Update on the Management of Surgical Site Infections
Abstract
:1. Introduction
2. Risk Factors
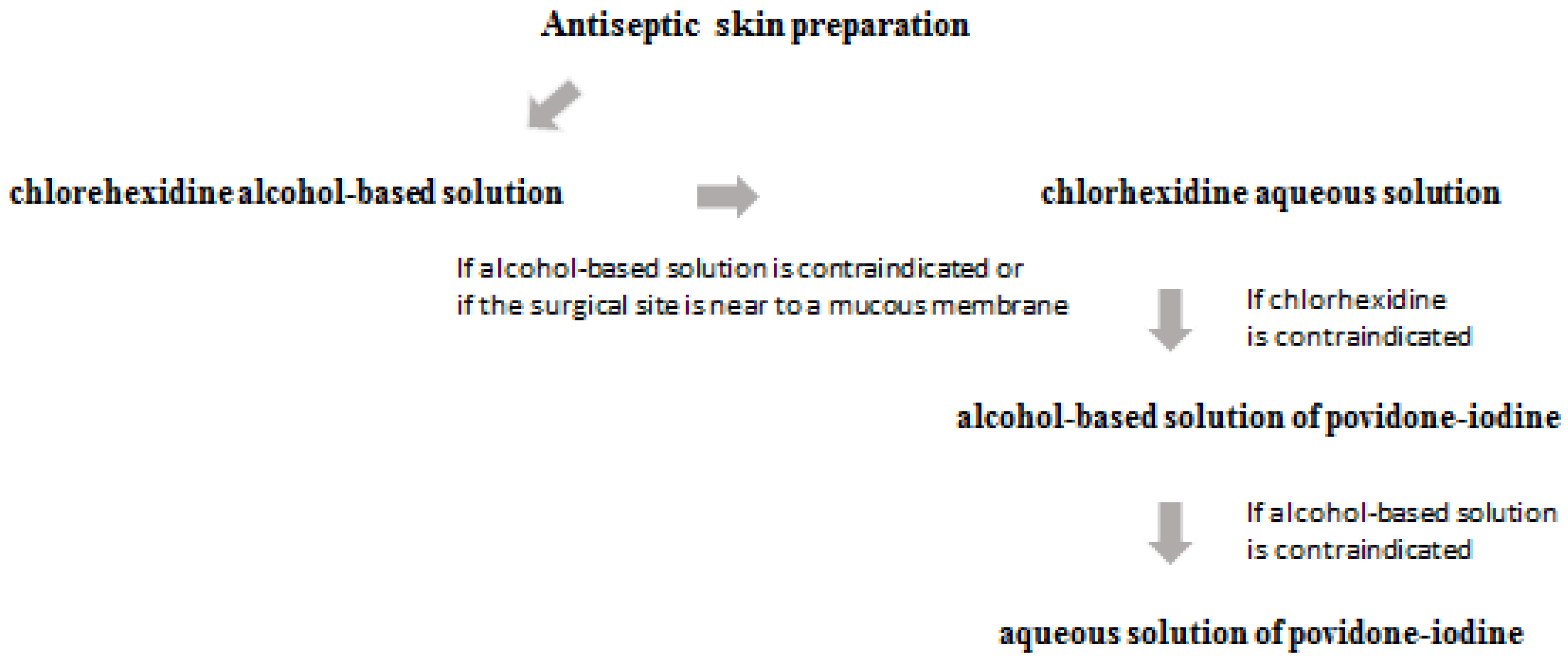
3. Prevention and Prophylaxis
4. Treatment
4.1. Available Treatments for Gram-Positive Bacteria: Old and Novel Drugs
4.2. Available Treatments for Gram-Negative Bacteria: Old and Novel Drugs
4.3. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- National Healthcare Safety Network, Centers for Disease Control and Prevention. Surgical Site Infection [SSI] Event. Available online: http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf (accessed on 1 September 2022).
- Anderson, D.J.; Podgorny, K.; Berríos-Torres, S.I.; Bratzler, D.W.; Dellinger, E.P.; Greene, L.; Nyquist, A.C.; Saiman, L.; Yokoe, D.S.; Maragakis, L.L.; et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update. Infect. Control. Hosp. Epidemiol. 2014, 35, 605–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R.; Hospital Infection Control Practices Advisory Committee. Guideline for Prevention of Surgical Site Infection, 1999. Infect. Control. Hosp. Epidemiol. 1999, 20, 247–278. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health careassociated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, S.; Robertson, C.; Pan, J.; Kennedy, S.; Dancer, S.; Haahr, L.; Manoukian, S.; Mason, H.; Kavanagh, K.; Cook, B.; et al. Epidemiology of healthcare-associated infection reported from a hospital-wide incidence study: Considerations for infection prevention and control planning. J. Hosp. Infect. 2021, 114, 10–22. [Google Scholar] [CrossRef] [PubMed]
- McKibben, L.; Horan, T.; Tokars, J.I.; Fowler, G.; Cardo, D.M.; Pearson, M.L.; Brennan, P.J.; Healthcare Infection Control Practices Advisory Committee. Heathcare Infection Control Practices Advisory Committee. Guidance on public reporting of healthcare-associated infections: Recommendations of the Healthcare Infection Control Practices Advisory Committee. Am. J. Infect. Control. 2005, 33, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Network NHS. Surgical Site Infection [SSI] Event; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- De Lissovoy, G.; Fraeman, K.; Hutchins, V.; Murphy, D.; Song, D.; Vaughn, B.B. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am. J. Infect. Control. 2009, 37, 387–397. [Google Scholar] [CrossRef]
- Bozic, K.J.; Ries, M.D. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J. Bone Jt. Surg. Am. 2005, 87, 1746–1751. [Google Scholar]
- Engemann, J.J.; Carmeli, Y.; Cosgrove, S.E.; Fowler, V.G.; Bronstein, M.Z.; Trivette, S.L.; Briggs, J.P.; Sexton, D.J.; Kaye, K.S. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 2003, 36, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.J.; Kirkland, K.B.; Kaye, K.S.; Thacker, P.A.; Kanafani, Z.A.; Auten, G.; Sexton, D.J. Underresourced hospital infection control and prevention programs: Penny wise, pound foolish? Infect. Control. Hosp. Epidemiol. 2007, 28, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of prevention. Available online: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf (accessed on 1 October 2022).
- Stone, P.W.; Braccia, D.; Larson, E. Systematic review of economic analyses of health care–associated infections. Am. J. Infect. Control. 2005, 33, 501–509. [Google Scholar] [PubMed]
- Neumayer, L.; Hosokawa, P.; Itani, K.; El-Tamer, M.; Henderson, W.G.; Khuri, S.F. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: Results from the patient safety in surgery study. J. Am. Coll. Surg. 2007, 204, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.A., Jr.; Henderson, W.G.; Englesbe, M.J.; Hall, B.L.; O’Reilly, M.; Bratzler, D.; Dellinger, E.P.; Neumayer, L.; Bass, B.L.; Hutter, M.M.; et al. Surgical site infection prevention: The importance of operative duration and blood transfusion—Results of the first American College of Surgeons-National Surgical Quality Improvement Program Best Practices Initiative. J. Am. Coll. Surg. 2008, 207, 810–820. [Google Scholar]
- Cullen, K.A.; Hall, M.J.; Golosinskiy, A. Ambulatory Surgery in the United States, 2006. National Health Statistics Reports 11. Revised, 4 September 2009. Available online: http://www.cdc.gov/nchs/data/nhsr/nhsr011.pdf (accessed on 1 October 2022).
- US Department of Health and Human Services. National Action Plan to Prevent Health Care–Associated Infections: Road Map to Elimination. 2013. Available online: https://health.gov/hcq/prevent-hai-action-plan.asp (accessed on 1 October 2022).
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect. Control. Hosp. Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Bath, M.F.; Davies, J.; Suresh, R.; Machesney, M.R. Surgical site infections: A scoping review on current intraoperative prevention measures. Ann. R. Coll. Surg. Engl. 2022, 104, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Parente, D.M.; Cunha, C.B.; Mylonakis, E.; Timbrook, T.T. The Clinical Utility of Methicillin-Resistant Staphylococcus aureus [MRSA] Nasal Screening to rule out MRSA Pneumonia: A diagnostic meta-analysis with antimicrobial stewardship implications. Clin. Infect. Dis. 2018, 67, 1–7. [Google Scholar] [CrossRef]
- Mergenhagen, K.A.; Starr, K.E.; Wattengel, B.A.; Lesse, A.J.; Sumon, Z.; Sellik, J.A. Determining the Utility of Methicillin-Resistant Staphylococcus aureus Nares Screening in Antimicrobial Stewardship. Clin. Infect. Dis. 2019, 71, 1142–1148. [Google Scholar]
- Surgical Site Infections: Prevention and Treatment. In National Institute for Health and Care Excellence (NICE); National Library of Medicine: Bethesda, MD, USA, 2020.
- Cimochowski, G.E.; Harostock, M.D.; Brown, R.; Bernardi, M.; Alonzo, N.; Coyle, K. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann. Thorac. Surg. 2001, 71, 1572–1578. [Google Scholar]
- Perl, T.M.; Cullen, J.J.; Wenzel, R.P.; Zimmerman, M.B.; Pfaller, M.A.; Sheppard, D.; Twombley, J.; French, P.P.; Herwaldt, L.A.; Mupirocin and the Risk of Staphylococcus Aureus Study Team. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 2002, 346, 1871–1877. [Google Scholar]
- Segers, P.; Speekenbrink, R.G.; Ubbink, D.T.; van Ogtrop, M.L.; Bas, A. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: A randomized controlled trial. JAMA 2006, 296, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Zemer-Wassercug, N.; Talker, O.; Lishtzinsky, Y.; Lev, B.; Samra, Z.; Leibovici, L.; Bishara, J. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin. Microbiol. Infect. 2010, 17, 1581–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunne, M.W.; Puttagunta, S.; Giordano, P.; Krievins, D.; Zelasky, M.; Baldassarre, J. A randomized Clinical Trial of Single-Dose versus weekly Dalbavancin for treatment of acute bacterial skin and skn structure infection. Clin. Infect. Dis. 2016, 62, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.B.; Gorwitz, R.J.; Jernigan, J.A. Mupirocin resistance. Clin. Infect. Dis. 2009, 49, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Tashiro, S.; Mihara, T.; Kon, J.; Sakurai, K.; Tanaka, Y.; Morita, T.; Enoki, Y.; Taguchi, K.; Matsumoto, K.; et al. Efficacy of surgical skin preparation with chlorhexidine in alcohol according to the concentration required to prevent surgical site infection: Meta-analysis. BJS Open. 2022, 6, zrac111. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; SpeekBerríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection. JAMA Surg. 2017, 152, 784–791. [Google Scholar]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Kadri, S.S.; Lai, Y.L.; Warner, S.; Strich, J.R.; Babiker, A.; Ricotta, E.E.; Demirkale, C.Y.; Dekker, J.P.; Palmore, T.N.; Rhee, C.; et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: A retrospective cohort analysis of prevalence, predictors and mortality risk in US hospitals. Lancet Infect. Dis. 2021, 21, 241–251. [Google Scholar] [CrossRef]
- Nambiar, K.; Seifert, H.; Rieg, S.; Kern, W.V.; Scarborough, M.; Gordon, N.C.; Kim, H.B.; Song, K.H.; Tilley, R.; Gott, H.; et al. Survival following Staphilococcus aureus bloodstream infection; a prospective multinational cohort study assessing the impact of place of care. J. Infect. 2018, 77, 516–525. [Google Scholar] [CrossRef]
- Gupta, K.; Strymish, J.; Abi-Haidar, Y.; Williams, S.A.; Itani, K.M. Preoperative nasal methicillin-resistant Staphylococcus aureus status, surgical prophylaxis, and risk-adjusted postoperative outcomes in veterans. Infect. Control. Hosp. Epidemiol. 2011, 32, 791–796. [Google Scholar] [CrossRef]
- Allareddy, V.; Das, A.; Lee, M.K.; Nalliah, R.P.; Rampa, S.; Allareddy, V.; Rotta, A.T. Prevalence, predictors, and outcomes of methicillin-resistant Staphylococcus aureus infections in patients undergoing major surgical procedures in the United States: A population-based study. Am. J. Surg. 2015, 210, 59–67. [Google Scholar] [CrossRef]
- Kalra, L.; Camacho, F.; Whitener, C.J.; Du, P.; Miller, M.; Zalonis, C.; Julian, K.G. Risk of methicillin-resistant Staphylococcus aureus surgical site infection in patients with nasal MRSA colonization. Am. J. Infect. Control. 2013, 41, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Fernandez-Fernandez, R.; Ruiz-Ripa, L.; Gomez, P.; Zarazaga, M.; Torres, C. Human mecC-Carrying MRSA: Clinical Implications and Risk Factors. Microorganisms 2020, 8, 1615. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Paterson, D.L.; et al. Effect of Vancomycin or Daptomycin with vs. without an antistaphylococcal β-lactam on mortality, bacteremia, relapse or treatment failure in patients with MRSA bacteremia. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Voth, D.; Nichol, K.; Karlowsky, J.A.; Noreddin, A.M.; Hoban, D.J. Pharmacodynamic activity of Ceftobiprole compared with Vancomycin versus methicillin-resistant Staphylococcus aureus [MRSA], Vancomycin-intermediate Staphylococcus aureus [VISA] and Vancomycin-resistant Staphylococcus aureus [VRSA] using an in vitro model. J. Antimicrob. Chemother. 2009, 64, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Nannini, E.C.; Stryjewski, M.E.; Singh, K.V.; Rude, T.H.; Corey, G.R.; Fowler, V.G., Jr.; Murray, B.E. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 2206–2208. [Google Scholar] [CrossRef] [Green Version]
- del Río, A.; Gasch, O.; Moreno, A.; Peña, C.; Cuquet, J.; Soy, D.; Mestres, C.A.; Suárez, C.; Pare, J.C.; Tubau, F.; et al. Efficacy and Safety of Fosfomycin plus Imipenem as rescue Therapy for complicated bacteremia and endocarditis due to Methicillin-resistant staphylococcus aureus: A multicenter clinical trial. Clin. Infect. Dis. 2014, 59, 1105–1112. [Google Scholar]
- Horner, C.; Mushtaq, S.; Livermore, D.M.; Committee, B.R.S.S. Activity of Ceftaroline versus Ceftobiprole against staphylococci and pneumococci in the UK and Ireland: Analysis of BSAC surveillance data. J. Antimicrob. Chemother. 2020, 76, 1659. [Google Scholar] [CrossRef]
- Kebriaei, R.; Rice, S.A.; Singh, N.B.; Stamper, K.C.; Nguyen, L.; Sheikh, Z.; Rybak, M.J. Combinations of [lipo] glycopeptides with β-lactams against MRSA: Susceptibility insights. J. Antimicrob. Chemother. 2020, 75, 2894–2901. [Google Scholar] [CrossRef]
- Rizzetto, G.; Molinelli, E.; Radi, G.; Diotallevi, F.; Cirioni, O.; Brescini, L.; Giacometti, A.; Offidani, A.; Simonetti, O. Role of Daptomycin in Cutaneous Wound Healing: A Narrative Review. Antibiotics 2022, 11, 944. [Google Scholar] [CrossRef]
- Pascale, R.; Maccaro, A.; Mikus, E.; Baldassarre, M.; Tazza, B.; Esposito, F.; Rinaldi, M.; Tenti, E.; Ambretti, S.; Albertini, A.; et al. A retrospective multicentre study on dalbavancin effectiveness and cost-evaluation in sternotomic wound infection treatment: DALBA SWIT Study. J. Glob. Antimicrob. Resist. 2022, 30, 390–394. [Google Scholar] [CrossRef]
- Fowler Jr, V.G.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Siempos, I.I.; Vardakas, K.Z. Linezolid versus glycopeptide or beta-lactam for treatment of gram-positive bacterial infections: Meta-analysis of randomized controlled trials. Lancet Infect. Dis. 2008, 8, 53–66. [Google Scholar] [CrossRef]
- Boucher, H.W.; Wilcox, M.; Talbot, G.H.; Puttagunta, S.; Das, A.F.; Dune, M.W. Once-weekly Dalbavancin versus daily conventional therapy for skin infection. N. Engl. J. Med. 2014, 370, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Solache, M.; Rice, L.B. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ch’ng, J.H.; Chong, K.K.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA [EARS-Net]-Annual Epidemiological Repot 2019; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf (accessed on 1 October 2022).
- European Committee of Antimicrobial Susceptibility Testing. EUCAST Expert Rules v 3.2 on Enterococcus. Available online: https://www.eucast.org/expert_rules_and_expected_phenotypes (accessed on 1 September 2022).
- Miller, W.R.; Murray, B.E.; Rice, L.B.; Arias, C.A. Vancomycin-resistant enterococci: Therapeutic challenges in the 21st century. Infect. Dis. Clin. N. Am. 2016, 30, 415–439. [Google Scholar] [CrossRef]
- Mishra, N.N.; Bayer, A.S.; Tran, T.T.; Shamoo, Y.; Mileykovskaya, E.; Dowhan, W.; Guan, Z.; Arias, C.A. Daptomycin resistance in enterococci is assoiated with distinct alterations of cell membrane phospholipid content. PLoS ONE 2012, 56, 838–844. [Google Scholar] [CrossRef]
- El Amin, N.A.; Jalal, S.; Wretlind, B. Alterations in GyrA and ParC associated with fluoroquinolone resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 1999, 43, 947–949. [Google Scholar] [CrossRef] [Green Version]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.; Karve, S.; Peeters, P.; Baelen, E.; Potter, D.; Rojas-Farreras, S.; Pascual, E.; Rodríguez-Baño, J. The impact of initial antibiotic treatment failure: Real-world insights in healthcare-associated or nosocomial pneumonia. J. Infect. 2018, 77, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Henderson, A.; Harris, P.N.A. Current evidence for therapy of ceftriaxone-resistant gram-negative bacteremia. Curr. Opin. Infect. Dis. 2019, 33, 78–85. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Giacobbe, D.R.; Falcone, M.; Tiseo, G.; Giannella, M.; Pascale, R.; Meschiari, M.; Digaetano, M.; Oliva, A.; et al. Ceftolozane/Tazobactam for treatment of severe ESBL-producing enterobacterales infections: A multicenter nationwide clinical experience [CEFTABUSE II Study]. Open Forum Infect. Dis. 2020, 7, ofaa139. [Google Scholar] [CrossRef]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolazone/Tazobactam: Second-generation β-Lactam/β-Lactamase inhibitor combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Potoski, B.A.; Marini, R.V.; Doi, Y.; Kreiswirth, B.N.; Clancy, C.J. Ceftazidime-Avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumonia bacteremia. Antimicrob. Agents Chemother. 2017, 61, e00883-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; De Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of Ceftazidime-Avibactam salvage therapy in patients wth infections caused by KPC-producing Klebsiella pneumonia. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Onorato, L.; Di Caprio, G.; Signoriello, S.; Coppola, N. Efficacy of Ceftazidime-Avibactam in monotherapy or combination therapy against carbapenem-resistant gram-negative bacteria: A meta-analysis. Int. J. Antimicrob. Agents 2019, 54, 735–740. [Google Scholar] [CrossRef]
- Compain, F.; Arthur, M. Impaired inhibition by Avibactam and resistance to the Ceftazidime-Avibactam combination due to the D179Y substitution in the KPC-2 β-lactamase. Antimicrob. Agents Chemother. 2017, 61, e00451-17. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Rybak, M.J. Meropenem and vaborbactam: Stepping up the battle against carbapenem resistant Enterobacteriaceae. Pharmacotherapy 2018, 38, 444–461. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Kazmierczak, K.M.; Hawser, S.P.; Magnet, S.; Young, K.; Motyl, M.R.; Sahm, D.F. In vitro activity of Imipenem/Relebactam against gram negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J. Antimicrob. Chemother. 2018, 73, 1872–1879. [Google Scholar] [CrossRef]
- Jean, S.S.; Gould, I.M.; Lee, W.S.; Hsueh, P.R. New Drugs for Multidrug-Resistant Gram-negative organism: Time for stewardship. Drugs 2019, 79, 705–714. [Google Scholar] [CrossRef]
- Lodise, T.P.; Smith, N.M.; O’Donnell, N.; Eakin, A.E.; Holden, P.N.; Boissonneault, K.R.; Zhou, J.; Tao, X.; Bulitta, J.B.; Fowler Jr, V.G.; et al. Determining the optimal dosing of a novel combination regimen of CZA with AZT against NDM-1 producing Enterobacteriaceae using a hollow-fibre infection model. J. Antimicrob. Chemother. 2020, 75, 2622–2632. [Google Scholar] [CrossRef]
- Tonziello, G.; Caraffa, E.; Pinchera, B.; Granata, G.; Petrosillo, N. Present and future of siderophore-based therapeutic and diagnostic approaches in infectious diseases. Infect. Dis. Rep. 2019, 11, 8208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohira, N.; Hackel, M.A.; Ishioka, Y.; Kuroiwa, M.; Sahm, D.F.; Sato, T.; Maki, H.; Yamano, Y. Reduced susceptibility mechanism to Cefiderocol, a siderophore cephalosporin, among clinical isolates from global surveillance program [SIDERO-WT-2014]. J. Glob. Antimicrob. Resist. 2020, 22, 738–741. [Google Scholar] [CrossRef]
- Breidenstein, E.B.M.; De La Fuente-Nunez, C.; Hancock, R. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, M.; Tazrout, S.; Bour, M.; Triponey, P.; Muller, C.; Jeannot, K.; Plésiat, P. Reassessment of the cooperativity between efflux system Mex-AB-OprM and cephalosporinase AmpC in the resistance of Pseudomonas aeruginosa to β-lactams. J. Antimicrob. Chemother. 2021, 76, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Nicolau, D.P.; Hopkins, K.L.; Meunier, D. Carbapenem-Resistant Enterobacterales, Carbapenem Resistant Organisms, Carbapenemase-Producing Enterobacterales, and Carbapenemase-Producing Organisms: Terminology Past its “Sell-By Date” in an Era of New Antibiotics and Regional Carbapenemase Epidemiology. Clin. Infect. Dis. 2020, 71, 1776–1782. [Google Scholar] [CrossRef]
- Rempenault, C.; Pagis, V.; Noussair, L.; Berbescu, S.; Duran, C.; Bouchand, F.; de Laroche, M.; Salomon, E.; Nich, C.; Bauer, T.; et al. Treatment of bone and joint infections by ceftazidime/avibactam and ceftolozane/tazobactam: A cohort study. J. Glob. Antimicrob. Resist. 2021, 25, 282–286. [Google Scholar] [CrossRef]
- Hassan, S.; Kahn, M.D.; Saraiya, N.; Nori, P. Treatment of a complex orthopaedic infection due to extensively drug-resistant Pseudomonas aeruginosa. BMJ Case Rep. 2018, 2018, bcr2017223202. [Google Scholar]
- Mora-Guzmán, I.; Rubio-Perez, I.; Maqueda González, R.; Domingo Garcia, D.; Martín-Pérez, E. Surgical site infection by carbapenemase-producing Enterobacteriaceae. A challenge for today’s surgeons. Cirugía Española 2020, 98, 342–349. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Romanelli, F.; Stolfa, S.; Belati, A.; Diella, L.; Ronga, L.; Fico, C.; Monno, L.; Mosca, A.; Saracino, A. Recurrent neurosurgical site infection by extensively drug-resistant P. aeruginosa treated with cefiderocol: A case report and literature review. Infect. Dis. 2021, 53, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Imipenem/Cilastatina/Relebactam: A review in gram-negative bacterial infections. Drugs 2021, 81, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M., Jr.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs. Colistin pls Imipenem in patients with Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabayoje, D.A.; NicFhogartaigh, C.; Cherian, B.P.; Tan, M.G.M.; Wareham, D.W. Compassionate use of cefiderocol for carbapenem-resistant Acinetobacter baumannii prosthetic joint infection. JAC Antimicrob. Resist. 2021, 3 (Suppl. 1), i21–i24. [Google Scholar] [CrossRef]
- Hetzler, L.; Kollef, M.H.; Yuenger, V.; Micek, S.T.; Betthauser, K.D. New antimicrobial treatment options for severe Gram-negative infections. Curr. Opin. Crit. Care 2022, 28, 522–533. [Google Scholar] [CrossRef]
- Shao, H.; Song, Y.; HE, J.; HU, L. Pharmacokinetics and drug concentration monitoring of high dose tigecycline in patients with septic shock. J. China Pharm. Univ. 2017, 48, 721–726. [Google Scholar]
- Prasad, P.; Sun, J.; Danner, R.L.; Natanson, C. Excess deaths associated with tigecycline after approval based on non-inferiority trials. Clin. Infect. Dis. 2012, 54, 1699–1709. [Google Scholar] [CrossRef] [Green Version]
- Penwell, W.F.; Shapiro, A.B.; Giacobbe, R.A.; Gu, R.F.; Gao, N.; Thresher, J.; McLaughlin, R.E.; Huband, M.D.; DeJonge, B.L.; Ehmann, D.E.; et al. Molecular Mechanism of Sulbactam Antibacterial Activity and resistance determinants in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 1680–1689. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.Y.; Lee, S.H.; Lee, S.Y.; Yang, S.; Noh, H.; Chung, E.K.; Lee, J.I. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: A systematic review and Bayesian network meta-analysis. Crit. Care 2017, 21, 319. [Google Scholar] [CrossRef] [Green Version]
- Mohd Sazlly Lim, S.; Heffernan, A.J.; Roberts, J.A.; Sime, F.B. Semi-mechanistic PK/PD modelling of fosfomycin and sulbactam combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e02472-20. [Google Scholar]
- Simonetti, O.; Morroni, G.; Ghiselli, R.; Orlando, F.; Brenciani, A.; Xhuvelaj, L.; Provinciali, M.; Offidani, A.; Guerrieri, M.; Giacometti, A.; et al. In vitro and in vivo activity of fosfomycin alone and in combination with rifampin and tigecycline against Gram-positive cocci isolated from surgical wound infections. J. Med. Microbiol. 2018, 67, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kusachi, S.; Nagao, J.; Saida, Y.; Watanabe, M.; Okamoto, Y.; Asai, K.; Nakamura, Y.; Enomoto, T.; Arima, Y.; Kiribayashi, T.; et al. Antibiotic time-lag combination therapy with fosfomycin for postoperative intra-abdominal abscesses. J. Infect. Chemother. 2011, 17, 91–96. [Google Scholar] [CrossRef]
- Giamarellou, H.; Karaiskos, I. Current and Potential Therapeutic Options for Infections Caused by Difficult-to-Treat and Pandrug Resistant Gram-Negative Bacteria in Critically Ill Patients. Antibiotics 2022, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Bush, K. In vitro susceptibility of β-lactamase-producing carbapenem-resistant Enterobacteriaceae [CRE] to eravacycline. J. Antibiot. 2016, 69, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Gardovskis, J.; Lawrence, K.; Montravers, P.; Sway, A.; Evans, D.; Tsai, L. IGNITE4: Results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs. meropenem in the treatment of complicated intraabdominal infections. Clin. Infect. Dis. 2019, 69, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, K.; Liang, B.; Zhang, G.; Wang, J.; Zhu, M.; Cai, Y. Efficacy and Safety of Plazomicin in the Treatment of Enterobacterales Infections: A Meta-analysis of Randomized Controlled Trials. Open Forum Infect. Dis. 2022, 9, ofac429. [Google Scholar] [CrossRef]
- Boyd, S.E.; Livermore, D.M.; Hooper, D.C.; Hope, W.W. Metallo-β-lactamases:structure, function, epidemiology, treatment options and the 2-development pipeline. Antimicrob. Agents Chemother. 2020, 64, e00397-20. [Google Scholar]
- Hamrick, J.C.; Docquier, J.D.; Uehara, T.; Myers, C.L.; Six, D.A.; Chatwin, C.L.; John, K.J.; Vernacchio, S.F.; Cusick, S.M.; Trout, R.E.; et al. VNRX-5133 [Taniborbactam], a broad-spectrum inhibitor of serine and metallo-b-lactamases, restores activity of Cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, e01963-19. [Google Scholar] [CrossRef] [Green Version]
- Seifert, H.; Müller, C.; Stefanik, D.; Higgins, P.G.; Miller, A.; Kresken, M. In vitro activity of Sulbactam/Durlobactam against global isolates of carbapenem-resistant Acinetobacter baumanniii. J. Antimicrob. Chemother. 2020, 75, 2616–2621. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinchera, B.; Buonomo, A.R.; Schiano Moriello, N.; Scotto, R.; Villari, R.; Gentile, I. Update on the Management of Surgical Site Infections. Antibiotics 2022, 11, 1608. https://doi.org/10.3390/antibiotics11111608
Pinchera B, Buonomo AR, Schiano Moriello N, Scotto R, Villari R, Gentile I. Update on the Management of Surgical Site Infections. Antibiotics. 2022; 11(11):1608. https://doi.org/10.3390/antibiotics11111608
Chicago/Turabian StylePinchera, Biagio, Antonio Riccardo Buonomo, Nicola Schiano Moriello, Riccardo Scotto, Riccardo Villari, and Ivan Gentile. 2022. "Update on the Management of Surgical Site Infections" Antibiotics 11, no. 11: 1608. https://doi.org/10.3390/antibiotics11111608




