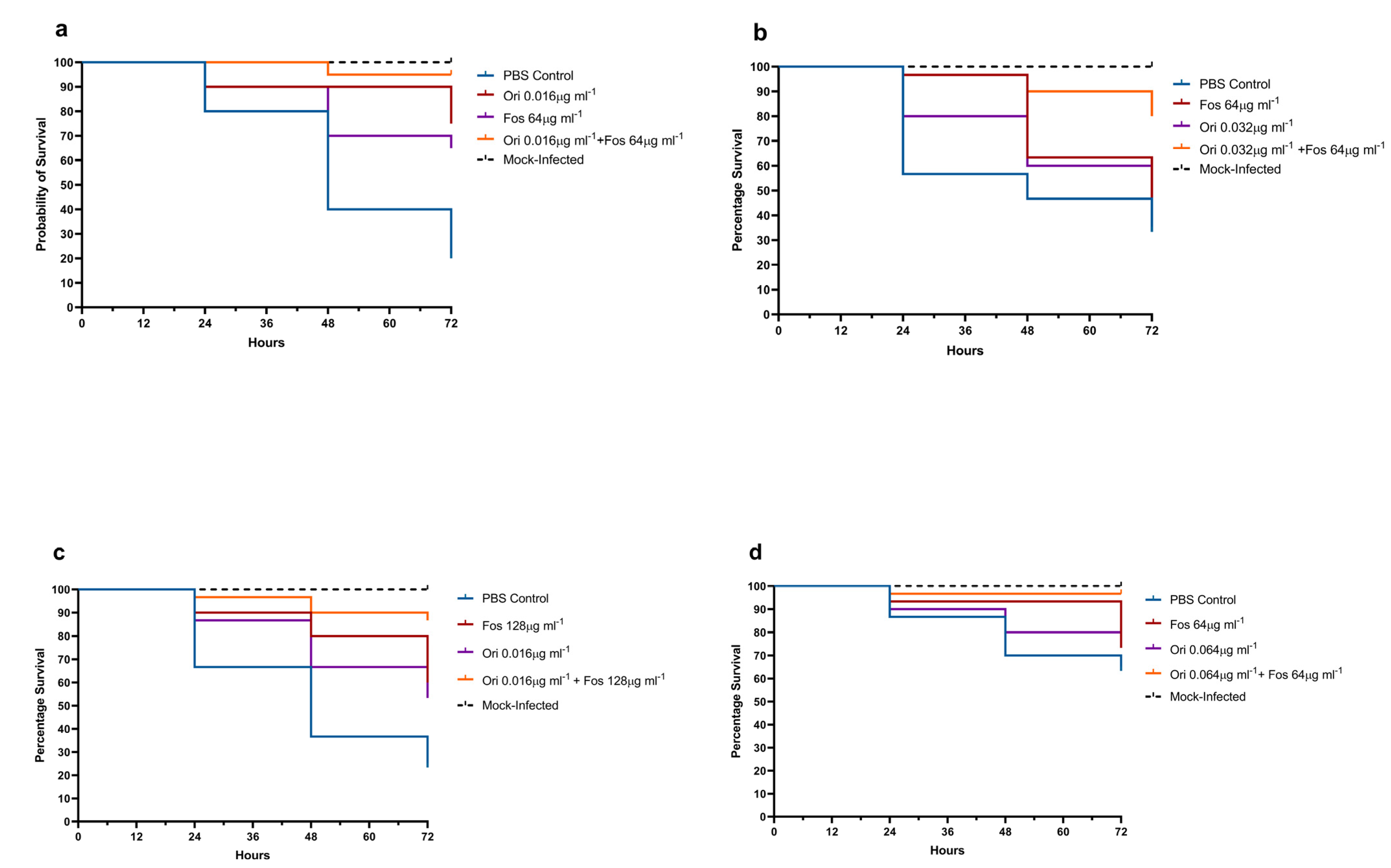
2.6. G. mellonella Treatment Assay Methods
3. Results
3.1. Synergism between Fosfomycin and Oritavancin: In Vitro Analysis
3.2. Biofilm Assays
3.3. In Vivo G. mellonella Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp.-Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Ntziora, F.; Falagas, M.E. Linezolid: Effectiveness and safety for approved and off-label indications. Expert Opin. Pharm. 2007, 8, 2381–2400. [Google Scholar] [CrossRef] [PubMed]
- Turnidge, J.; Kahlmeter, G.; Cantón, R.; MacGowan, A.; Giske, C.G.; European Committee on Antimicrobial Susceptibility Testing. Daptomycin in the Treatment of Enterococcal Bloodstream Infections and Endocarditis: A EUCAST Position Paper. Clin. Microbiol. Infect. 2020, 26, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Sader, H.S.; Flamm, R.K.; Castanheira, M.; Mendes, R.E. Oritavancin in Vitro Activity against Gram-Positive Organisms from European and United States Medical Centers: Results from the SENTRY Antimicrobial Surveillance Program for 2010–2014. Diagn. Microbiol. Infect. Dis. 2018, 91, 199–204. [Google Scholar] [CrossRef]
- Antonello, R.M.; Di Bella, S.; Maraolo, A.E.; Luzzati, R. Fosfomycin in continuous or prolonged infusion for systemic bacterial infections: A systematic review of its dosing regimen proposal from in vitro, in vivo and clinical studies. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1117–1126. [Google Scholar] [CrossRef]
- Antonello, R.M.; Principe, L.; Maraolo, A.E.; Viaggi, V.; Pol, R.; Fabbiani, M.; Montagnani, F.; Lovecchio, A.; Luzzati, R.; Di Bella, S. Fosfomycin as Partner Drug for Systemic Infection Management. A Systematic Review of Its Synergistic Properties from In Vitro and In Vivo Studies. Antibiotics 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Babiker, A.; Clarke, L.; Doi, Y.; Shields, R.K. Fosfomycin for Treatment of Multidrug-Resistant Pathogens Causing Urinary Tract Infection: A Real-World Perspective and Review of the Literature. Diagn. Microbiol. Infect. Dis. 2019, 95, 114856. [Google Scholar] [CrossRef]
- Zheng, J.-X.; Sun, X.; Lin, Z.-W.; Qi, G.-B.; Tu, H.-P.; Wu, Y.; Jiang, S.-B.; Chen, Z.; Deng, Q.-W.; Qu, D.; et al. In Vitro Activities of Daptomycin Combined with Fosfomycin or Rifampin on Planktonic and Adherent Linezolid-Resistant Isolates of Enterococcus Faecalis. J. Med. Microbiol. 2019, 68, 493–502. [Google Scholar] [CrossRef]
- Lagatolla, C.; Milic, J.; Imperi, F.; Cervoni, M.; Bressan, R.; Luzzati, R.; Di Bella, S. Synergistic Activity of Fosfomycin and Chloramphenicol against Vancomycin-Resistant Enterococcus Faecium (VREfm) Isolates from Bloodstream Infections. Diagn. Microbiol. Infect. Dis. 2021, 99, 115241. [Google Scholar] [CrossRef]
- M100. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 27 August 2022).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. EUCAST, Basel. 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 22 September 2022).
- Doern, C.D. When Does 2 plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, S.M.; Foster, J.M.; Emerson, J.; Burns, J.L. Clinically Feasible Biofilm Susceptibility Assay for Isolates of Pseudomonas Aeruginosa from Patients with Cystic Fibrosis. J. Clin. Microbiol. 2004, 42, 1915–1922. [Google Scholar] [CrossRef]
- Antonello, R.M.; Di Bella, S.; Betts, J.; La Ragione, R.; Bressan, R.; Principe, L.; Morabito, S.; Gigliucci, F.; Tozzoli, R.; Busetti, M.; et al. Zidovudine in Synergistic Combination with Fosfomycin: An in Vitro and in Vivo Evaluation against Multidrug-Resistant Enterobacterales. Int. J. Antimicrob. Agents 2021, 58, 106362. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; McManus, D.; Topal, J.E. Combination Therapy of Chloramphenicol and Daptomycin for the Treatment of Infective Endocarditis Secondary to Multidrug Resistant Enterococcus faecium. Hosp. Pharm. 2022, 57, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, N.J.; Davis, S.L.; Zervos, M.J.; Herc, E.S. Combatting resistant enterococcal infections: A pharmacotherapy review. Expert Opin. Pharmacother. 2018, 19, 979–992. [Google Scholar] [CrossRef]
- Boukthir, S.; Dejoies, L.; Zouari, A.; Collet, A.; Potrel, S.; Auger, G.; Cattoir, V. In vitro activity of eravacycline and mechanisms of resistance in enterococci. Int. J. Antimicrob. Agents 2020, 56, 106215. [Google Scholar] [CrossRef]
- Riccardi, N.; Monticelli, J.; Antonello, R.M.; Di Lallo, G.; Frezza, D.; Luzzati, R.; Di Bella, S. Therapeutic Options for Infections due to vanB Genotype Vancomycin-Resistant Enterococci. Microb. Drug. Resist. 2021, 27, 536–545. [Google Scholar] [CrossRef]
- O’Driscoll, T.; Crank, C.W. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect. Drug Resist. 2015, 8, 217–230. [Google Scholar]
- Mihailescu, R.; Furustrand Tafin, U.; Corvec, S.; Oliva, A.; Betrisey, B.; Borens, O.; Trampuz, A. High Activity of Fosfomycin and Rifampin against Methicillin-Resistant Staphylococcus Aureus Biofilm in Vitro and in an Experimental Foreign-Body Infection Model. Antimicrob. Agents Chemother. 2014, 58, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Yim, J.; Raut, A.; Rybak, M.J. Oritavancin Combinations with β-Lactams against Multidrug-Resistant Staphylococcus Aureus and Vancomycin-Resistant Enterococci. Antimicrob. Agents Chemother. 2016, 60, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Deraedt, M.F.; Harrington, A.T.; Danziger, L.H.; Wenzler, E. Efficacy of Oritavancin Alone and in Combination against Vancomycin-Susceptible and -Resistant Enterococci in an in-Vivo Galleria Mellonella Survival Model. Int. J. Antimicrob. Agents 2019, 54, 197–201. [Google Scholar] [CrossRef]
| MIC µg/mL | |||||
|---|---|---|---|---|---|
| Drugs Alone | Drugs in Combination | ||||
| FOF | ORI | FOF | ORI | FICI | |
| Ef-1 (vanA) | 64 | 0.512 | 32 | 0.016 | 0.531 |
| Ef-2 (vanA) | 128 | 0.512 | 16 | 0.128 | 0.375 |
| Ef-5 (vanA) | 64 | 0.512 | 16 | 0.064 | 0.375 |
| Ef-8 (vanA) | 512 | 0.512 | 128 | 0.016 | 0.281 |
| Ef-10 (vanA) | 256 | 0.512 | 64 | 0.064 | 0.375 |
| Ef-3 (vanB) | 512 | 0.512 | 64 | 0.016 | 0.156 |
| Ef-4 (vanB) | 512 | 0.256 | 64 | 0.032 | 0.250 |
| Ef-6 (vanB) | 64 | 0.032 | 32 | 0.008 | 0.500 |
| Ef-7 (vanB) | 256 | 0.512 | 32 | 0.032 | 0.188 |
| Ef-9 (vanB) | 512 | 0.512 | 64 | 0.004 | 0.133 |
| BIC µg/mL | |||||
|---|---|---|---|---|---|
| Drugs Alone | Drugs in Combination | ||||
| FOF | ORI | FOF | ORI | FICI | |
| Ef-3 (vanB) | 2048 | 8 | 64 | 0.5 | 0.094 |
| Ef-10 (vanA) | >2048 | 2 | 256 | 1 | 0.563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagatolla, C.; Mehat, J.W.; La Ragione, R.M.; Luzzati, R.; Di Bella, S. In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus faecium. Antibiotics 2022, 11, 1334. https://doi.org/10.3390/antibiotics11101334
Lagatolla C, Mehat JW, La Ragione RM, Luzzati R, Di Bella S. In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus faecium. Antibiotics. 2022; 11(10):1334. https://doi.org/10.3390/antibiotics11101334
Chicago/Turabian StyleLagatolla, Cristina, Jai W. Mehat, Roberto Marcello La Ragione, Roberto Luzzati, and Stefano Di Bella. 2022. "In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus faecium" Antibiotics 11, no. 10: 1334. https://doi.org/10.3390/antibiotics11101334
APA StyleLagatolla, C., Mehat, J. W., La Ragione, R. M., Luzzati, R., & Di Bella, S. (2022). In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus faecium. Antibiotics, 11(10), 1334. https://doi.org/10.3390/antibiotics11101334








