Genomic Characterization of Colistin-Resistant Isolates from the King Fahad Medical City, Kingdom of Saudi Arabia
Abstract
:1. Introduction
2. Results
2.1. Clinical Bacterial Isolates
2.2. Antibiotic Susceptibility Testing (AST)
2.3. Genome Assembly Results
2.4. Investigation of Genes and Gene Mutations Associated with the Antibiotic Resistance Phenotype (The Resistome) from the WGS Data
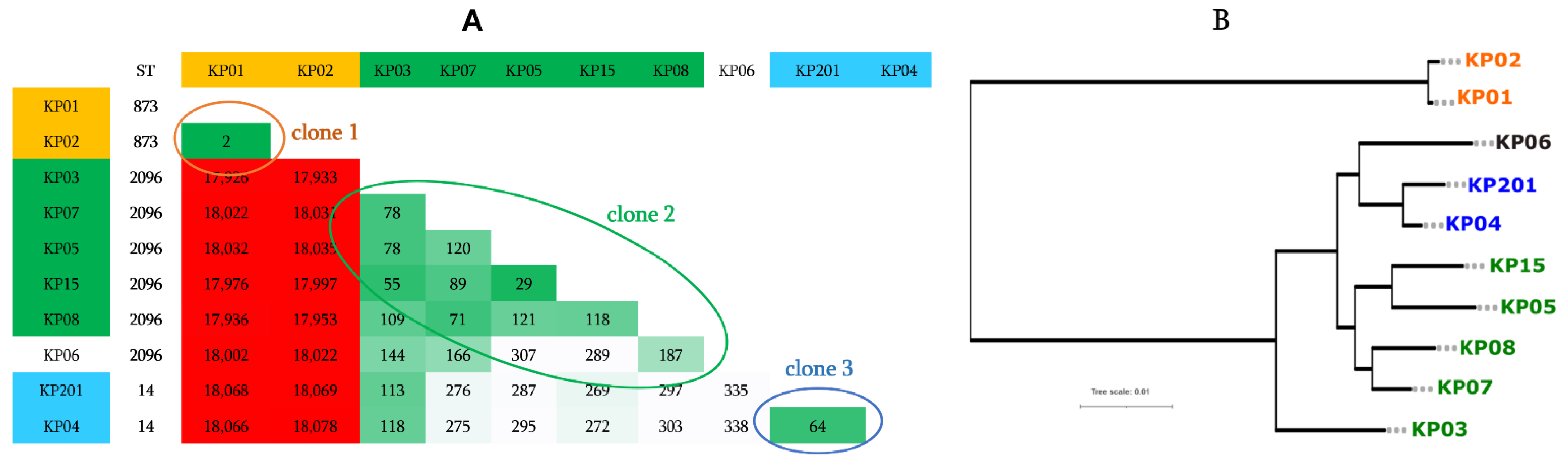
2.5. Genotype, Comparative Genomics, and Phylogenomic Relationship of Isolates
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lushniak, B.D. Antibiotic Resistance: A Public Health Crisis. Public Health Rep. 2014, 129, 314–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitout, J.D.D.; Nordmann, P.; Poirel, L. Carbapenemase-Producing Klebsiella Pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girmenia, C.; Serrao, A.; Canichella, M. Epidemiology of Carbapenem Resistant Klebsiella Pneumoniae Infections in Mediterranean Countries. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Brunel, J.-M.; Dubus, J.-C.; Reynaud-Gaubert, M.; Rolain, J.-M. Colistin: An Update on the Antibiotic of the 21st Century. Expert Rev. Anti-Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.G.; Langlykke, A.F. Antibiotic Activity of Bacillus Polymyxa. J. Bacteriol. 1947, 54, 24. [Google Scholar]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Baron, S.; Hadjadj, L.; Rolain, J.M.; Olaitan, A.O. Molecular Mechanisms of Polymyxin Resistance: Knowns and Unknowns. Int. J. Antimicrob. Agents 2016, 48, 583–591. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Li, J. Emergence of Polymyxin Resistance in Gram-Negative Bacteria. Int. J. Antimicrob. Agents 2016, 48, 581–582. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Xiao, Y. Prevalence and Transmission of Mobilized Colistin Resistance (Mcr) Gene in Bacteria Common to Animals and Humans. Biosaf. Heal. 2020, 2, 71–78. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Walsh, T.R.; Yi, L.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Y.; Chan, E.W.; Zhou, H.; Chen, S. Dissemination of the Mcr-1 Colistin Resistance Gene. Lancet. Infect. Dis. 2016, 16, 291–292. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel Plasmid-Mediated Colistin Resistance Gene Mcr-3 in Escherichia Coli. MBio 2017, 8, e00543-17. [Google Scholar] [CrossRef] [Green Version]
- Bradley, P.; Zhang, Q.; Shaw, L.P.; Iqbal, Z.; Jin, L.; Dorai-Schneiders, T.; Wang, Q.; van Dorp, L.; Rieux, A.; Wang, X.; et al. The Global Distribution and Spread of the Mobilized Colistin Resistance Gene Mcr-1. Nat. Commun. 2018, 9, 1–9. [Google Scholar]
- Somily, A.M.; Arshad, M.Z.; Garaween, G.A.; Senok, A.C. Phenotypic and Genotypic Characterization of Extended-Spectrum β-Lactamases Producing Escherichia Coli and Klebsiella Pneumoniae in a Tertiary Care Hospital in Riyadh, Saudi Arabia. Ann. Saudi Med. 2015, 35, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Diene, S.M.; Rolain, J.-M. Investigation of Antibiotic Resistance in the Genomic Era of Multidrug-Resistant Gram-Negative Bacilli, Especially Enterobacteriaceae, Pseudomonas and Acinetobacter. Expert Rev. Anti. Infect. Ther. 2013, 11, 277–296. [Google Scholar] [CrossRef]
- Aruhomukama, D.; Sserwadda, I.; Mboowa, G. Investigating Colistin Drug Resistance: The Role of High-Throughput Sequencing and Bioinformatics. F1000Research 2019, 8, 1–16. [Google Scholar] [CrossRef]
- Di Pilato, V.; Arena, F.; Tascini, C.; Cannatelli, A.; Henrici De Angelis, L.; Fortunato, S. Mcr-1.2, a New Mcr Variant Carried on a Transferable Plasmid from a Colistin-Resistant KPC Carbapenemase-Producing Klebsiella Pneumoniae Strain of Sequence Type 512. Antimicrob. Agents Chemother. 2016, 60, 5612–5615. [Google Scholar] [CrossRef] [Green Version]
- Cabello, F.C.; Godfrey, H.P. Comment on: Transferable Resistance to Colistin: A New but Old Threat. J. Antimicrob. Chemother. 2016, 72, 636–637. [Google Scholar] [CrossRef] [Green Version]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of Polymyxin Resistance: Acquired and Intrinsic Resistance in Bacteria. Front. Microbiol. 2014, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Gharaibeh, M.H.; Shatnawi, S.Q. An Overview of Colistin Resistance, Mobilized Colistin Resistance Genes Dissemination, Global Responses, and the Alternatives to Colistin: A Review. Vet. World 2019, 12, 1735–1746. [Google Scholar] [CrossRef] [Green Version]
- Kharaba, A. Prevalence and Outcomes of Colistin-Resistant Acinetobacter Infection in Saudi Critical Care Units. Saudi Crit. Care J. 2017, 1, 25. [Google Scholar] [CrossRef]
- Al Mayahi, Z.; Kamel, S.; Amer, H.; Beatty, M. Outbreak of Colistin-Resistant Organisms at a Tertiary Hospital in Riyadh, Saudi Arabia, 2016. Pan Afr. Med. J. 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Charretier, Y.; Diene, S.M.; Baud, D.; Chatellier, S.; Santiago-Allexant, E.; Van Belkum, A.; Guigon, G.; Schrenzel, J. Colistin Heteroresistance and Involvement of the PmrAB Regulatory System in Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2018, 62, e00788-18. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, S.M.; Brannon, M.K.; Dasgupta, N.; Pier, M.; Sgambati, N.; Miller, A.K.; Selgrade, S.E.; Miller, S.I.; Denton, M.; Conway, S.P.; et al. PmrB Mutations Promote Polymyxin Resistance of Pseudomonas Aeruginosa Isolated from Colistin-Treated Cystic Fibrosis Patients. Antimicrob. Agents Chemother. 2012, 56, 1019–1030. [Google Scholar] [CrossRef] [Green Version]
- Von Dach, E.; Diene, S.M.; Fankhauser, C.; Schrenzel, J.; Harbarth, S.; François, P. Comparative Genomics of Community-Associated Methicillin-Resistant Staphylococcus Aureus Shows the Emergence of Clone ST8-USA300 in Geneva, Switzerland. J. Infect. Dis. 2016, 213, 1370–1379. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamad, A.; Pal, T.; Leskafi, H.; Abbas, H.; Hejles, H.; Alsubikhy, F.; Darwish, D.; Ghazawi, A.; Sonnevend, A. Molecular Characterization of Clinical and Environmental Carbapenem Resistant Acinetobacter Baumannii Isolates in a Hospital of the Eastern Region of Saudi Arabia. J. Infect. Public Health 2020, 13, 632–636. [Google Scholar] [CrossRef]
- Biswas, S.; Rolain, J.M. Use of MALDI-TOF Mass Spectrometry for Identification of Bacteria That Are Difficult to Culture. J. Microbiol. Methods 2013, 92, 14–24. [Google Scholar] [CrossRef]
- Ravi, R.K.; Walton, K.; Khosroheidari, M. Miseq: A next Generation Sequencing Platform for Genomic Analysis. Methods Mol. Biol. 2018, 1706, 223–232. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Coil, D.; Jospin, G.; Darling, A.E. A5-Miseq: An Updated Pipeline to Assemble Microbial Genomes from Illumina MiSeq Data. Bioinformatics 2015, 31, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [Green Version]
- Roosaare, M.; Puustusmaa, M.; Möls, M.; Vaher, M.; Remm, M. PlasmidSeeker: Identification of Known Plasmids from Bacterial Whole Genome Sequencing Reads. PeerJ 2018, 6, e4588. [Google Scholar] [CrossRef]
- Zuo, G.; Hao, B. CVTree3 Web Server for Whole-Genome-Based and Alignment-Free Prokaryotic Phylogeny and Taxonomy. Genom. Proteom. Bioinforma 2015, 13, 321–331. [Google Scholar] [CrossRef]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and Open Software for Comparing Large Genomes. Genome Biol. 2004, 5, R12. [Google Scholar] [CrossRef] [Green Version]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef]



| Isolate Names | ST Type | Resistance to | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactams | Aminoglycosides | Macrolides | Tetracyclines | Sulfonamides | Phenicols | Colistin * | ||||||||||||||||||||||||
| 138 | 142 | 227 | 278 | |||||||||||||||||||||||||||
| A | A | A | D | |||||||||||||||||||||||||||
| ADC-73 | ADC-30 | BlaA1 | BlaA2 | Mbl | OXA-23 | OXA-66 | OXA-72 | OXA-181 | TEM-1D | Zn_hydrolase | ArmA | Aph3’’Ia | AphA6 | AadA1-pm | Ant3’’Ih-Aac6-IId | Aac3-I | Aac6-Iaf | StrA | StrB | MphE | MsrE | TetB | SulI | CatB8 | PmrB | |||||
| AB01 | ST604 | + | - | + | + | + | + | + | - | - | + | + | + | + | - | - | - | - | - | + | + | + | + | + | - | - | T | . | . | . |
| AB02 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | + | - | - | + | + | + | + | + | - | - | T | . | . | G | ||
| AB07 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | + | - | - | + | + | + | + | + | - | - | T | . | . | G | ||
| AB08 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | - | - | - | - | - | + | + | + | + | + | - | - | T | . | . | G |
| AB12 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | - | - | - | - | - | + | + | + | + | + | - | - | T | . | . | . |
| AB14 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | + | T | . | V | . |
| AB15 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | - | - | + | + | + | + | + | - | - | T | . | . | . | |||
| AB16 | ST2 | + | - | + | + | + | + | + | - | - | + | + | - | + | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB17 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | + | T | . | V | . |
| AB18 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB19 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB20 | ST2 | + | - | + | + | + | + | + | - | - | - | + | - | + | + | + | + | - | - | + | + | + | + | + | + | + | T | V | . | . |
| AB21 | ST2 | + | - | + | + | + | + | + | - | - | + | - | + | + | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB22 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB23 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB24 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | + | T | . | V | . |
| AB26 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB29 | ST45 | - | + | + | + | + | - | + | + | - | - | + | - | - | - | + | - | + | + | - | - | + | + | - | + | - | . | V | . | . |
| AB30 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | + | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB33 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB54 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | T | . | . | G |
| AB191 | ST2 | - | - | + | + | + | + | + | - | - | - | + | - | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB192 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | + | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB193 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | + | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB194 | ST2 | + | - | - | + | + | + | + | - | - | - | + | - | - | + | - | - | - | - | + | + | - | - | + | + | - | T | . | . | . |
| AB195 | ST2 | + | - | - | + | + | + | + | - | - | - | + | + | - | + | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB196 | ST2 | + | - | + | + | + | + | + | - | - | + | + | + | + | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB197 | ST2 | + | - | - | + | + | + | + | - | + | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB198 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | + | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB199 | ST2 | + | - | - | + | + | + | + | - | - | - | + | - | - | + | - | - | - | - | + | + | - | - | + | + | - | T | . | . | . |
| AB201 | ST2 | + | - | - | + | + | + | + | - | - | - | + | - | - | - | - | - | - | - | + | + | + | - | + | + | - | T | . | . | . |
| AB202 | ST2 | + | - | - | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB203 | ST2 | + | - | - | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB204 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | + | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB1911 | ST2 | + | - | + | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | - | - | + | + | - | - | - | T | . | . | . |
| AB1912 | ST2 | + | - | - | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| AB1913 | ST2 | + | - | - | + | + | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | + | + | + | - | T | . | . | . |
| Isolate | ST | β-Lactams | Aminoglycosides | Quinolones | Macrolides | PHE | FOS | SUL | TET | TRP | Colistin * | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AmpH | TEM-150 | OXA-1 | OXA-181 | SHV-106 | SHV-110 | CTX-M-15 | CTX-M-9 | OXA-48 | PBP | AadA2 | Ant3″Ih | Aac3-IIa | Aph3-VIb | ArmA | StrAB | Sat-2A | OqxA | OqxBgb | QnrB1 | MphD | MphE | MsrE | CatA1 | CatB4 | FosA2 | SulI | Tet-34 | TetA | TetD | DfrA12 | DfrA14 | Mcr-1 | MgrB | PmrA | PmrB | PhoQ | ||
| KP01 | 873 | + | + | + | - | - | + | + | - | + | + | - | + | + | + | - | + | - | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | Q22P | - | - | - | |
| KP02 | 873 | + | + | + | - | - | + | + | - | + | + | - | + | + | + | - | + | - | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | Q22P | - | - | - | |
| KP03 | 2096 | + | - | + | + | + | - | - | - | - | + | - | + | - | - | - | - | + | + | + | - | + | - | - | - | + | + | - | + | - | - | - | - | - | - | G53V | - | - |
| KP04 | 14 | + | - | + | + | + | - | + | - | - | + | + | + | - | - | + | - | + | + | + | - | + | + | + | + | + | + | + | + | - | - | + | - | - | ΔMgrB | - | - | - |
| KP05 | 2096 | + | + | + | + | + | - | + | - | - | + | + | + | - | - | - | - | + | + | + | - | + | - | + | - | + | + | + | + | - | + | + | - | + | - | - | - | L52V |
| KP06 | 2096 | + | + | + | - | + | - | + | + | + | + | + | + | - | - | + | - | + | + | + | - | - | + | + | + | + | + | + | + | - | + | + | + | - | - | - | - | - |
| KP07 | 2096 | + | + | + | + | + | - | + | - | - | + | + | + | - | - | + | - | + | + | + | - | + | + | + | - | + | + | + | + | - | + | + | - | - | - | - | - | G385S |
| KP08 | 2096 | + | + | + | + | + | - | + | - | - | + | + | + | - | - | + | - | + | + | + | - | + | + | + | - | + | + | + | + | - | + | + | - | - | - | - | T128P; T157P | D495- |
| KP15 | 2096 | + | + | + | + | + | + | - | - | + | + | + | - | - | - | - | + | + | + | - | + | + | + | - | + | + | + | + | - | + | + | - | + | - | - | - | L52V | |
| KP201 | 14 | + | - | + | + | + | + | - | - | + | + | + | - | - | - | - | + | + | + | - | + | + | + | + | + | + | + | + | - | - | + | - | - | ΔMgrB | - | - | - | |
| Resistance to | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST Type | Β-LACT | AGLY | QNE | PHE | SUL | TET | COL * | |||||||||||||||
| AQU1 | OXA-10 | OXA-50 | GES-1 | VEB-1 | OprD | AadA1-pm | AadB | Aph3-IIb | Aph6-I | AphA15 | 4 Copies of OqxBgb | QepA | CatB7 | SulI | TetA | Tcr-3 | TetR | PmrA | PmrB | PhoQ | ||
| PA24 | 357 | + | + | + | - | + | ΔOprD | + | + | + | + | - | + | + | + | + | + | + | + | - | S2P; A4T; V15I; G68S; Y345H | - |
| PA31 | 235 | + | - | + | + | - | Loss | - | - | + | + | + | + | + | + | + | - | + | - | L71R | S2P; A4T; W25C; G68S; Y345H; G362S | Y85F |
| PA191 | 357 | + | + | + | - | + | ΔOprD | + | - | + | + | - | + | + | + | + | + | - | + | - | S2P; A4T; V15I; G68S | - |
| PA193 | 357 | + | + | + | - | + | ΔOprD | + | + | + | + | - | + | + | + | + | + | - | + | - | S2P; A4T; V15I; G68S; L96V; Y345H; G419S | ΔPhoQ |
| PA201 | Unknown | + | - | + | - | - | ΔOprD | - | - | + | + | - | + | + | + | - | - | + | - | L71R | A4T; G131D; Y345H; P369A; D461N; A462T | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okdah, L.; AlDosary, M.S.; AlMazyed, A.; Alkhurayb, H.M.; Almossallam, M.; Al Obaisi, Y.s.; Marie, M.A.; Abdelrahman, T.; Diene, S.M. Genomic Characterization of Colistin-Resistant Isolates from the King Fahad Medical City, Kingdom of Saudi Arabia. Antibiotics 2022, 11, 1597. https://doi.org/10.3390/antibiotics11111597
Okdah L, AlDosary MS, AlMazyed A, Alkhurayb HM, Almossallam M, Al Obaisi Ys, Marie MA, Abdelrahman T, Diene SM. Genomic Characterization of Colistin-Resistant Isolates from the King Fahad Medical City, Kingdom of Saudi Arabia. Antibiotics. 2022; 11(11):1597. https://doi.org/10.3390/antibiotics11111597
Chicago/Turabian StyleOkdah, Liliane, Mohammed Saeed AlDosary, Abeer AlMazyed, Hussain Mushabbab Alkhurayb, Meshari Almossallam, Yousef sultan Al Obaisi, Mohammed Ali Marie, Tamir Abdelrahman, and Seydina M. Diene. 2022. "Genomic Characterization of Colistin-Resistant Isolates from the King Fahad Medical City, Kingdom of Saudi Arabia" Antibiotics 11, no. 11: 1597. https://doi.org/10.3390/antibiotics11111597






