Sterilization Procedures for Titanium Alloy Surfaces Leads to Higher Expression of Biofilm-Related Staphylococcus aureus Genes
Abstract
1. Introduction
2. Material and Methods
2.1. Titanium Material and Sterilization
2.2. Biofilm Formation & Bacteria
2.3. CFU
2.4. Primers
| Gene | Forward Primer | Reverse Primer |
| ica A | 5′-CGC ACT CAA TCA AGG CAT TA-3′ | 5′-CCA GCA AGT GTC TGA CTT CG-3′ |
| ica B | 5′-CAC ATA CCC ACG ATT TGC AT-3′ | 5′-TCG GAG TGA CTG CTT TTT CC-3′ |
| ica C | 5′-CTT GGG TAT TTG CAC GCA TT-3′ | 5′-GCA ATA TCA TGC CGA CAC CT-3′ |
| ica D | 5′-ACC CAA CGC TAA AAT CAT CG-3′ | 5′-GCG AAA ATG CCC ATA GTT TC-3′ |
| SarA | 5′-AAG GAC AAT CAC ATC ACG AAG-3′ | 5′-GAA CGC TCT AAT TCA GCG G-3′ |
| SodA | 5′-GTT TCA TCA CGA CAA ACA TCA C-3 | 5′-TGA CAT CCT CAT CGC TTC C-3 |
| SigB | 5′-AGA AGC AAT GGA AAT GGG AC-3′ | 5′-CTT AAA CCG ATA CGC TCA CC-3′ |
| 16s | 5′-GAA AGC CAC GGC TAA CTA CG-3′ | 5′-CAT TTC ACC GCT ACA CAT GG-3′ |
2.5. RNA Extraction
2.6. cDNA Synthesis from Bacterial RNA
2.7. Bacterial Biofilm Gene Expression Using Real-Time Quantitative Polymerase Chain Reaction (Real-Time qPCR)
2.8. Statistical Analysis
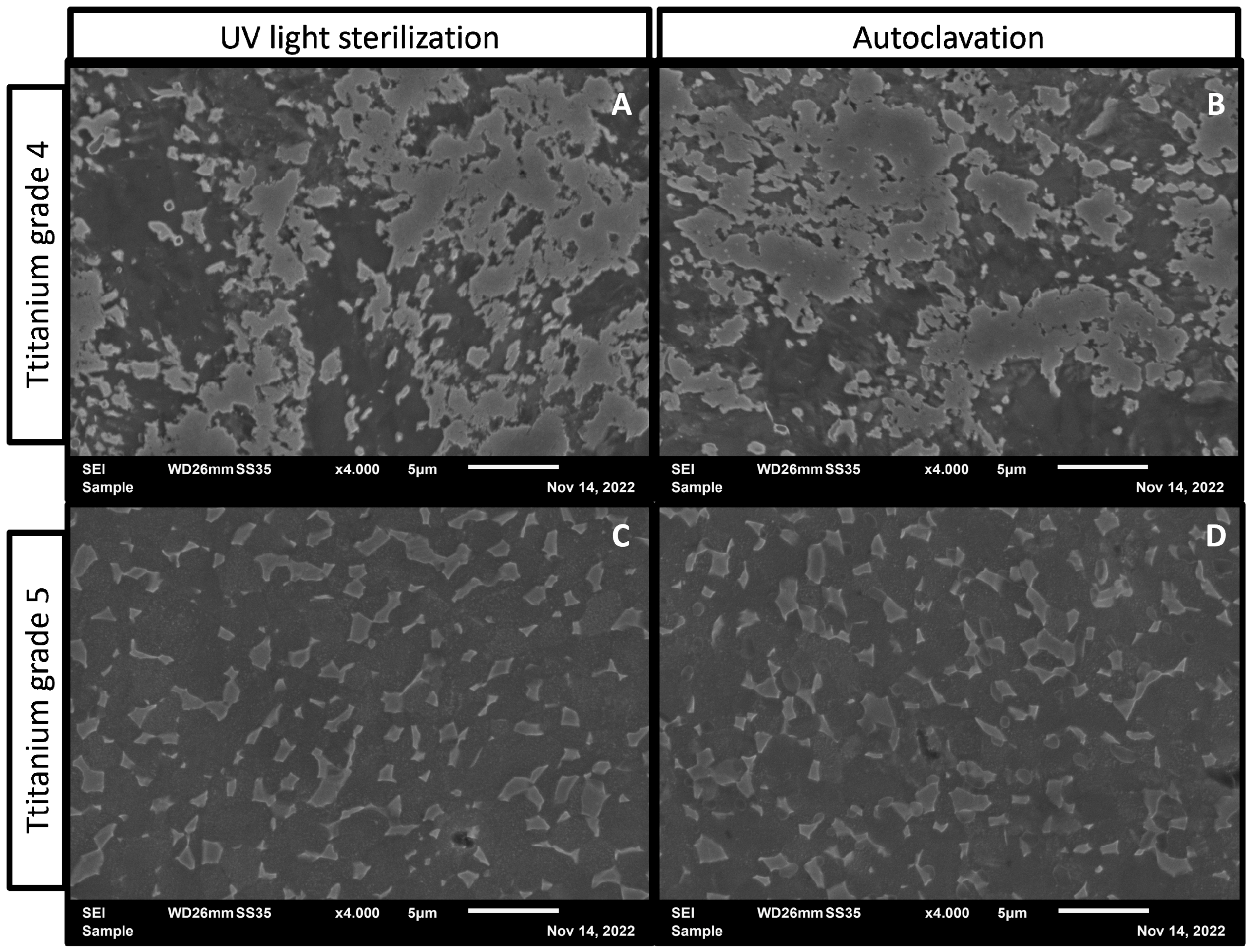
2.9. Scanning Electron Microscopy
3. Results
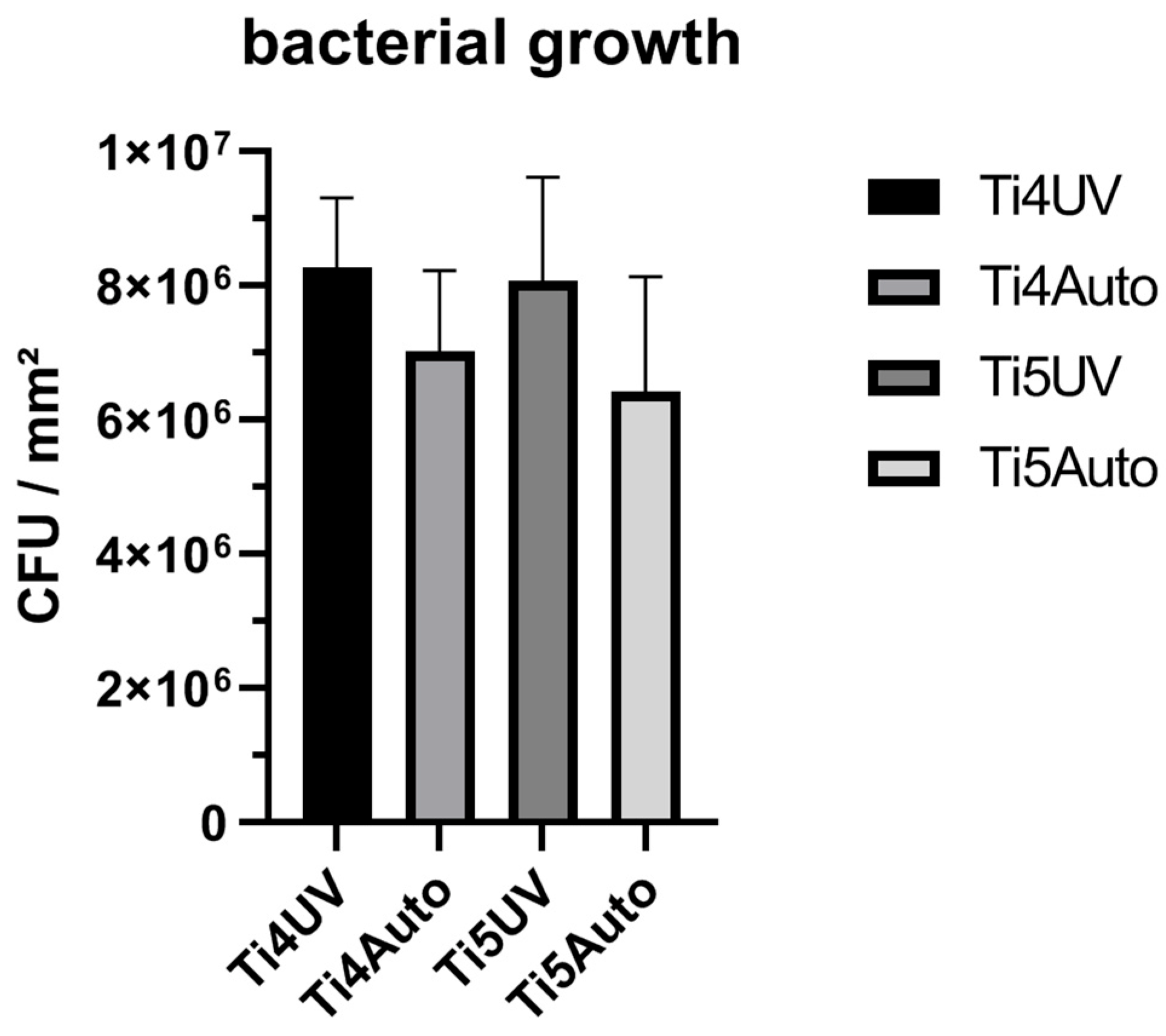
3.1. CFU
3.2. Gene Expression—Influence of Sterilization Methods on Biofilm Formation and Stress Induction
3.3. Surface Characterization after Sterilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet Neuronal Interact 2009, 9, 61–71. [Google Scholar] [PubMed]
- Ahmed, S.S.; Haddad, F.S. Prosthetic joint infection. Bone Jt. Res. 2019, 8, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef]
- Schwarz, F.; Wieland, M.; Schwartz, Z.; Zhao, G.; Rupp, F.; Geis-Gerstorfer, J.; Schedle, A.; Broggini, N.; Bornstein, M.M.; Buser, D.; et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 544–557. [Google Scholar] [CrossRef]
- Schwartz, Z.; Boyan, B.D. Underlying mechanisms at the bone-biomaterial interface. J. Cell Biochem. 1994, 56, 340–347. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Biomaterial and implant surfaces: On the role of cleanliness, contamination, and preparation procedures. J. Biomed. Mater. Res. 1988, 22, 145–158. [Google Scholar] [CrossRef]
- ISO 17664-1:2021; Processing of Health Care Products. ISO: Geneva, Switzerland, 2021.
- Vezeau, P.J.; Koorbusch, G.F.; Draughn, R.A.; Keller, J.C. Effects of multiple sterilization on surface characteristics and in vitro biologic responses to titanium. J. Oral Maxillofac. Surg. 1996, 54, 738–746. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Begum, F.; Kayani, B.; Haddad, F.S. Risk factors, diagnosis and management of prosthetic joint infection after total hip arthroplasty. Expert Rev. Med. Devices 2019, 16, 1063–1070. [Google Scholar] [CrossRef]
- Andrey, D.O.; Jousselin, A.; Villanueva, M.; Renzoni, A.; Monod, A.; Barras, C.; Rodriguez, N.; Kelley, W.L. Impact of the Regulators SigB, Rot, SarA and sarS on the Toxic Shock Tst Promoter and TSST-1 Expression in Staphylococcus aureus. PLoS ONE 2015, 10, e0135579. [Google Scholar] [CrossRef]
- Arciola, C.R.; Baldassarri, L.; Montanaro, L. In catheter infections by Staphylococcus epidermidis the intercellular adhesion (ica) locus is a molecular marker of the virulent slime-producing strains. J. Biomed. Mater. Res. 2002, 59, 557–562. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell Infect Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Ballal, A.; Manna, A.C. Regulation of superoxide dismutase (sod) genes by SarA in Staphylococcus aureus. J. Bacteriol. 2009, 191, 3301–3310. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.-F.; Zhang, K.-F.; Feng, X.-Q.; Su, Z.-X.; Ma, J.-T. Synthesis of Ag modified vanadium oxide nanotubes and their antibacterial properties. Mater. Res. Bull. 2008, 43, 2810–2817. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Pan, F.; Altenried, S.; Zuber, F.; Wagner, R.S.; Su, Y.H.; Rottmar, M.; Maniura-Weber, K.; Ren, Q. Photo-activated titanium surface confers time dependent bactericidal activity towards Gram positive and negative bacteria. Colloids Surf. B Biointerfaces 2021, 206, 111940. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Pal, R.; Cameotra, S.S. Antibacterial potential of Al2O3 nanoparticles against multidrug resistance strains of Staphylococcusaureus isolated from skin exudates. J. Nanoparticle Res. 2013, 15, 1970. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Ranjan, S.; Ramalingam, C. Titanium dioxide nanoparticles induce bacterial membrane rupture by reactive oxygen species generation. Environ. Chem. Lett. 2016, 14, 487–494. [Google Scholar] [CrossRef]
- Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.; Xie, K.; Yao, J.; Wang, L.; Liu, C.; Tang, Y. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8, 576969. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiegel, C.; Nogler, M.; Coraça-Huber, D.C. Sterilization Procedures for Titanium Alloy Surfaces Leads to Higher Expression of Biofilm-Related Staphylococcus aureus Genes. Antibiotics 2022, 11, 1647. https://doi.org/10.3390/antibiotics11111647
Spiegel C, Nogler M, Coraça-Huber DC. Sterilization Procedures for Titanium Alloy Surfaces Leads to Higher Expression of Biofilm-Related Staphylococcus aureus Genes. Antibiotics. 2022; 11(11):1647. https://doi.org/10.3390/antibiotics11111647
Chicago/Turabian StyleSpiegel, Christopher, Michael Nogler, and Débora C. Coraça-Huber. 2022. "Sterilization Procedures for Titanium Alloy Surfaces Leads to Higher Expression of Biofilm-Related Staphylococcus aureus Genes" Antibiotics 11, no. 11: 1647. https://doi.org/10.3390/antibiotics11111647
APA StyleSpiegel, C., Nogler, M., & Coraça-Huber, D. C. (2022). Sterilization Procedures for Titanium Alloy Surfaces Leads to Higher Expression of Biofilm-Related Staphylococcus aureus Genes. Antibiotics, 11(11), 1647. https://doi.org/10.3390/antibiotics11111647






