Recombinant Helicobacter pylori Vaccine Delivery Vehicle: A Promising Tool to Treat Infections and Combat Antimicrobial Resistance
Abstract
:1. Introduction
2. Treatment of Helicobacter pylori Infection and Problems Posed by Eradication Failure
3. Current Status of Vaccines for Helicobacter pylori
4. Helicobacter pylori as a Platform for the Delivery of Oral Vaccines
4.1. Genetic Tools and Technologies for Recombinant Helicobacter pylori Vaccine Delivery Vehicle
4.2. Recombinant Protein Expression Systems for Helicobacter pylori
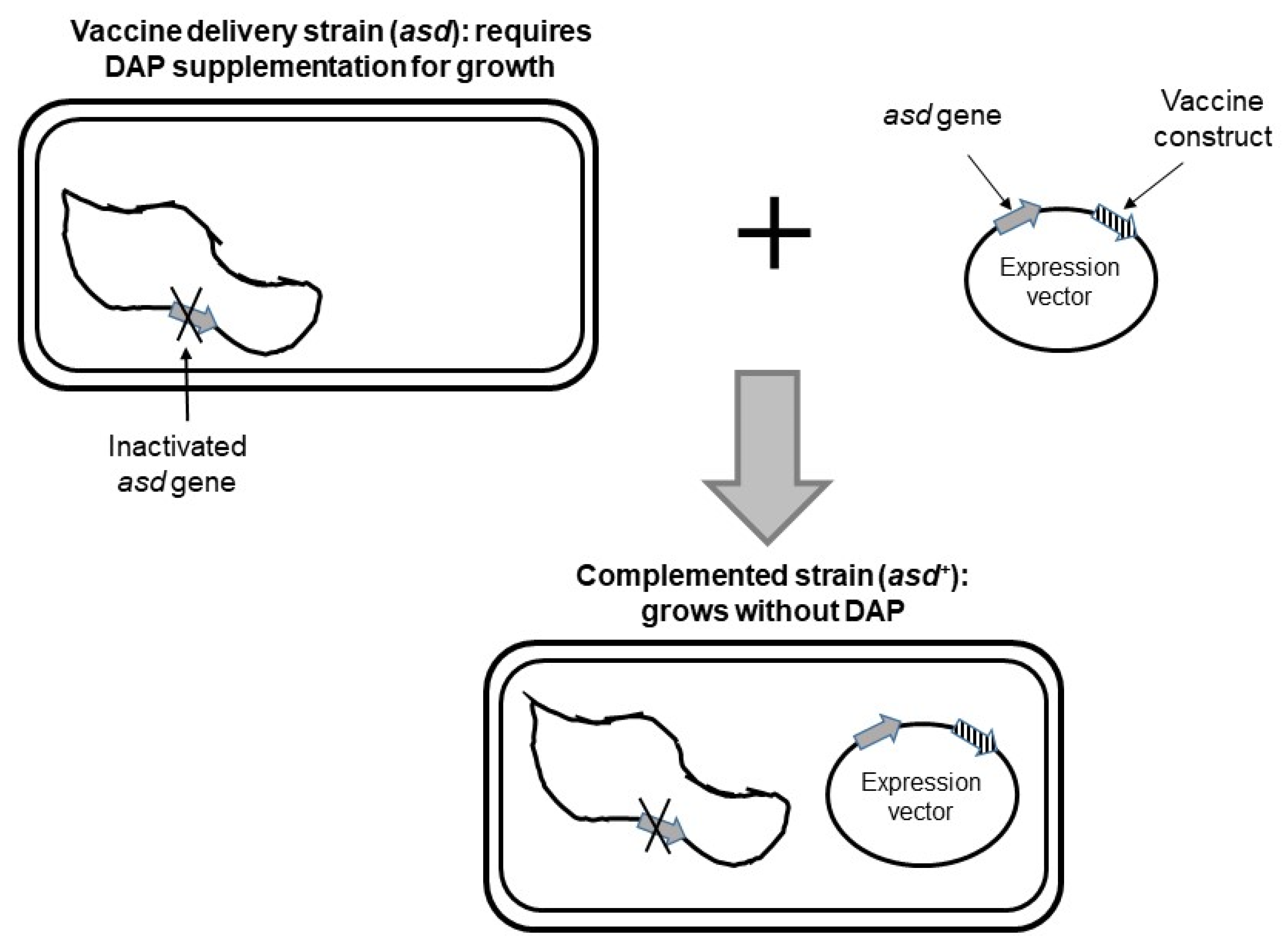
4.3. Selection of Recombinant Bacteria and Stabilization of Antigen-Encoding Plasmids
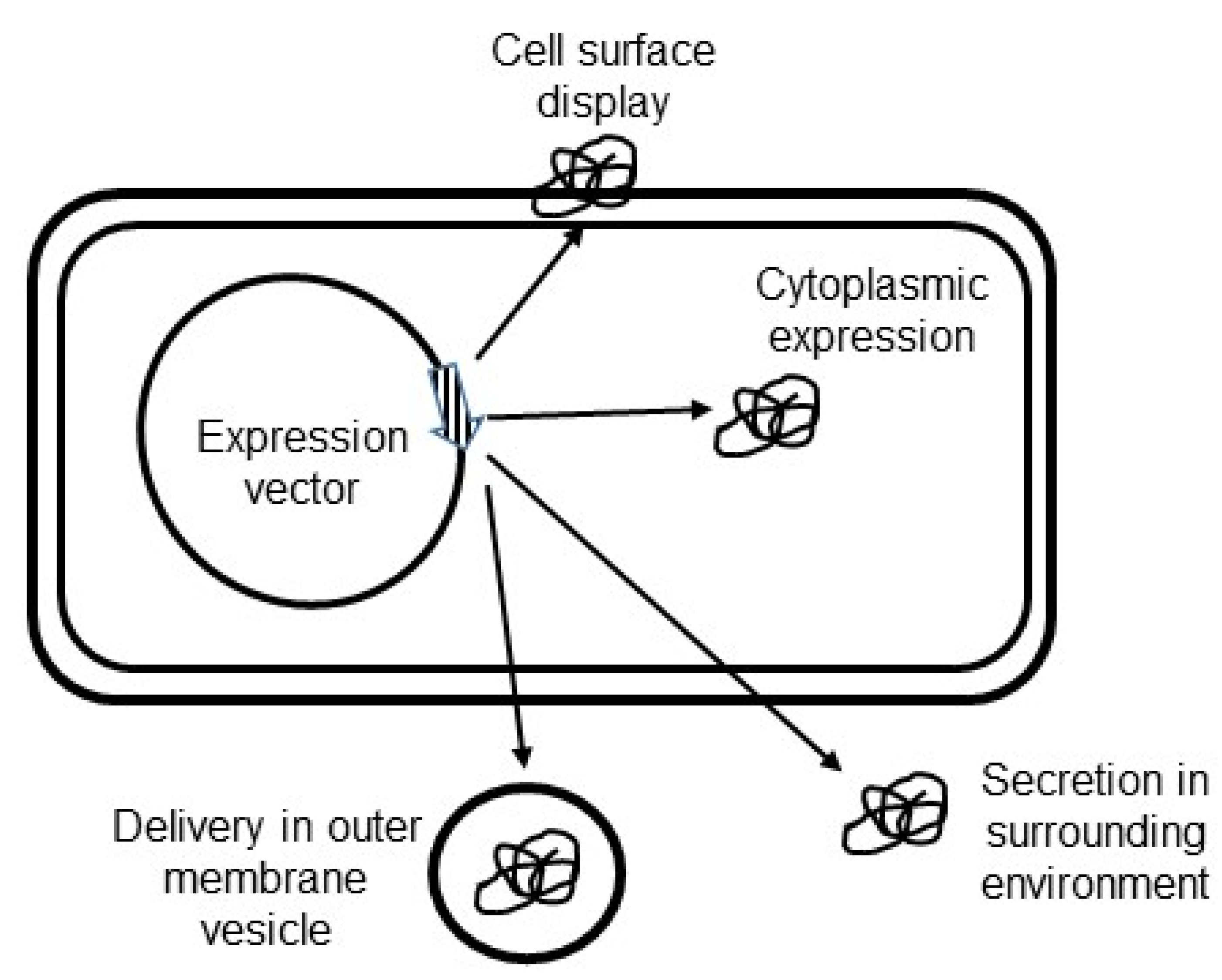
4.4. Targeting of Antigen Constructs in Helicobacter pylori
4.5. Delivery of DNA Vaccines by Helicobacter pylori
4.6. Overcoming Oral Tolerance and Immune Suppression by Helicobacter pylori
4.7. Bio-Containment of Recombinant Helicobacter pylori Vaccine Carrier
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moodley, Y.; Linz, B.; Bond, R.P.; Nieuwoudt, M.; Soodyall, H.; Schlebusch, C.M.; Bernhoft, S.; Hale, J.; Suerbaum, S.; Mugisha, L.; et al. Age of the association between Helicobacter pylori and man. PLoS Pathog. 2012, 8, e1002693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, M.M.; Sharaf, R.R.; Aziz, R.K. Helicobacter pylori: A poor man’s gut pathogen? Gut. Pathog. 2010, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, V.; Romano, M.; Bouquet, P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J. Gastroenterol. 2011, 17, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef]
- Wang, A.Y.; Peura, D.A. The prevalence and incidence of Helicobacter pylori-associated peptic ulcer disease and upper gastrointestinal bleeding throughout the world. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.I.; Ajayi, A.; Jolaiya, T.; Onyekwere, C.; Setshedi, M.; Schulz, C.; Otegbayo, J.A.; Ndip, R.; Dieye, Y.; Alboraie, M.; et al. Helicobacter pylori Infection in Africa: Update of the Current Situation and Challenges. Dig. Dis. 2022, 40, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.R.; Sachs, G.; Marcus, E.A. The role of acid inhibition in Helicobacter pylori eradication. F1000Research 2016, 5, 1747. [Google Scholar] [CrossRef] [Green Version]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of Bismuth in the Eradication of Helicobacter pylori. Am. J. Ther. 2017, 24, e751–e757. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.T. High-dose dual therapy versus bismuth-containing quadruple therapy for the treatment of Helicobacter pylori infection—A review of the strengths, weaknesses, and proposed solutions. Tzu-Chi Med. J. 2022, 34, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Malizia, T.; Tejada, M.; Marchetti, F.; Favini, P.; Pizzarelli, G.; Campa, M.; Senesi, S. Synergic interactions of macrolides and proton-pump inhibitors against Helicobacter pylori: A comparative in-vitro study. J. Antimicrob. Chemother. 1998, 41, 29–35. [Google Scholar] [CrossRef]
- Dos Santos Viana, I.; Cordeiro Santos, M.L.; Santos Marques, H.; Lima de Souza Goncalves, V.; Bittencourt de Brito, B.; Franca da Silva, F.A.; Oliveira, E.S.N.; Dantas Pinheiro, F.; Fernandes Teixeira, A.; Tanajura Costa, D.; et al. Vaccine development against Helicobacter pylori: From ideal antigens to the current landscape. Expert Rev. Vaccines 2021, 20, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P. “Rescue” regimens after Helicobacter pylori treatment failure. World J. Gastroenterol. 2008, 14, 5385–5402. [Google Scholar] [CrossRef]
- Chen, X.G.; Correa, P.; Offerhaus, J.; Rodriguez, E.; Janney, F.; Hoffmann, E.; Fox, J.; Hunter, F.; Diavolitsis, S. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am. J. Clin. Pathol. 1986, 86, 575–582. [Google Scholar] [CrossRef]
- Ruggiero, P. Helicobacter pylori and inflammation. Curr. Pharm. Des. 2010, 16, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Mentucci, F.; Voli, A.; Petrella, A.; Porta, A.; Tosco, A. Helicobacter pylori Pathogen-Associated Molecular Patterns: Friends or Foes? Int. J. Mol. Sci. 2022, 23, 3531. [Google Scholar] [CrossRef] [PubMed]
- Karkhah, A.; Ebrahimpour, S.; Rostamtabar, M.; Koppolu, V.; Darvish, S.; Vasigala, V.K.R.; Validi, M.; Nouri, H.R. Helicobacter pylori evasion strategies of the host innate and adaptive immune responses to survive and develop gastrointestinal diseases. Microbiol. Res. 2019, 218, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mejias-Luque, R.; Gerhard, M. Immune Evasion Strategies and Persistence of Helicobacter pylori. Curr. Top. Microbiol. Immunol. 2017, 400, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.T.B. Strategies used by Helicobacter pylori to establish persistent infection. World J. Gastroenterol. 2017, 23, 2870–2882. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Leone, I.; Suraci, E.; Imeneo, M.; Luzza, F. Helicobacter pylori and T Helper Cells: Mechanisms of Immune Escape and Tolerance. J. Immunol. Res. 2015, 2015, 981328. [Google Scholar] [CrossRef] [PubMed]
- Denic, M.; Touati, E.; De Reuse, H. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2020, 25, e12736. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.; Ali, A.; Akbar, H.; Sayaf, A.M.; Khan, A.; Wei, D.Q. Immunoinformatics approaches to explore Helicobacter Pylori proteome (Virulence Factors) to design B and T cell multi-epitope subunit vaccine. Sci. Rep. 2019, 9, 13321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urrutia-Baca, V.H.; Gomez-Flores, R.; De La Garza-Ramos, M.A.; Tamez-Guerra, P.; Lucio-Sauceda, D.G.; Rodriguez-Padilla, M.C. Immunoinformatics Approach to Design a Novel Epitope-Based Oral Vaccine Against Helicobacter pylori. J. Comput. Biol. 2019, 26, 1177–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, M.; Mao, X.H.; Li, J.X.; Tong, W.D.; Wang, B.; Zhang, Y.J.; Guo, G.; Zhao, Z.J.; Li, L.; Wu, D.L.; et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Formichella, L.; Romberg, L.; Bolz, C.; Vieth, M.; Geppert, M.; Gottner, G.; Nolting, C.; Walter, D.; Schepp, W.; Schneider, A.; et al. A novel line immunoassay based on recombinant virulence factors enables highly specific and sensitive serologic diagnosis of Helicobacter pylori infection. Clin. Vaccine Immunol. 2013, 20, 1703–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, S.F.; Moise, L.; Lee, D.S.; Kim, W.; Zhang, S.; Lee, J.; Rogers, A.B.; Martin, W.; De Groot, A.S. HelicoVax: Epitope-based therapeutic Helicobacter pylori vaccination in a mouse model. Vaccine 2011, 29, 2085–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Dai, L.X.; Pan, X.; Wang, H.; Li, B.; Zhu, J.; Li, M.Y.; Shi, X.L.; Wang, B.N. Protection against Helicobacter pylori infection in BALB/c mice by oral administration of multi-epitope vaccine of CTB-UreI-UreB. Pathog. Dis. 2015, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, C.; Cheng, W.; Duan, G.; Shi, Q.; Chen, S.; Fan, Q. Delivery of Helicobacter pylori HpaA to gastrointestinal mucosal immune sites using Lactococcus lactis and its immune efficacy in mice. Biotechnol. Lett. 2018, 40, 585–590. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Ye, J.; Ning, L.; Luo, J.; Zhang, L.; Jiang, Y.; Xi, Y.; Ning, Y. Antibody Production and Th1-biased Response Induced by an Epitope Vaccine Composed of Cholera Toxin B Unit and Helicobacter pylori Lpp20 Epitopes. Helicobacter 2016, 21, 234–248. [Google Scholar] [CrossRef]
- Sutton, P.; Boag, J.M. Status of vaccine research and development for Helicobacter pylori. Vaccine 2019, 37, 7295–7299. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Selgrad, M.; Wex, T.; Romi, B.; Borgogni, E.; Spensieri, F.; Zedda, L.; Ruggiero, P.; Pancotto, L.; Censini, S.; et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: A randomised, placebo-controlled phase 1/2 study. Lancet Gastroenterol. Hepatol. 2018, 3, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Ren, B.; Zhou, X.; Cheng, L. Helicobacter pylori in the Oral Cavity: Current Evidence and Potential Survival Strategies. Int. J. Mol. Sci. 2022, 23, 13646. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, T.; Laforsch, S.; Hurwitz, R.; Brombacher, F.; Meyer, T.F. Immunity against Helicobacter pylori: Significance of interleukin-4 receptor alpha chain status and gender of infected mice. Infect. Immun. 2001, 69, 556–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Yang, H.; Tang, F.; Yin, R.; Liu, H.; Gong, X.; Wei, J.; Zhang, Y.; Xu, G.; Liu, K. Oral Immunization with a Multivalent Epitope-Based Vaccine, Based on NAP, Urease, HSP60, and HpaA, Provides Therapeutic Effect on H. pylori Infection in Mongolian gerbils. Front. Cell Infect. Microbiol. 2017, 7, 349. [Google Scholar] [CrossRef] [Green Version]
- Marshall, B.; Schoep, T. Helicobacter pylori as a vaccine delivery system. Helicobacter 2007, 12, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Axon, A.T. Are all helicobacters equal? Mechanisms of gastroduodenal pathology and their clinical implications. Gut 1999, 45, I1–I4. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.P.; Holck, S. Possible evidence of invasiveness of Helicobacter (Campylobacter) pylori. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Necchi, V.; Candusso, M.E.; Tava, F.; Luinetti, O.; Ventura, U.; Fiocca, R.; Ricci, V.; Solcia, E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 2007, 132, 1009–1023. [Google Scholar] [CrossRef]
- Nagai, S.; Mimuro, H.; Yamada, T.; Baba, Y.; Moro, K.; Nochi, T.; Kiyono, H.; Suzuki, T.; Sasakawa, C.; Koyasu, S. Role of Peyer’s patches in the induction of Helicobacter pylori-induced gastritis. Proc. Natl. Acad. Sci. USA 2007, 104, 8971–8976. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Lehours, P. Review - Helicobacter, inflammation, immunology and vaccines. Helicobacter 2020, 25, e12737. [Google Scholar] [CrossRef]
- Bijlsma, J.J.; Vandenbroucke-Grauls, C.M.; Phadnis, S.H.; Kusters, J.G. Identification of virulence genes of Helicobacter pylori by random insertion mutagenesis. Infect. Immun. 1999, 67, 2433–2440. [Google Scholar] [CrossRef] [Green Version]
- Salama, N.R.; Shepherd, B.; Falkow, S. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J. Bacteriol. 2004, 186, 7926–7935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dailidiene, D.; Dailide, G.; Kersulyte, D.; Berg, D.E. Contraselectable streptomycin susceptibility determinant for genetic manipulation and analysis of Helicobacter pylori. Appl. Environ. Microbiol. 2006, 72, 5908–5914. [Google Scholar] [CrossRef] [Green Version]
- Debowski, A.W.; Gauntlett, J.C.; Li, H.; Liao, T.; Sehnal, M.; Nilsson, H.O.; Marshall, B.J.; Benghezal, M. Xer-cise in Helicobacter pylori: One-step transformation for the construction of markerless gene deletions. Helicobacter 2012, 17, 435–443. [Google Scholar] [CrossRef]
- Debowski, A.W.; Carnoy, C.; Verbrugghe, P.; Nilsson, H.O.; Gauntlett, J.C.; Fulurija, A.; Camilleri, T.; Berg, D.E.; Marshall, B.J.; Benghezal, M. Xer recombinase and genome integrity in Helicobacter pylori, a pathogen without topoisomerase IV. PLoS ONE 2012, 7, e33310. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Ellermeier, C.D.; Janakiraman, A.; Slauch, J.M. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 2002, 290, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Heuermann, D.; Haas, R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 1998, 257, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Humbert, O.; Salama, N.R. The Helicobacter pylori HpyAXII restriction-modification system limits exogenous DNA uptake by targeting GTAC sites but shows asymmetric conservation of the DNA methyltransferase and restriction endonuclease components. Nucleic Acids Res. 2008, 36, 6893–6906. [Google Scholar] [CrossRef] [PubMed]
- Bauerfeind, P.; Garner, R.; Dunn, B.E.; Mobley, H.L. Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut 1997, 40, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.; De Reuse, H. Staying alive overdosed: How does Helicobacter pylori control urease activity? Int. J. Med. Microbiol. 2005, 295, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.M.; McDaniel, T.K.; Whitmire, J.M.; Gancz, H.; Guidotti, S.; Censini, S.; Merrell, D.S. Expanding the Helicobacter pylori genetic toolbox: Modification of an endogenous plasmid for use as a transcriptional reporter and complementation vector. Appl. Environ. Microbiol. 2007, 73, 7506–7514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, A.R.; Woodruff, A.J.; Connolly, L.E.; Sause, W.E.; Ottemann, K.M. Recombination-based in vivo expression technology identifies Helicobacter pylori genes important for host colonization. Infect. Immun. 2008, 76, 5632–5644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandermeulen, G.; Marie, C.; Scherman, D.; Preat, V. New generation of plasmid backbones devoid of antibiotic resistance marker for gene therapy trials. Mol. Ther. 2011, 19, 1942–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.H.; Mairhofer, J. Marker-free plasmids for biotechnological applications - implications and perspectives. Trends Biotechnol. 2013, 31, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Mignon, C.; Sodoyer, R.; Werle, B. Antibiotic-free selection in biotherapeutics: Now and forever. Pathogens 2015, 4, 157–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyed, N.; Zahedifard, F.; Habibzadeh, S.; Yousefi, R.; Lajevardi, M.S.; Gholami, E.; Rafati, S. Antibiotic-Free Nanoplasmids as Promising Alternatives for Conventional DNA Vectors. Vaccines 2022, 10, 1710. [Google Scholar] [CrossRef]
- Galan, J.E.; Nakayama, K.; Curtiss, R., 3rd. Cloning and characterization of the asd gene of Salmonella typhimurium: Use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene 1990, 94, 29–35. [Google Scholar] [CrossRef]
- Mairhofer, J.; Pfaffenzeller, I.; Merz, D.; Grabherr, R. A novel antibiotic free plasmid selection system: Advances in safe and efficient DNA therapy. Biotechnol. J. 2008, 3, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.; Carnes, A.E.; Hodgson, C.P.; Williams, J.A. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine 2009, 27, 6454–6459. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Good, L. Plasmid selection in Escherichia coli using an endogenous essential gene marker. BMC Biotechnol. 2008, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Castro, C.P.; Drumond, M.M.; Batista, V.L.; Nunes, A.; Mancha-Agresti, P.; Azevedo, V. Vector Development Timeline for Mucosal Vaccination and Treatment of Disease Using Lactococcus lactis and Design Approaches of Next Generation Food Grade Plasmids. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.; Cortes-Perez, N.G.; de Moreno de Leblanc, A.; Loiseau, J.; Aucouturier, A.; Langella, P.; LeBlanc, J.G.; Bermudez-Humaran, L.G. Use of genetically modified lactic acid bacteria and bifidobacteria as live delivery vectors for human and animal health. Gut Microbes 2022, 14, 2110821. [Google Scholar] [CrossRef] [PubMed]
- Clark-Curtiss, J.E.; Curtiss, R., 3rd. Salmonella Vaccines: Conduits for Protective Antigens. J. Immunol. 2018, 200, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Karita, M.; Etterbeek, M.L.; Forsyth, M.H.; Tummuru, M.K.; Blaser, M.J. Characterization of Helicobacter pylori dapE and construction of a conditionally lethal dapE mutant. Infect. Immun. 1997, 65, 4158–4164. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Dieye, Y.; Hoekman, A.J.; Clier, F.; Juillard, V.; Boot, H.J.; Piard, J.C. Ability of Lactococcus lactis to export viral capsid antigens: A crucial step for development of live vaccines. Appl. Environ. Microbiol. 2003, 69, 7281–7288. [Google Scholar] [CrossRef] [Green Version]
- Saier, M.H., Jr. Protein secretion and membrane insertion systems in gram-negative bacteria. J. Membr. Biol. 2006, 214, 75–90. [Google Scholar] [CrossRef]
- Bumann, D.; Aksu, S.; Wendland, M.; Janek, K.; Zimny-Arndt, U.; Sabarth, N.; Meyer, T.F.; Jungblut, P.R. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 2002, 70, 3396–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.; Weeks, D.L.; Shin, J.M.; Scott, D.R.; Young, M.K.; Sachs, G. Proteins released by Helicobacter pylori in vitro. J. Bacteriol. 2002, 184, 6155–6162. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Lim, J.M.; Weinberg, M.V.; Wells, L.; Hoover, T.R. Direct analysis of the extracellular proteome from two strains of Helicobacter pylori. Proteomics 2007, 7, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Alm, R.A.; Bina, J.; Andrews, B.M.; Doig, P.; Hancock, R.E.; Trust, T.J. Comparative genomics of Helicobacter pylori: Analysis of the outer membrane protein families. Infect. Immun. 2000, 68, 4155–4168. [Google Scholar] [CrossRef] [Green Version]
- Panthel, K.; Meinel, K.M.; Sevil Domenech, V.E.; Trulzsch, K.; Russmann, H. Salmonella type III-mediated heterologous antigen delivery: A versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int. J. Med. Microbiol. 2008, 298, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Russman, H. Inverted pathogenicity: The use of pathogen-specific molecular mechanisms for prevention or therapy of disease. Int. J. Med. Microbiol. 2004, 293, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Li, X.; Wang, J.; Wang, Y.; Zhang, C.; Dai, S.; Wang, X.; Deng, X.; Zhao, L.; Shan, B. Outer Membrane Vesicles Secreted by Helicobacter pylori Transmitting Gastric Pathogenic Virulence Factors. ACS Omega 2022, 7, 240–258. [Google Scholar] [CrossRef]
- Mullaney, E.; Brown, P.A.; Smith, S.M.; Botting, C.H.; Yamaoka, Y.Y.; Terres, A.M.; Kelleher, D.P.; Windle, H.J. Proteomic and functional characterization of the outer membrane vesicles from the gastric pathogen Helicobacter pylori. Proteomics Clin. Appl. 2009, 3, 785–796. [Google Scholar] [CrossRef]
- Blaas, S.H.; Stieber-Gunckel, M.; Falk, W.; Obermeier, F.; Rogler, G. CpG-oligodeoxynucleotides stimulate immunoglobulin A secretion in intestinal mucosal B cells. Clin. Exp. Immunol. 2009, 155, 534–540. [Google Scholar] [CrossRef]
- Becker, P.D.; Noerder, M.; Guzman, C.A. Genetic immunization: Bacteria as DNA vaccine delivery vehicles. Hum. Vaccin. 2008, 4, 189–202. [Google Scholar] [CrossRef]
- Kennemann, L.; Didelot, X.; Aebischer, T.; Kuhn, S.; Drescher, B.; Droege, M.; Reinhardt, R.; Correa, P.; Meyer, T.F.; Josenhans, C.; et al. Helicobacter pylori genome evolution during human infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5033–5038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulick, S.; Moccia, C.; Didelot, X.; Falush, D.; Kraft, C.; Suerbaum, S. Mosaic DNA imports with interspersions of recipient sequence after natural transformation of Helicobacter pylori. PLoS ONE 2008, 3, e3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahara, K.; Kawai, M.; Furuta, Y.; Takahashi, N.; Handa, N.; Tsuru, T.; Oshima, K.; Yoshida, M.; Azuma, T.; Hattori, M.; et al. Genome-wide survey of mutual homologous recombination in a highly sexual bacterial species. Genome Biol. Evol. 2012, 4, 628–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrer, S.; Holsten, L.; Weiss, E.; Benghezal, M.; Fischer, W.; Haas, R. Multiple pathways of plasmid DNA transfer in Helicobacter pylori. PLoS ONE 2012, 7, e45623. [Google Scholar] [CrossRef] [Green Version]
- Varga, M.G.; Shaffer, C.L.; Sierra, J.C.; Suarez, G.; Piazuelo, M.B.; Whitaker, M.E.; Romero-Gallo, J.; Krishna, U.S.; Delgado, A.; Gomez, M.A.; et al. Pathogenic Helicobacter pylori strains translocate DNA and activate TLR9 via the cancer-associated cag type IV secretion system. Oncogene 2016, 35, 6262–6269. [Google Scholar] [CrossRef] [Green Version]
- Tegtmeyer, N.; Linz, B.; Yamaoka, Y.; Backert, S. Unique TLR9 Activation by Helicobacter pylori Depends on the cag T4SS, But Not on VirD2 Relaxases or VirD4 Coupling Proteins. Curr. Microbiol. 2022, 79, 121. [Google Scholar] [CrossRef] [PubMed]
- Neuper, T.; Frauenlob, T.; Sarajlic, M.; Posselt, G.; Wessler, S.; Horejs-Hoeck, J. TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. pylori-Infected Human DCs. Int. J. Mol. Sci. 2020, 21, 3897. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Huang, J.C.; Cheng, H.H.; Wu, M.C.; Huang, M.Z.; Hsu, H.Y.; Chen, Y.A.; Hsu, C.Y.; Pan, Y.J.; Chu, Y.T.; et al. Helicobacter pylori cholesterol glucosylation modulates autophagy for increasing intracellular survival in macrophages. Cell Microbiol. 2018, 20, e12947. [Google Scholar] [CrossRef]
- Rezende, R.M.; Weiner, H.L. Oral tolerance: An updated review. Immunol. Lett. 2022, 245, 29–37. [Google Scholar] [CrossRef]
- Lawson, L.B.; Norton, E.B.; Clements, J.D. Defending the mucosa: Adjuvant and carrier formulations for mucosal immunity. Curr. Opin. Immunol. 2011, 23, 414–420. [Google Scholar] [CrossRef]
- Beddoe, T.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010, 35, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, E.; O’Neal, C.J.; Mitchell, D.D.; Robien, M.A.; Zhang, Z.; Pickens, J.C.; Tan, X.J.; Korotkov, K.; Roach, C.; Krumm, B.; et al. Structural biology and structure-based inhibitor design of cholera toxin and heat-labile enterotoxin. Int. J. Med. Microbiol. 2004, 294, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, S.; Magnani, J.L.; Twiddy, E.M.; Holmes, R.K.; Ginsburg, V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 1988, 56, 1748–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anosova, N.G.; Chabot, S.; Shreedhar, V.; Borawski, J.A.; Dickinson, B.L.; Neutra, M.R. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer’s patches. Mucosal Immunol. 2008, 1, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Connell, T.D. Cholera toxin, LT-I, LT-IIa and LT-IIb: The critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev. Vaccines 2007, 6, 821–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G.; Nawar, H.; Tapping, R.I.; Russell, M.W.; Connell, T.D. The Type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect. Immun. 2004, 72, 6351–6358. [Google Scholar] [CrossRef] [Green Version]
- Nawar, H.F.; Arce, S.; Russell, M.W.; Connell, T.D. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect. Immun. 2007, 75, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Kiyono, H.; Yamamoto, M.; Imaoka, K.; Fujihashi, K.; Van Ginkel, F.W.; Noda, M.; Takeda, Y.; McGhee, J.R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 1997, 94, 5267–5272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Takeda, Y.; Yamamoto, M.; Kurazono, H.; Imaoka, K.; Yamamoto, M.; Fujihashi, K.; Noda, M.; Kiyono, H.; McGhee, J.R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 1997, 185, 1203–1210. [Google Scholar] [CrossRef]
- Lee, C.H.; Masso-Welch, P.; Hajishengallis, G.; Connell, T.D. TLR2-dependent modulation of dendritic cells by LT-IIa-B5, a novel mucosal adjuvant derived from a type II heat-labile enterotoxin. J. Leukoc. Biol. 2011, 90, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Nashar, T.O.; Webb, H.M.; Eaglestone, S.; Williams, N.A.; Hirst, T.R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: Receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc. Natl. Acad. Sci. USA 1996, 93, 226–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plant, A.; Williams, R.; Jackson, M.E.; Williams, N.A. The B subunit of Escherichia coli heat labile enterotoxin abrogates oral tolerance, promoting predominantly Th2-type immune responses. Eur. J. Immunol. 2003, 33, 3186–3195. [Google Scholar] [CrossRef]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melit, L.E.; Marginean, C.O.; Marginean, C.D.; Marginean, M.O. The Relationship between Toll-like Receptors and Helicobacter pylori-Related Gastropathies: Still a Controversial Topic. J. Immunol. Res. 2019, 2019, 8197048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Li, Z.S.; Du, Y.Q.; Gong, Y.F.; Yang, H.; Sun, B.; Jin, J. Construction of recombinant attenuated Salmonella typhimurium DNA vaccine expressing H pylori ureB and IL-2. World J. Gastroenterol. 2007, 13, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimman, T.G.; Smit, E.; Klein, M.R. Evidence-based biosafety: A review of the principles and effectiveness of microbiological containment measures. Clin. Microbiol. Rev. 2008, 21, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Fischer, W.; Tegtmeyer, N.; Stingl, K.; Backert, S. Four Chromosomal Type IV Secretion Systems in Helicobacter pylori: Composition, Structure and Function. Front. Microbiol. 2020, 11, 1592. [Google Scholar] [CrossRef]
- Oyarzabal, O.A.; Rad, R.; Backert, S. Conjugative transfer of chromosomally encoded antibiotic resistance from Helicobacter pylori to Campylobacter jejuni. J. Clin. Microbiol. 2007, 45, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ierardi, E.; Losurdo, G.; Mileti, A.; Paolillo, R.; Giorgio, F.; Principi, M.; Di Leo, A. The Puzzle of Coccoid Forms of Helicobacter pylori: Beyond Basic Science. Antibiotics 2020, 9, 293. [Google Scholar] [CrossRef]
- Curtiss, R., III; Xin, W.; Li, Y.; Kong, W.; Wanda, S.Y.; Gunn, B.; Wang, S. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit. Rev. Immunol. 2010, 30, 255–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P. Biocontainment strategies for live lactic acid bacteria vaccine vectors. Bioeng. Bugs 2010, 1, 75–77. [Google Scholar] [CrossRef]

| Vaccine | Country | Status | Type | Route | Refs. |
|---|---|---|---|---|---|
| Recombinant UreB/LTB fusion | China | Phase III | Prophylacitc/Therapeutic | Oral | [24] |
| Imevax/IMX101 (H. pylori GGT) | Germany | Phase I | Prophylacitc/Therapeutic | Intradermal and sublingual | [25] |
| HelicoVax (HLA class II epitopes) | USA | Preclinical | Prophylactic | Intramuscular and intranasal | [26] |
| Recombinant CTB-UreI-UreB | China | Preclinical | Prophylactic | N/A | [27] |
| Recombinant Vibrio cholerae expressing H. pylori HpaA antigen | Sweden | Preclinical | Prophylactic | N/A | [28] |
| CTB-Lpp20 | China | Preclinical | Prophylactic/therapeutic | Intraperitoneal | [29] |
| Recombinant HtrA | Australia | Preclinical | Prophylactic | N/A | [30] |
| Recombinant VacA-CagA-NAP | Germany | Phase I/II | Prophylactic | Intramuscular | [31] |
| Attenuated Shigella expressing UreB-HspA fusion | China | Preclinical | Prophylactic | Oral | [32] |
| Recombinant Salmonella expressing Urease or Urease + CT fusion | Germany | Preclinical | Prophylactic | Oral | [33] |
| Multi epitope: NAP, Urease, HSP60, and HpaA | China | Preclinical | Profilacitc/Therapeutic | Oral | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieye, Y.; Nguer, C.M.; Thiam, F.; Diouara, A.A.M.; Fall, C. Recombinant Helicobacter pylori Vaccine Delivery Vehicle: A Promising Tool to Treat Infections and Combat Antimicrobial Resistance. Antibiotics 2022, 11, 1701. https://doi.org/10.3390/antibiotics11121701
Dieye Y, Nguer CM, Thiam F, Diouara AAM, Fall C. Recombinant Helicobacter pylori Vaccine Delivery Vehicle: A Promising Tool to Treat Infections and Combat Antimicrobial Resistance. Antibiotics. 2022; 11(12):1701. https://doi.org/10.3390/antibiotics11121701
Chicago/Turabian StyleDieye, Yakhya, Cheikh Momar Nguer, Fatou Thiam, Abou Abdallah Malick Diouara, and Cheikh Fall. 2022. "Recombinant Helicobacter pylori Vaccine Delivery Vehicle: A Promising Tool to Treat Infections and Combat Antimicrobial Resistance" Antibiotics 11, no. 12: 1701. https://doi.org/10.3390/antibiotics11121701





