CcpA Regulates Staphylococcus aureus Biofilm Formation through Direct Repression of Staphylokinase Expression
Abstract
1. Introduction
2. Results
2.1. Deletion of ccpA Impairs Biofilm Formation of S. aureus Strain XN108
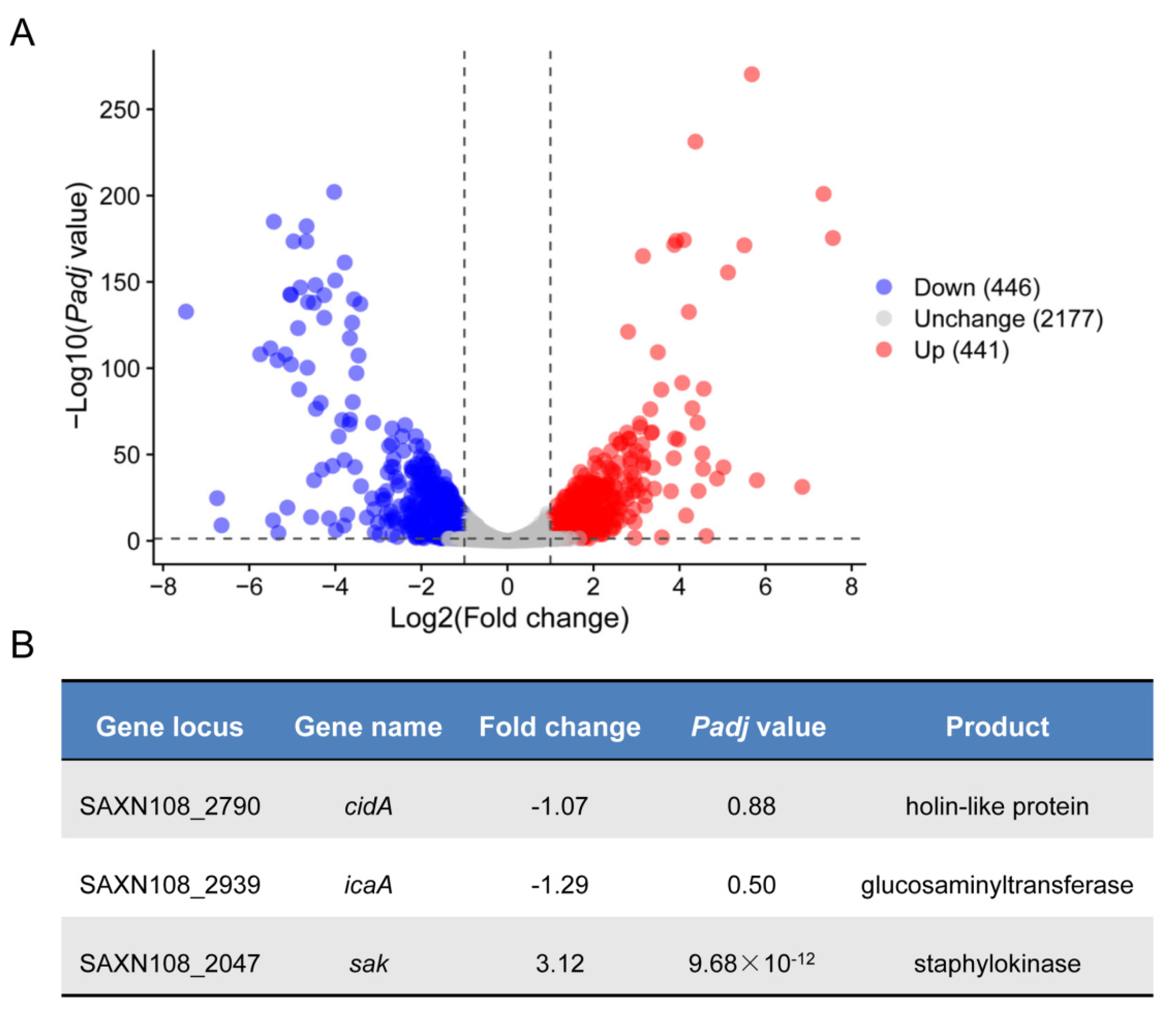
2.2. Transcriptomic Analysis Reveals Potential CcpA Regulon in S. aureus Strain XN108
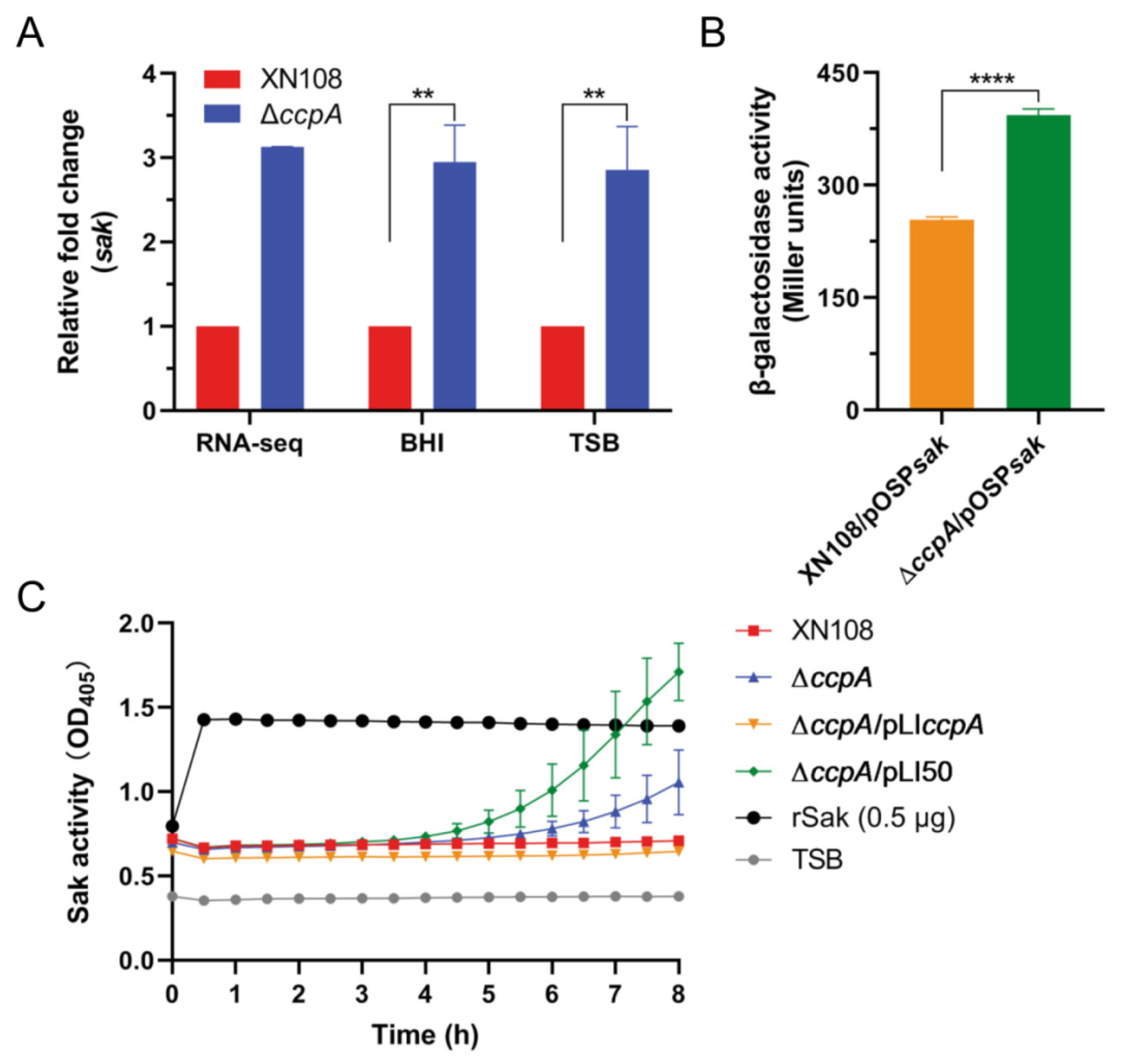
2.3. Deletion of ccpA Promotes the Sak Production in S. aureus Strain XN108
2.4. CcpA Specifically Binds to the Promoter Region of Sak
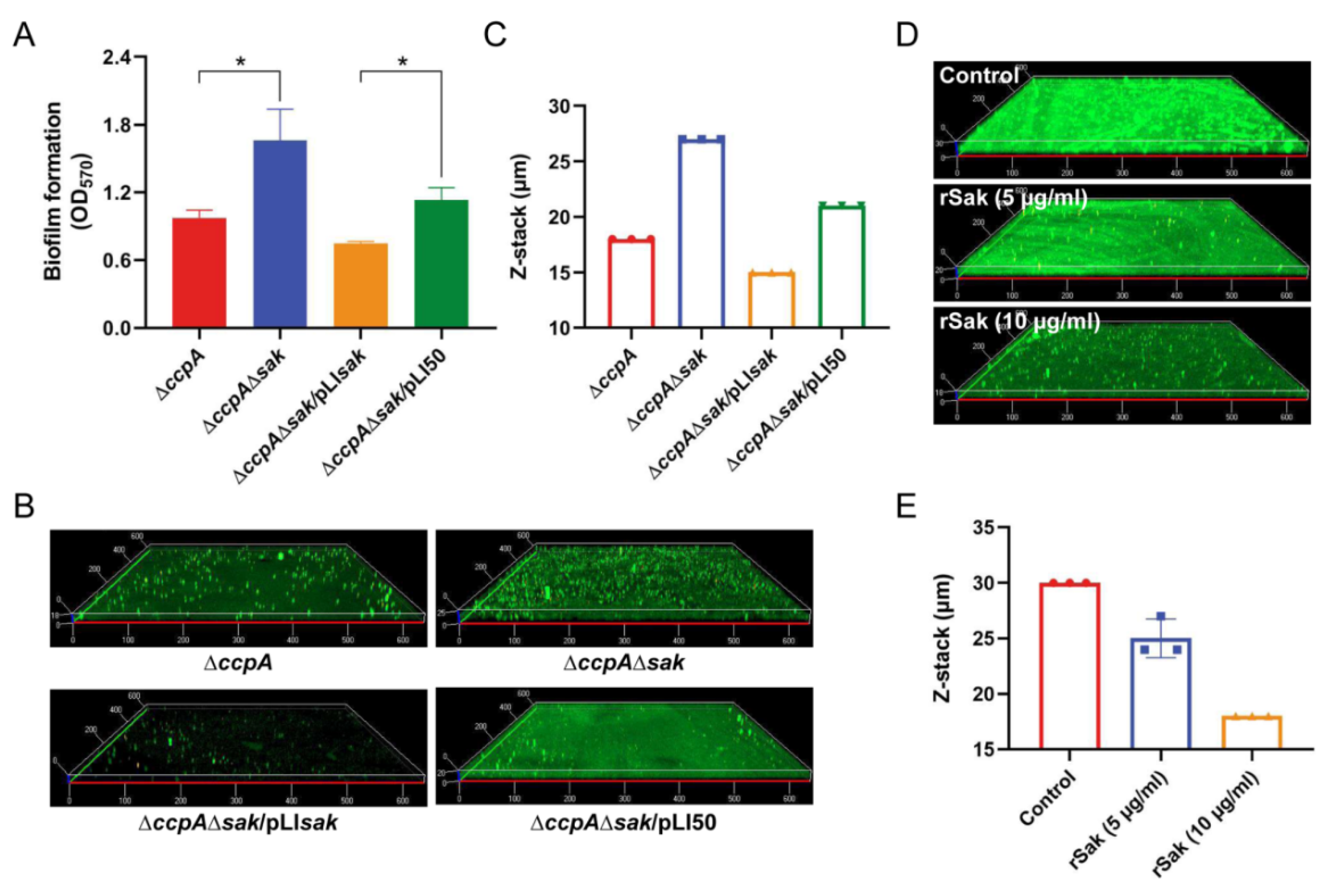
2.5. CcpA-Controlled Biofilm Is Involved in Direct Repression of Sak Production
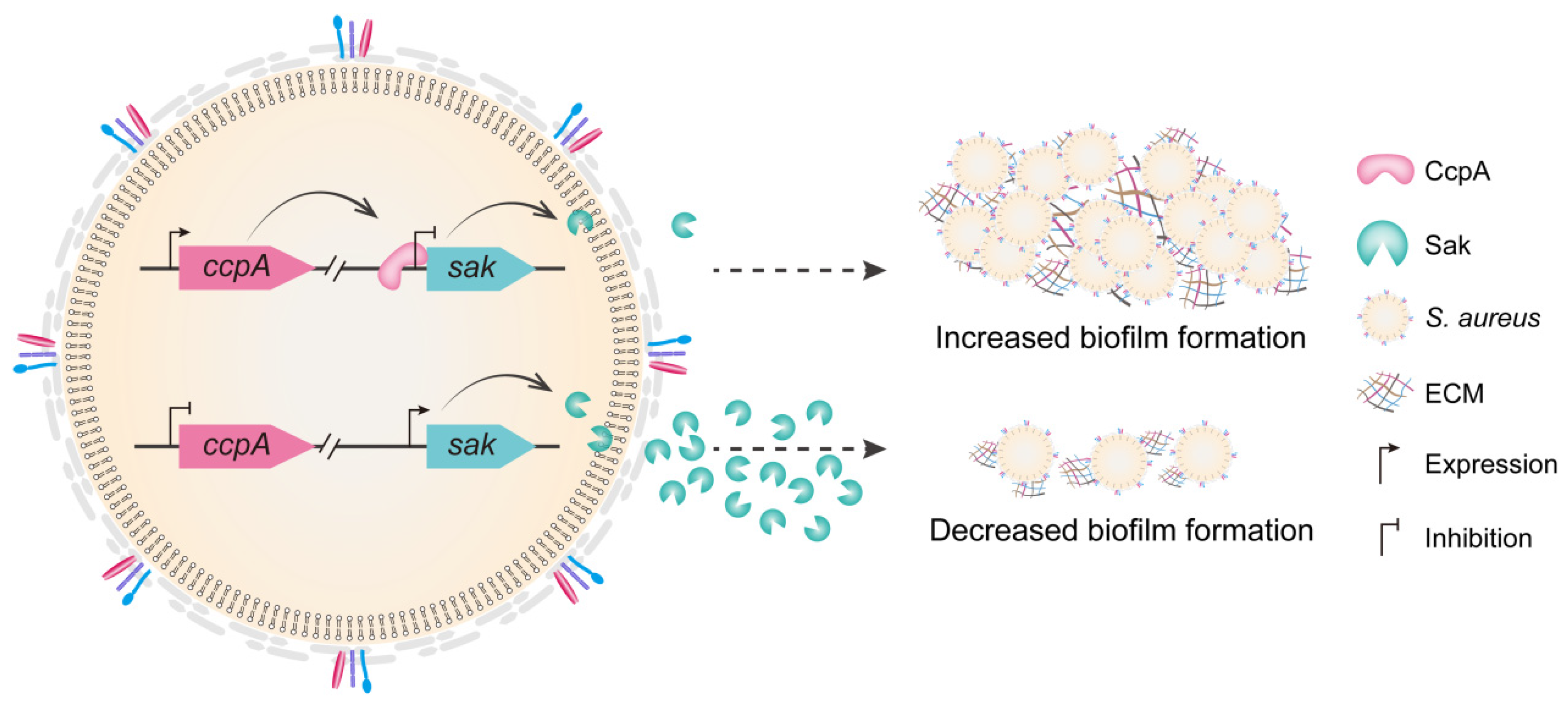
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Growth Conditions
4.2. Construction of Gene Deletion Mutants and Complemented Strains in S. aureus
4.3. S. aureus Growth Profiling
4.4. Biofilm Formation
4.5. Confocal Laser Scanning Microscopy (CLSM)
4.6. Total Cellular RNA Isolation and RNA-seq
4.7. RT-qPCR Analysis
4.8. β-Galactosidase Activity Assay
4.9. Measurement of Sak Secretion
4.10. Electrophoretic Mobility Shift Assay (EMSA)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Kranjec, C.; Morales Angeles, D.; Torrissen Marli, M.; Fernandez, L.; Garcia, P.; Kjos, M.; Diep, D.B. Staphylococcal Biofilms: Challenges and Novel Therapeutic Perspectives. Antibiotics 2021, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Amod, A.; Pandey, P.; Bose, P.; Pingali, M.S.; Shivalkar, S.; Varadwaj, P.K.; Sahoo, A.K.; Samanta, S.K. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 2022, 17, 022003. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Zhou, S.; Rao, Y.; Li, J.; Huang, Q.; Rao, X. Staphylococcus aureus small-colony variants: Formation, infection, and treatment. Microbiol. Res. 2022, 260, 127040. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ji, Y. Environmental factors modulate biofilm formation by Staphylococcus aureus. Sci. Prog. 2020, 103, 36850419898659. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Seki, M.; Suzuki, Y.; Kinjo, Y.; Mizunoe, Y.; Sugimoto, S. Staphylococcus aureus utilizes environmental RNA as a building material in specific polysaccharide-dependent biofilms. NPJ Biofilms Microbiomes 2022, 8, 17. [Google Scholar] [CrossRef]
- Warner, J.B.; Lolkema, J.S. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Stulke, J.; Hillen, W. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 2000, 54, 849–880. [Google Scholar] [CrossRef] [PubMed]
- Troitzsch, A.; Loi, V.V.; Methling, K.; Zuhlke, D.; Lalk, M.; Riedel, K.; Bernhardt, J.; Elsayed, E.M.; Bange, G.; Antelmann, H.; et al. Carbon Source-Dependent Reprogramming of Anaerobic Metabolism in Staphylococcus aureus. J. Bacteriol. 2021, 203, e00639-20. [Google Scholar] [CrossRef] [PubMed]
- Rudra, P.; Boyd, J.M. Metabolic control of virulence factor production in Staphylococcus aureus. Curr. Opin. Microbiol. 2020, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Seidl, K.; Goerke, C.; Wolz, C.; Mack, D.; Berger-Bachi, B.; Bischoff, M. Staphylococcus aureus CcpA affects biofilm formation. Infect. Immun. 2008, 76, 2044–2050. [Google Scholar] [CrossRef]
- Sadykov, M.R.; Windham, I.H.; Widhelm, T.J.; Yajjala, V.K.; Watson, S.M.; Endres, J.L.; Bavari, A.I.; Thomas, V.C.; Bose, J.L.; Bayles, K.W. CidR and CcpA Synergistically Regulate Staphylococcus aureus cidABC Expression. J. Bacteriol. 2019, 201, e00371-19. [Google Scholar] [CrossRef]
- Seidl, K.; Stucki, M.; Ruegg, M.; Goerke, C.; Wolz, C.; Harris, L.; Berger-Bachi, B.; Bischoff, M. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 2006, 50, 1183–1194. [Google Scholar] [CrossRef]
- Bronesky, D.; Desgranges, E.; Corvaglia, A.; Francois, P.; Caballero, C.J.; Prado, L.; Toledo-Arana, A.; Lasa, I.; Moreau, K.; Vandenesch, F.; et al. A multifaceted small RNA modulates gene expression upon glucose limitation in Staphylococcus aureus. EMBO J. 2019, 38, e99363. [Google Scholar] [CrossRef]
- Nedaeinia, R.; Faraji, H.; Javanmard, S.H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Goli, M.; Mashkani, B.; Nedaeinia, M.; Haghighi, M.H.H.; Ranjbar, M. Bacterial staphylokinase as a promising third-generation drug in the treatment for vascular occlusion. Mol. Biol. Rep. 2020, 47, 819–841. [Google Scholar] [CrossRef]
- Vakili, B.; Nezafat, N.; Negahdaripour, M.; Yari, M.; Zare, B.; Ghasemi, Y. Staphylokinase Enzyme, An Overview of Structure, Function and Engineered Forms. Curr. Pharm. Biotechnol. 2017, 18, 1026–1037. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Yuan, W.; Shang, W.; Cheng, H.; Yuan, J.; Zhu, J.; Hu, Z.; Li, S.; Chen, W.; et al. First report of a sequence type 239 vancomycin-intermediate Staphylococcus aureus isolate in Mainland China. Diagn. Microbiol. Infect. Dis. 2013, 77, 64–68. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yuan, W.; Hu, Q.; Shang, W.; Hu, X.; Tong, Y.; Rao, X. Complete Genome Sequence of Staphylococcus aureus XN108, an ST239-MRSA-SCCmec III Strain with Intermediate Vancomycin Resistance Isolated in Mainland China. Genome Announc. 2014, 2, e00449-14. [Google Scholar] [CrossRef] [PubMed]
- Patzold, L.; Brausch, A.C.; Bielefeld, E.L.; Zimmer, L.; Somerville, G.A.; Bischoff, M.; Gaupp, R. Impact of the Histidine-Containing Phosphocarrier Protein HPr on Carbon Metabolism and Virulence in Staphylococcus aureus. Microorganisms 2021, 9, 466. [Google Scholar] [CrossRef]
- Seidl, K.; Muller, S.; Francois, P.; Kriebitzsch, C.; Schrenzel, J.; Engelmann, S.; Bischoff, M.; Berger-Bachi, B. Effect of a glucose impulse on the CcpA regulon in Staphylococcus aureus. BMC Microbiol. 2009, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Seidl, K.; Bischoff, M.; Berger-Bachi, B. CcpA mediates the catabolite repression of tst in Staphylococcus aureus. Infect. Immun. 2008, 76, 5093–5099. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Tsunemoto, H.; Seif, Y.; Sastry, A.V.; Szubin, R.; Xu, S.; Machado, H.; Olson, C.A.; Anand, A.; Pogliano, J.; et al. Revealing 29 sets of independently modulated genes in Staphylococcus aureus, their regulators, and role in key physiological response. Proc. Natl. Acad. Sci. USA 2020, 117, 17228–17239. [Google Scholar] [CrossRef] [PubMed]
- Bulock, L.L.; Ahn, J.; Shinde, D.; Pandey, S.; Sarmiento, C.; Thomas, V.C.; Guda, C.; Bayles, K.W.; Sadykov, M.R. Interplay of CodY and CcpA in Regulating Central Metabolism and Biofilm Formation in Staphylococcus aureus. J. Bacteriol. 2022, 204, e0061721. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Peetermans, M.; Liesenborghs, L.; Na, M.L.; Bjornsdottir, H.; Zhu, X.F.; Jacobsson, G.; Johansson, B.R.; Geoghegan, J.A.; Foster, T.J.; et al. Staphylokinase Control of Staphylococcus aureus Biofilm Formation and Detachment Through Host Plasminogen Activation. J. Infect. Dis. 2016, 213, 139–148. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Sun, Y.; Zhang, X.; Lu, H.; Li, J.; Cao, J.; Zhou, T. Characterization of the mechanism and impact of staphylokinase on the formation of Candida albicans and Staphylococcus aureus polymicrobial biofilms. J. Med. Microbiol. 2019, 68, 355–367. [Google Scholar] [CrossRef]
- Miwa, Y.; Nakata, A.; Ogiwara, A.; Yamamoto, M.; Fujita, Y. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 2000, 28, 1206–1210. [Google Scholar] [CrossRef]
- Mohammed, Y.H.E.; Manukumar, H.M.; Rakesh, K.P.; Karthik, C.S.; Mallu, P.; Qin, H.L. Vision for medicine: Staphylococcus aureus biofilm war and unlocking key’s for anti-biofilm drug development. Microb. Pathog. 2018, 123, 339–347. [Google Scholar] [CrossRef]
- Halsey, C.R.; Lei, S.; Wax, J.K.; Lehman, M.K.; Nuxoll, A.S.; Steinke, L.; Sadykov, M.; Powers, R.; Fey, P.D. Amino Acid Catabolism in Staphylococcus aureus and the Function of Carbon Catabolite Repression. mBio 2017, 8, e01434-16. [Google Scholar] [CrossRef]
- Yang, Y.P.; Zhang, L.; Huang, H.; Yang, C.; Yang, S.; Gu, Y.; Jiang, W.H. A Flexible Binding Site Architecture Provides New Insights into CcpA Global Regulation in Gram-Positive Bacteria. mBio 2017, 8, e02004-16. [Google Scholar] [CrossRef]
- Leiba, J.; Hartmann, T.; Cluzel, M.E.; Cohen-Gonsaud, M.; Delolme, F.; Bischoff, M.; Molle, V. A novel mode of regulation of the Staphylococcus aureus catabolite control protein A (CcpA) mediated by Stk1 protein phosphorylation. J. Biol. Chem. 2012, 287, 43607–43619. [Google Scholar] [CrossRef]
- DebRoy, S.; Saldana, M.; Travisany, D.; Montano, A.; Galloway-Pena, J.; Horstmann, N.; Yao, H.; Gonzalez, M.; Maass, A.; Latorre, M.; et al. A Multi-Serotype Approach Clarifies the Catabolite Control Protein A Regulon in the Major Human Pathogen Group A Streptococcus. Sci. Rep. 2016, 6, 32442. [Google Scholar] [CrossRef][Green Version]
- Willenborg, J.; de Greeff, A.; Jarek, M.; Valentin-Weigand, P.; Goethe, R. The CcpA regulon of Streptococcus suis reveals novel insights into the regulation of the streptococcal central carbon metabolism by binding of CcpA to two distinct binding motifs. Mol. Microbiol. 2014, 92, 61–83. [Google Scholar] [CrossRef]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Liesenborghs, L.; Verhamme, P.; Vanassche, T. Staphylococcus aureus, master manipulator of the human hemostatic system. J. Thromb. Haemost. 2018, 16, 441–454. [Google Scholar] [CrossRef]
- Fang, B.; Liu, B.; Sun, B. Transcriptional regulation of virulence factors Hla and phenol-soluble modulins α by AraC-type regulator Rbf in Staphylococcus aureus. Int. J. Med. Microbiol. 2020, 310, 151436. [Google Scholar] [CrossRef]
- Shang, W.; Rao, Y.; Zheng, Y.; Yang, Y.; Hu, Q.; Hu, Z.; Yuan, J.; Peng, H.; Xiong, K.; Tan, L.; et al. β-Lactam Antibiotics Enhance the Pathogenicity of Methicillin-Resistant Staphylococcus aureus via SarA-Controlled Lipoprotein-Like Cluster Expression. mBio 2019, 10, e00880-19. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Peng, H.; Shang, W.; Hu, Z.; Yang, Y.; Tan, L.; Li, M.; Zhou, R.; Rao, X. A vancomycin resistance-associated WalK(S221P) mutation attenuates the virulence of vancomycin-intermediate Staphylococcus aureus. J. Adv. Res. 2022, 40, 167–178. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Tran, P.M.; Feiss, M.; Kinney, K.J.; Salgado-Pabon, W. ΦSa3mw Prophage as a Molecular Regulatory Switch of Staphylococcus aureus β-Toxin Production. J. Bacteriol. 2019, 201, e00766-18. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shen, M.; Yang, Y.; Le, S.; Li, M.; Wang, J.; Zhao, Y.; Tan, Y.; Hu, F.; Lu, S. Adaptation of Pseudomonas aeruginosa to Phage PaP1 Predation via O-Antigen Polymerase Mutation. Front. Microbiol. 2018, 9, 1170. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Jacobsson, G.; Karlsson, M.; Zhu, X.; Wang, W.; Bremell, T.; Josefsson, E.; Jin, T. Staphylokinase promotes the establishment of Staphylococcus aureus skin infections while decreasing disease severity. J. Infect. Dis. 2013, 208, 990–999. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Zhu, K.; Peng, H.; Shang, W.; Zhao, Y.; Lu, S.; Rao, X.; Li, M.; Zhou, R.; Li, G. CcpA Regulates Staphylococcus aureus Biofilm Formation through Direct Repression of Staphylokinase Expression. Antibiotics 2022, 11, 1426. https://doi.org/10.3390/antibiotics11101426
Zheng M, Zhu K, Peng H, Shang W, Zhao Y, Lu S, Rao X, Li M, Zhou R, Li G. CcpA Regulates Staphylococcus aureus Biofilm Formation through Direct Repression of Staphylokinase Expression. Antibiotics. 2022; 11(10):1426. https://doi.org/10.3390/antibiotics11101426
Chicago/Turabian StyleZheng, Mingxia, Keting Zhu, Huagang Peng, Weilong Shang, Yan Zhao, Shuguang Lu, Xiancai Rao, Ming Li, Renjie Zhou, and Gang Li. 2022. "CcpA Regulates Staphylococcus aureus Biofilm Formation through Direct Repression of Staphylokinase Expression" Antibiotics 11, no. 10: 1426. https://doi.org/10.3390/antibiotics11101426
APA StyleZheng, M., Zhu, K., Peng, H., Shang, W., Zhao, Y., Lu, S., Rao, X., Li, M., Zhou, R., & Li, G. (2022). CcpA Regulates Staphylococcus aureus Biofilm Formation through Direct Repression of Staphylokinase Expression. Antibiotics, 11(10), 1426. https://doi.org/10.3390/antibiotics11101426








