Influence of Single Dose Enrofloxacin Injection on Development of Fluoroquinolone Resistance in Campylobacter jejuni in Calves
Abstract
:1. Introduction
2. Results
2.1. Campylobacter Status of Calves Prior to Challenge
2.2. Bovine Respiratory Disease Induction
2.3. Experimental Inoculation of Calves with FQ-Susceptible C. jejuni Resulted in Effective Intestinal Colonization
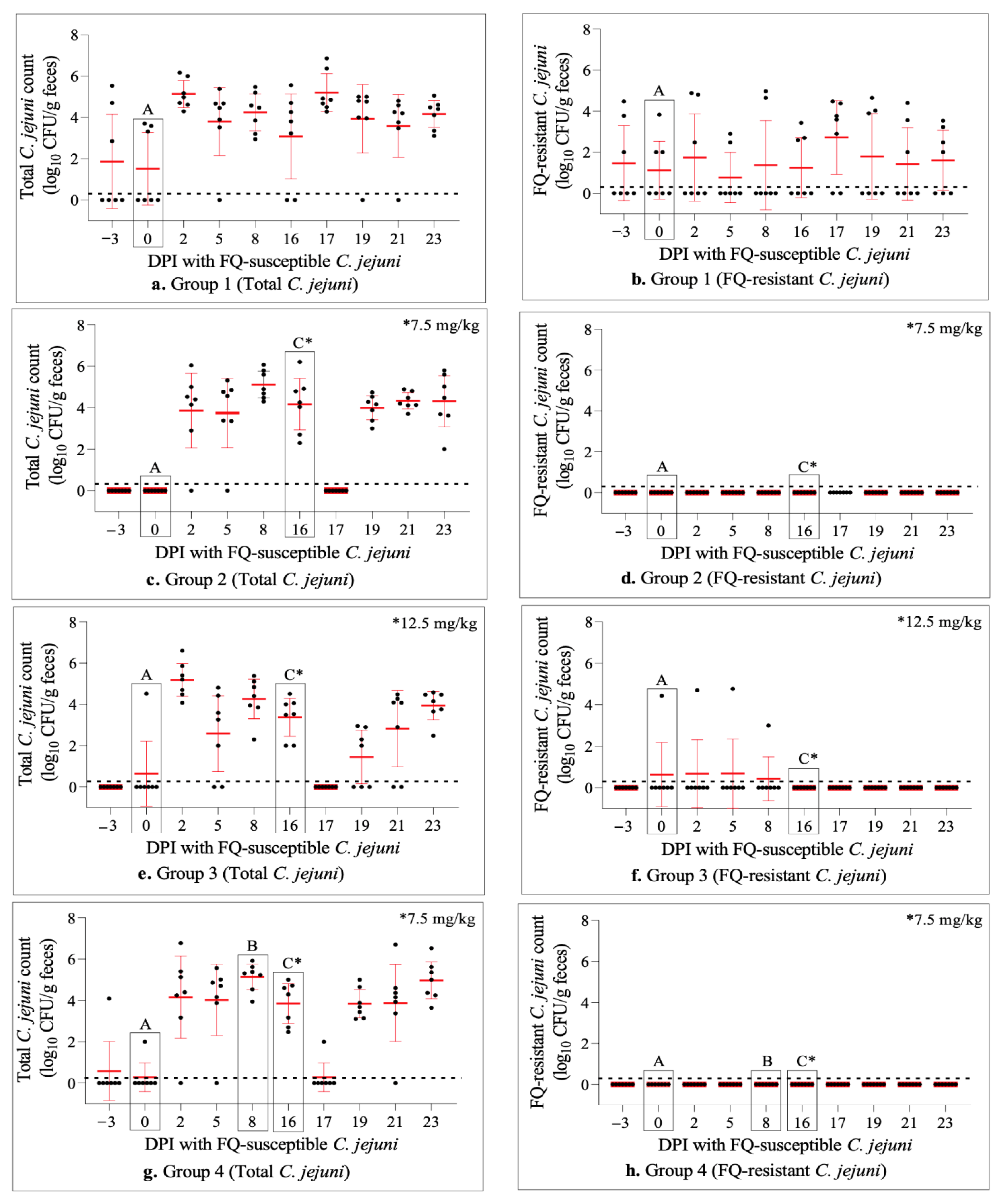
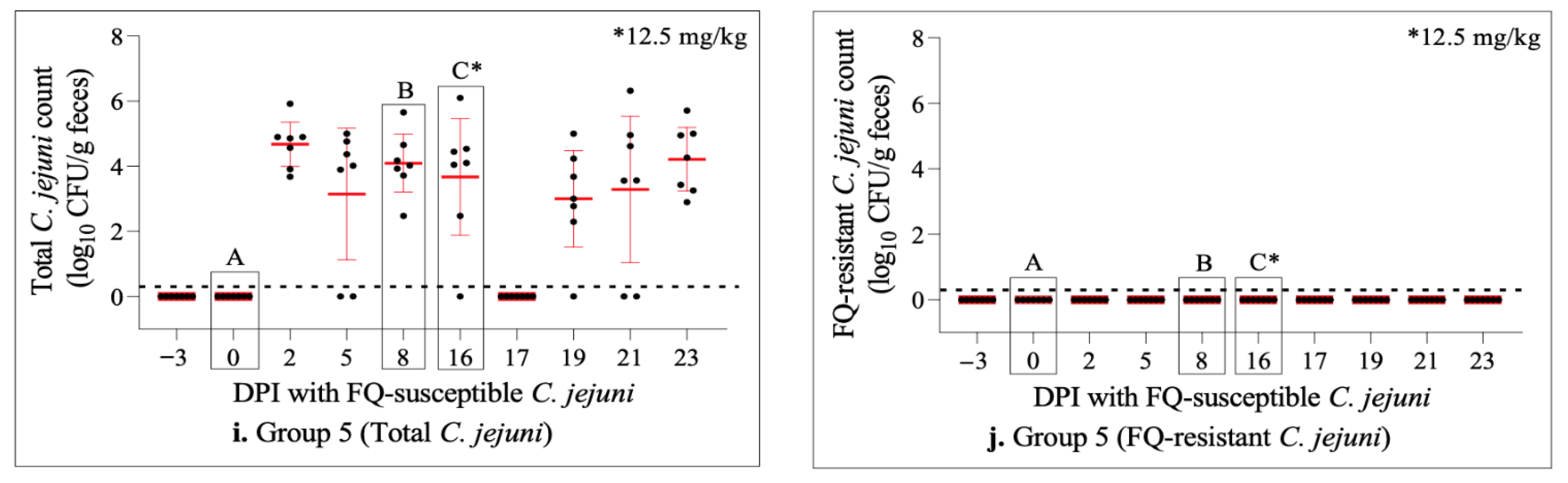
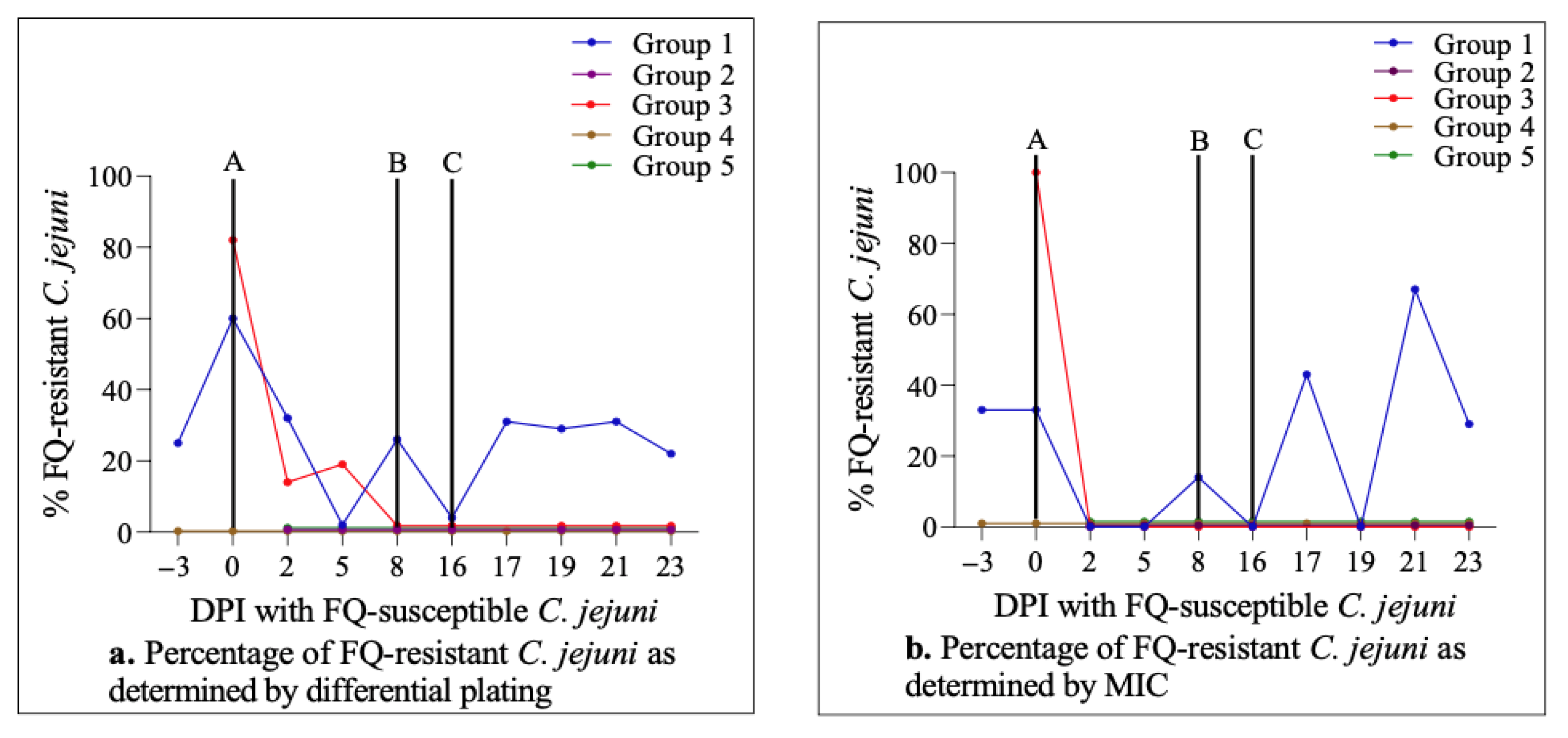
2.4. Enrofloxacin Treatment Did Not Induce FQ-Resistance Development in the Intestine of Calves Colonized with FQ-Susceptible C. jejuni
2.5. Antimicrobial Susceptibility Profiles of C. jejuni Strains from Calves
2.6. Dynamics of C. jejuni Population throughout the Study
3. Discussion
4. Materials and Methods
4.1. Animals and Study Design
4.2. Inoculation of C. jejuni
4.3. Inoculation with Mannheimia haemolytica
4.4. Enrofloxacin Injection
4.5. Collection of Fecal Samples
4.6. Bacterial Isolation and Identification
4.7. Pulsed-Field Gel Electrophoresis (PFGE)
4.8. Multilocus Sequence Typing (MLST)
4.9. Minimum Inhibitory Concentration (MIC) Determination
4.10. Necropsy
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geissler, A.L.; Carrillo, F.B.; Swanson, K.; Patrick, M.E.; Fullerton, K.E.; Bennett, C.; Barrett, K.; Mahon, B.E. Increasing Campylobacter infections, outbreaks, and antimicrobial resistance in the United States, 2004–2012. Clin. Infect. Dis. 2017, 65, 1624–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, M.C.; Vugia, D.J.; Shallow, S.; Marcus, R.; Segler, S.; McGivern, T.; Kassenborg, H.; Reilly, K.; Kennedy, M.; Angulo, F.; et al. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, Food Net 1996–1999. Clin. Infect. Dis. 2004, 38, S165–S174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef] [Green Version]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Skarp, C.; Hänninen, M.-L.; Rautelin, H. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Boysen, L.; Rosenquist, H.; Larsson, J.T.; Nielsen, E.M.; Sørensen, G.; Nordentoft, S.; Hald, T. Source attribution of human campylobacteriosis in Denmark. Epidemiol. Infect. 2013, 142, 1599–1608. [Google Scholar] [CrossRef] [Green Version]
- Thépault, A.; Rose, V.; Quesne, S.; Poezevara, T.; Béven, V.; Hirchaud, E.; Touzain, F.; Lucas, P.; Méric, G.; Mageiros, L.; et al. Ruminant and chicken: Important sources of campylobacteriosis in France despite a variation of source attribution in 2009 and 2015. Sci. Rep. 2018, 8, 9305. [Google Scholar] [CrossRef] [Green Version]
- De Haan, C.P.; Kivistö, R.I.; Hakkinen, M.; Corander, J.; Hänninen, M.-L. Multilocus sequence types of Finnish bovine Campylobacter jejuni isolates and their attribution to human infections. BMC Microbiol. 2010, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.M.; Balasegaram, S.; Willis, C.; Wimalarathna, H.M.L.; Maiden, M.C.; McCarthy, N.D. Partial failure of milk pasteurization as a risk for the transmission of Campylobacter from cattle to humans. Clin. Infect. Dis. 2015, 61, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.G.; Price, L.; Ahmed, R.; Woodward, D.L.; Melito, P.L.; Rodgers, F.G.; Jamieson, F.; Ciebin, B.; Li, A.; Ellis, A. Characterization of Waterborne Outbreak–associated Campylobacter jejuni, Walkerton, Ontario. Emerg. Infect. Dis. 2003, 9, 1232–1241. [Google Scholar] [CrossRef]
- Stanley, K.; Jones, K. Cattle and sheep farms as reservoirs of Campylobacter. J. Appl. Microbiol. 2003, 94, 104–113. [Google Scholar] [CrossRef]
- Krueger, N.A.; Anderson, R.C.; Krueger, W.K.; Horne, W.J.; Wesley, I.V.; Callaway, T.R.; Edrington, T.S.; Carstens, G.E.; Harvey, R.B.; Nisbet, D.J. Prevalence and concentration of Campylobacter in rumen contents and feces in pasture and feedlot-fed cattle. Foodborne Pathog. Dis. 2008, 5, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, K.; Wallace, J.; Currie, J.; Diggle, P.; Jones, K. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 1998, 85, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Wesley, I.V.; Wells, S.J.; Harmon, K.M.; Green, A.; Schroeder-Tucker, L.; Glover, M.; Siddique, I. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Appl. Environ. Microbiol. 2000, 66, 1994–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joens, L.A. Campylobacter and Helicobacter. In Pathogenesis of Bacterial Infections in Animals, 3rd ed.; Gyles, L., Prescott, J.F., Songer, J.G., Thoen, C.O., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2004; pp. 353–361. [Google Scholar]
- Englen, M.; Cray, P.; Ladely, S.; Dargatz, D. Antimicrobial resistance patterns of Campylobacter from feedlot cattle. J. Appl. Microbiol. 2005, 99, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englen, M.; Hill, A.; Dargatz, D.; Ladely, S.; Fedorka-Cray, P. Prevalence and antimicrobial resistance of Campylobacter in U.S. dairy cattle. J. Appl. Microbiol. 2006, 102, 1570–1577. [Google Scholar] [CrossRef]
- Tang, Y.; Sahin, O.; Pavlovic, N.; Lejeune, J.; Carlson, J.; Wu, Z.; Dai, L.; Zhang, Q. Rising fluoroquinolone resistance in Campylobacter isolated from feedlot cattle in the United States. Sci. Rep. 2017, 7, 494. [Google Scholar] [CrossRef] [Green Version]
- Acheson, D.; Allos, B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Sproston, E.L.; Wimalarathna, H.M.L.; Sheppard, S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018, 4, e000198. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Futur. Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef] [Green Version]
- Nickell, J.S.; White, B.J. Metaphylactic Antimicrobial therapy for bovine respiratory disease in stocker and feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Ives, S.E.; Richeson, J.T. Use of Antimicrobial metaphylaxis for the control of bovine respiratory disease in high-risk cattle. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, C.; Laudert, S.; Zimmermann, A. Metaphylactic treatment in undifferentiated bovine respiratory disease. Large Anim. Rev. 2002, 8, 37–44. [Google Scholar]
- USDA. Feedlot 2011 Part IV: Health and Health Management on U.S. Feedlots with a Capacity of 1000 or More Head #638.0913; USDA: Fort Collins, CO, USA, 2015.
- FDA. Extralabel Use and Antimicrobials. 2021. Available online: https://www.fda.gov/animal-veterinary/antimicrobial-resistance/extralabel-use-and-antimicrobials (accessed on 10 October 2021).
- Davis, J.L.; Smith, G.; Baynes, R.E.; Tell, L.A.; Webb, A.I.; Riviere, J.E. Update on drugs prohibited from extralabel use in food animals. J. Am. Vet. Med. Assoc. 2009, 235, 528–534. [Google Scholar] [CrossRef]
- McKellar, Q.; Gibson, I.; Monteiro, A.; Bregante, M. Pharmacokinetics of enrofloxacin and danofloxacin in plasma, inflammatory exudate, and bronchial secretions of calves following subcutaneous administration. Antimicrob. Agents Chemother. 1999, 43, 1988–1992. [Google Scholar] [CrossRef] [Green Version]
- TerHune, T.N.; Skogerboe, T.L.; Shostrom, V.K.; Weigel, D.J. Comparison of pharmacokinetics of danofloxacin and enrofloxacin in calves challenged with Mannheimia haemolytica. Am. J. Vet. Res. 2005, 66, 342–349. [Google Scholar] [CrossRef]
- Bayer HealthCare LLC Animal Health Division. BAYTRIL-Enrofloxacin Injection, Solution. 2021. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=401d2b8c-872b-4ffc-b100-22663b0f6bbb (accessed on 5 October 2021).
- Ocejo, M.; Oporto, B.; Hurtado, A. Occurrence of Campylobacter jejuni and Campylobacter coli in cattle and sheep in Northern Spain and changes in antimicrobial resistance in two studies 10-years apart. Pathogens 2019, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Oporto, B.; Esteban, J.; Aduriz, G.; Juste, R.; Hurtado, A. Prevalence and strain diversity of thermophilic campylobacters in cattle, sheep and swine farms. J. Appl. Microbiol. 2007, 103, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Haruna, M.; Sasaki, Y.; Murakami, M.; Mori, T.; Asai, T.; Ito, K.; Yamada, Y. Prevalence and antimicrobial resistance of Campylobacter isolates from beef cattle and pigs in Japan. J. Vet. Med. Sci. 2013, 75, 625–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanad, Y.M.; Kassem, I.I.; Abley, M.; Gebreyes, W.; LeJeune, J.T.; Rajashekara, G. Genotypic and phenotypic properties of cattle-associated Campylobacter and their implications to public health in the USA. PLoS ONE 2011, 6, e25778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulart, D.B.; Beyi, A.F.; Wu, Z.; Adiguzel, M.C.; Schroeder, A.; Singh, K.; Xu, C.; Ocal, M.M.; Dewell, R.; Dewell, G.A.; et al. Effect of danofloxacin treatment on the development of fluoroquinolone resistance in Campylobacter jejuni in cattle. Antibiotics 2022, 11, 531. [Google Scholar] [CrossRef]
- Love, W.J.; Lehenbauer, T.W.; Kass, P.H.; Van Eenennaam, A.; Aly, S.S. Development of a novel clinical scoring system for on-farm diagnosis of bovine respiratory disease in pre-weaned dairy calves. PeerJ 2014, 2, e238. [Google Scholar] [CrossRef] [Green Version]
- Lhermie, G.; Ferran, A.A.; Assié, S.; Cassard, H.; El Garch, F.; Schneider, M.; Woerhlé, F.; Pacalin, D.; Delverdier, M.; Bousquet-Mélou, A.; et al. Impact of yiming and dosage of a fluoroquinolone treatment on the microbiological, pathological, and clinical outcomes of calves challenged with Mannheimia haemolytica. Front. Microbiol. 2016, 7, 237. [Google Scholar] [CrossRef] [Green Version]
- Hurd, H.S.; Vaughn, M.B.; Holtkamp, D.; Dickson, J.; Warnick, L. Quantitative risk from fluoroquinolone-resistant Salmonella and Campylobacter due to treatment of dairy heifers with enrofloxacin for bovine respiratory disease. Foodborne Pathog. Dis. 2010, 7, 1305–1322. [Google Scholar] [CrossRef] [Green Version]
- Cha, W.; Mosci, R.E.; Wengert, S.L.; Vargas, C.V.; Rust, S.R.; Bartlett, P.C.; Grooms, D.L.; Manning, S.D. Comparing the genetic diversity and antimicrobial resistance profiles of Campylobacter jejuni recovered from cattle and humans. Front. Microbiol. 2017, 8, 818. [Google Scholar] [CrossRef] [Green Version]
- Beyi, A.F.; Mochel, J.P.; Magnin, G.; Hawbecker, T.; Slagel, C.; Dewell, G.; Dewell, R.; Sahin, O.; Coetzee, J.F.; Zhang, Q.; et al. Comparisons of plasma and fecal pharmacokinetics of danofloxacin and enrofloxacin in healthy and Mannheimia haemolytica infected calves. Sci. Rep. 2022, 12, 5107. [Google Scholar] [CrossRef]
- Smith, A.B.; Renter, D.G.; Cernicchiaro, N.; Shi, X.; Nickell, J.S.; Keil, D.J.; Nagaraja, T. A Randomized trial to assess the effect of fluoroquinolone metaphylaxis on the fecal prevalence and quinolone susceptibilities of Salmonella and Campylobacter in Feedlot Cattle. Foodborne Pathog. Dis. 2017, 14, 600–607. [Google Scholar] [CrossRef]
- Humphrey, T.J.; Jørgensen, F.; Frost, J.A.; Wadda, H.; Domingue, G.; Elviss, N.C.; Griggs, D.J.; Piddock, L.J.V. Prevalence and subtypes of ciprofloxacin-resistant Campylobacter spp. in commercial poultry flocks before, during, and after treatment with fluoroquinolones. Antimicrob. Agents Chemother. 2005, 49, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Sahin, O.; Lin, J.; Michel, L.O.; Zhang, Q. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob. Agents Chemother. 2003, 47, 390–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Boven, M.; Veldman, K.T.; de Jong, M.C.M.; Mevius, D.J. Rapid selection of quinolone resistance in Campylobacter jejuni but not in Escherichia coli in individually housed broilers. J. Antimicrob. Chemother. 2003, 52, 719–723. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Bodeis, S.M.; English, L.L.; White, D.G.; Walker, R.D.; Zhao, S.; Simjee, S.; Wagner, D.D. Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J. Infect. Dis. 2002, 185, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Griggs, D.J.; Johnson, M.M.; Frost, J.A.; Humphrey, T.; Jørgensen, F.; Piddock, L.J.V. Incidence and mechanism of ciprofloxacin resistance in Campylobacter spp. isolated from commercial poultry flocks in the United Kingdom before, during, and after fluoroquinolone treatment. Antimicrob. Agents Chemother. 2005, 49, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Sahin, O.; Kassem, I.I.; Shen, Z.; Lin, J.; Rajashekara, G.; Zhang, Q. Campylobacter in poultry: Ecology and potential interventions. Avian Dis. 2015, 59, 185–200. [Google Scholar] [CrossRef]
- Nielsen, E. Occurrence and strain diversity of thermophilic campylobacters in cattle of different age groups in dairy herds. Lett. Appl. Microbiol. 2002, 35, 85–89. [Google Scholar] [CrossRef]
- Han, J.; Sahin, O.; Barton, Y.-W.; Zhang, Q. Key Role of Mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLOS Pathog. 2008, 4, e1000083. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Sahin, O.; Lin, J.; Zhang, Q. Role of the CmeABC efflux pump in the emergence of fluoroquinolone-resistant Campylobacter under selection pressure. J. Antimicrob. Chemother. 2006, 58, 1154–1159. [Google Scholar] [CrossRef]
- Farnell, M.; Donoghue, A.; Cole, K.; Reyes-Herrera, I.; Blore, P.; Donoghue, D. Campylobacter susceptibility to ciprofloxacin and corresponding fluoroquinolone concentrations within the gastrointestinal tracts of chickens. J. Appl. Microbiol. 2005, 99, 1043–1050. [Google Scholar] [CrossRef]
- Drlica, K.; Zhao, X. Mutant selection window hypothesis updated. Clin. Infect. Dis. 2007, 44, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.M.; Jacob, M.E.; Theriot, C.M.; Callahan, B.J.; Prange, T.; Papich, M.; Foster, D.M. Dosing regimen of enrofloxacin impacts intestinal pharmacokinetics and the fecal microbiota in steers. Front. Microbiol. 2018, 9, 2190. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.S.L.; Birtles, A.; Bolton, F.J.; French, N.P.; Robinson, S.E.; Newbold, L.S.; Upton, M.; Fox, A.J. Longitudinal study of the molecular epidemiology of Campylobacter jejuni in cattle on dairy farms. Appl. Environ. Microbiol. 2008, 74, 3626–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thépault, A.; Poezevara, T.; Quesne, S.; Rose, V.; Chemaly, M.; Rivoal, K. Prevalence of thermophilic Campylobacter in cattle production at slaughterhouse level in France and link between C. jejuni bovine strains and campylobacteriosis. Front. Microbiol. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Meinersmann, R.J.; Sahin, O.; Wu, Z.; Dai, L.; Carlson, J.; Lawrence, J.P.; Genzlinger, L.; LeJeune, J.T.; Zhang, Q. Wide but variable distribution of a hypervirulent Campylobacter jejuni clone in beef and dairy cattle in the United States. Appl. Environ. Microbiol. 2017, 83, e01425-17. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Wang, Y.; Sahin, O.; Shen, Z.; Guo, B.; Shen, J.; Zhang, Q. A Fluoroquinolone resistance associated mutation in gyrA affects DNA supercoiling in Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2012, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Hanthorn, C.J.; Dewell, R.D.; Cooper, V.L.; Frana, T.S.; Plummer, P.J.; Wang, C.; Dewell, G.A. Randomized clinical trial to evaluate the pathogenicity of Bibersteinia trehalosi in respiratory disease among calves. BMC Vet. Res. 2014, 10, 89. [Google Scholar] [CrossRef]
| Groups | Inoculation with FQ-S C. jejuni * | Challenge with M. haemolytica # | Enrofloxacin Injection (Dose mg/kg) § |
|---|---|---|---|
| 1 | Yes | No | No |
| 2 | Yes | No | Yes (7.5) |
| 3 | Yes | No | Yes (12.5) |
| 4 | Yes | Yes | Yes (7.5) |
| 5 | Yes | Yes | Yes (12.5) |
| Pre-Inoculation (DPI –3 and 0), n = 3 | Post-Inoculation (DPI 2–16), n = 85 | Post-Injection (DPI 17–23), n = 77 | ||||||
|---|---|---|---|---|---|---|---|---|
| Genotype * | CIP § | MIC * | Genotype | CIP | MIC | Genotype | CIP | MIC |
| a (0) | --- | --- | a (43) | S | 0.12 (32); 0.25 (11) | a (3) | S | 0.12 (3); |
| b (0) | --- | --- | b (1) | S | 0.12 (1) | b (3) | S | 0.12 (2); 0.5 (1) |
| c (0) | --- | --- | c (20) | S | 0.015 (1); 0.12 (12); 0.25 (6); 0.5 (1) | c (30) | S | 0.06 (2); 0.12 (21); 0.25 (5); 0.5 (2); |
| d (0) | --- | --- | d (0) | --- | --- | d (0) | --- | --- |
| e (0) | --- | --- | e (0) | --- | --- | e (0) | --- | --- |
| f (0) | --- | --- | f (0) | --- | --- | f (0) | --- | --- |
| g (0) | --- | --- | g (0) | --- | --- | g (0) | --- | --- |
| h (1) | R | 8 (1) | h (0) | --- | --- | h (0) | --- | --- |
| i (0) | --- | --- | i (0) | --- | --- | i (1) | S | 0.12 (1) |
| j (2) ¶ | S | 0.12 (2) | j (7) | S | 0.06 (2); 0.12 (5) | j (24) | S | 0.015 (1); 0.06 (10); 0.12 (12); 0.25 (1) |
| k (0) ¶ | --- | --- | k (14) | S | 0.12 (10); 0.25 (3); 2 (1) | k (16) | S | 0.12 (12); 0.25 (4) |
| Pre-Inoculation (DPI –3 and 0), n = 6 | Post-Inoculation (DPI 2–23), n = 46 | ||||
|---|---|---|---|---|---|
| Genotype * | CIP § | MIC * | Genotype | CIP | MIC |
| a (0) | --- | --- | a (17) | S/R | 0.12 (13); 0.25 (1); 4 (1); 8 (2) |
| b (0) | --- | --- | b (2) | S | 0.06 (1); 0.12 (1) |
| c (0) | --- | --- | c (13) | S/R | 0.12 (10); 0.25 (2); 4 (1) |
| d (2) | S | 0.06 (2) | d (2) | S | 0.12 (1); 0.25 (1) |
| e (2) | R | 4 (1); 8 (1) | e (5) | R | 4 (1); 8 (4) |
| f (2) | S | 0.06 (1); 0.12 (1) | f (0) | --- | --- |
| g (0) | --- | --- | g (1) | R | 8 (1) |
| h (0) | --- | --- | h (1) | S | 0.25 (1) |
| i (0) | --- | --- | i (0) | --- | --- |
| j (0) ¶ | --- | --- | j (3) | S | 0.06 (3) |
| k (0) ¶ | --- | --- | k (2) | S | 0.12 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulart, D.B.; Beyi, A.F.; Wu, Z.; Adiguzel, M.C.; Wilson, S.; Xu, C.; Pang, J.; Dewell, R.; Dewell, G.A.; Plummer, P.J.; et al. Influence of Single Dose Enrofloxacin Injection on Development of Fluoroquinolone Resistance in Campylobacter jejuni in Calves. Antibiotics 2022, 11, 1407. https://doi.org/10.3390/antibiotics11101407
Goulart DB, Beyi AF, Wu Z, Adiguzel MC, Wilson S, Xu C, Pang J, Dewell R, Dewell GA, Plummer PJ, et al. Influence of Single Dose Enrofloxacin Injection on Development of Fluoroquinolone Resistance in Campylobacter jejuni in Calves. Antibiotics. 2022; 11(10):1407. https://doi.org/10.3390/antibiotics11101407
Chicago/Turabian StyleGoulart, Debora Brito, Ashenafi Feyisa Beyi, Zuowei Wu, Mehmet Cemal Adiguzel, Samantha Wilson, Changyun Xu, Jinji Pang, Renee Dewell, Grant A. Dewell, Paul J. Plummer, and et al. 2022. "Influence of Single Dose Enrofloxacin Injection on Development of Fluoroquinolone Resistance in Campylobacter jejuni in Calves" Antibiotics 11, no. 10: 1407. https://doi.org/10.3390/antibiotics11101407
APA StyleGoulart, D. B., Beyi, A. F., Wu, Z., Adiguzel, M. C., Wilson, S., Xu, C., Pang, J., Dewell, R., Dewell, G. A., Plummer, P. J., Zhang, Q., & Sahin, O. (2022). Influence of Single Dose Enrofloxacin Injection on Development of Fluoroquinolone Resistance in Campylobacter jejuni in Calves. Antibiotics, 11(10), 1407. https://doi.org/10.3390/antibiotics11101407






