Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review
Abstract
:1. Introduction
1.1. Heterocyclic Compounds
1.2. Application of Heterocyclic Compounds
2. Antibacterial Activity of Heterocyclic Compounds
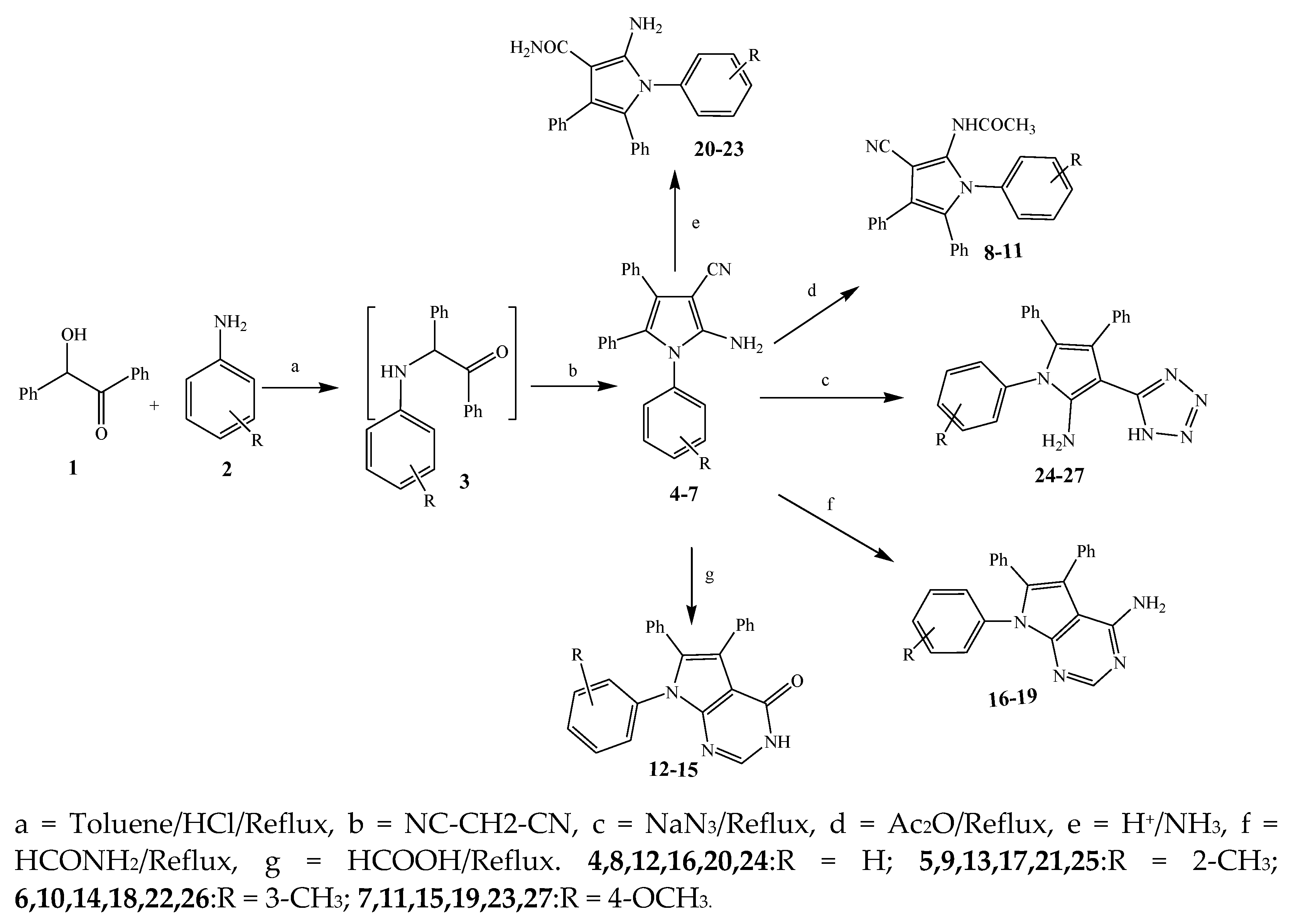
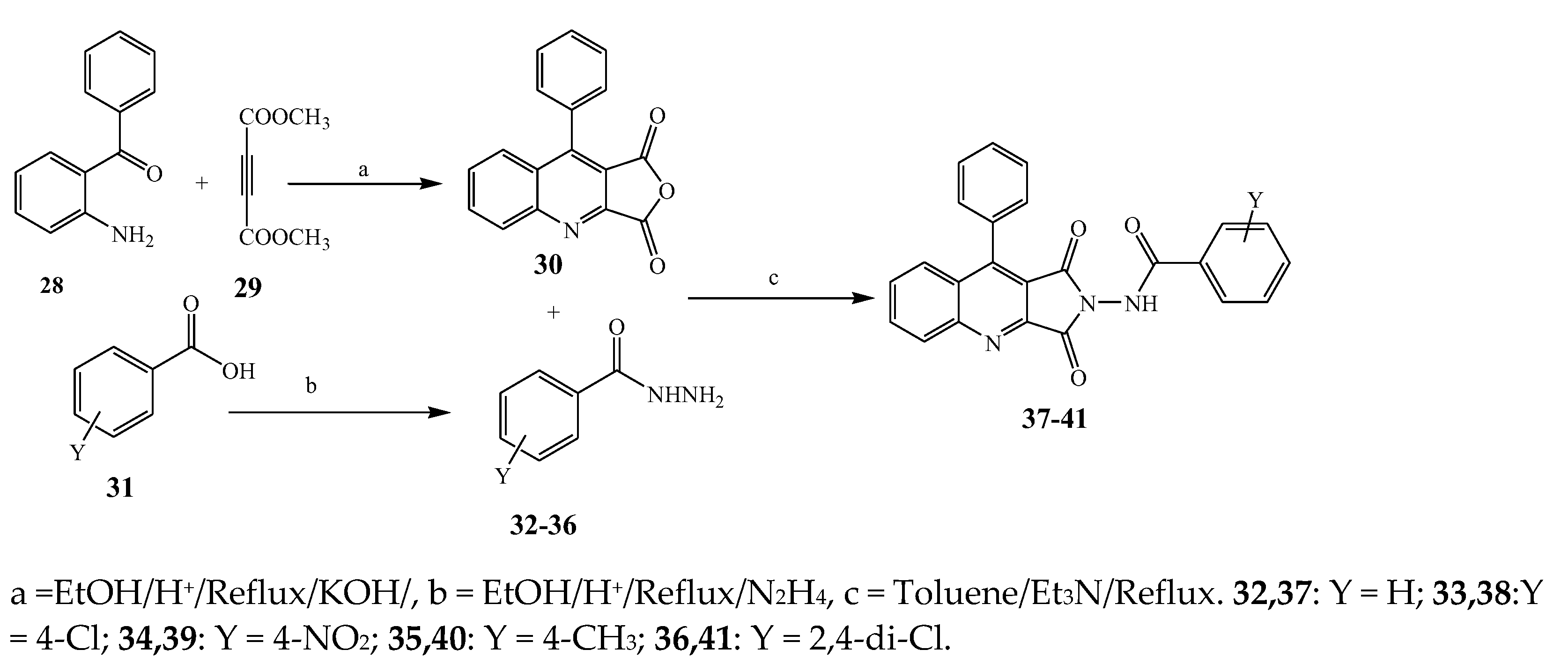
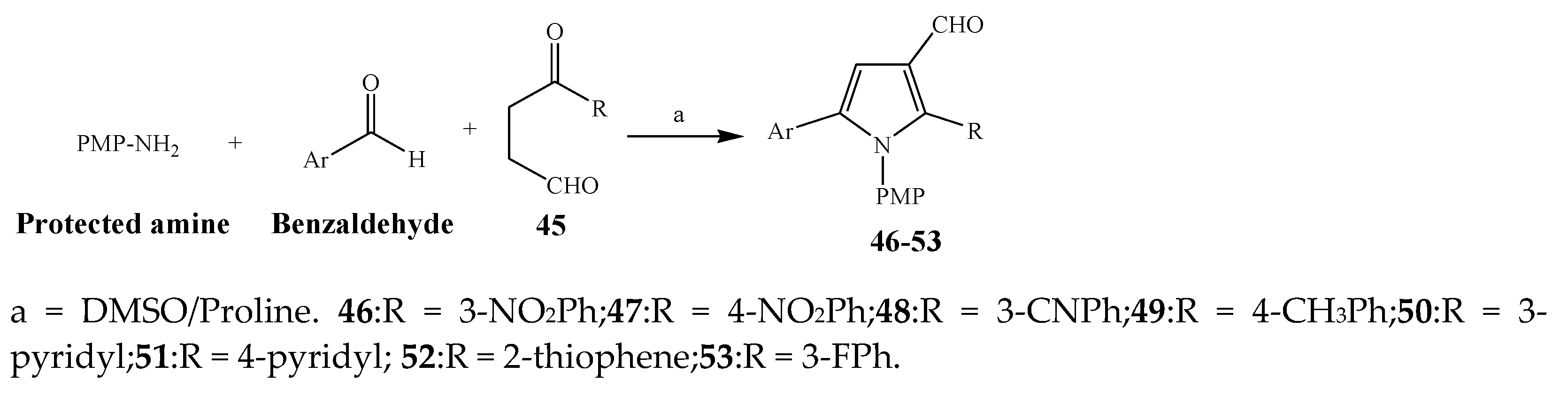
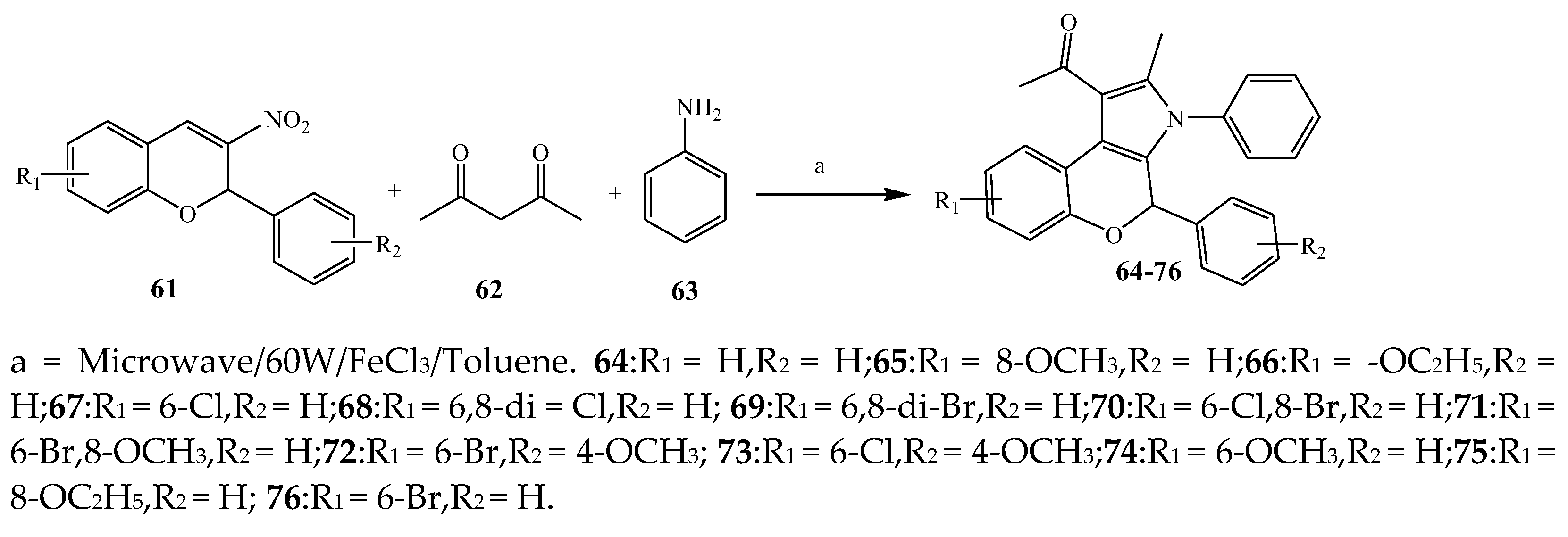
2.1. Synthesis and Antibacterial Activity of the Pyrrole
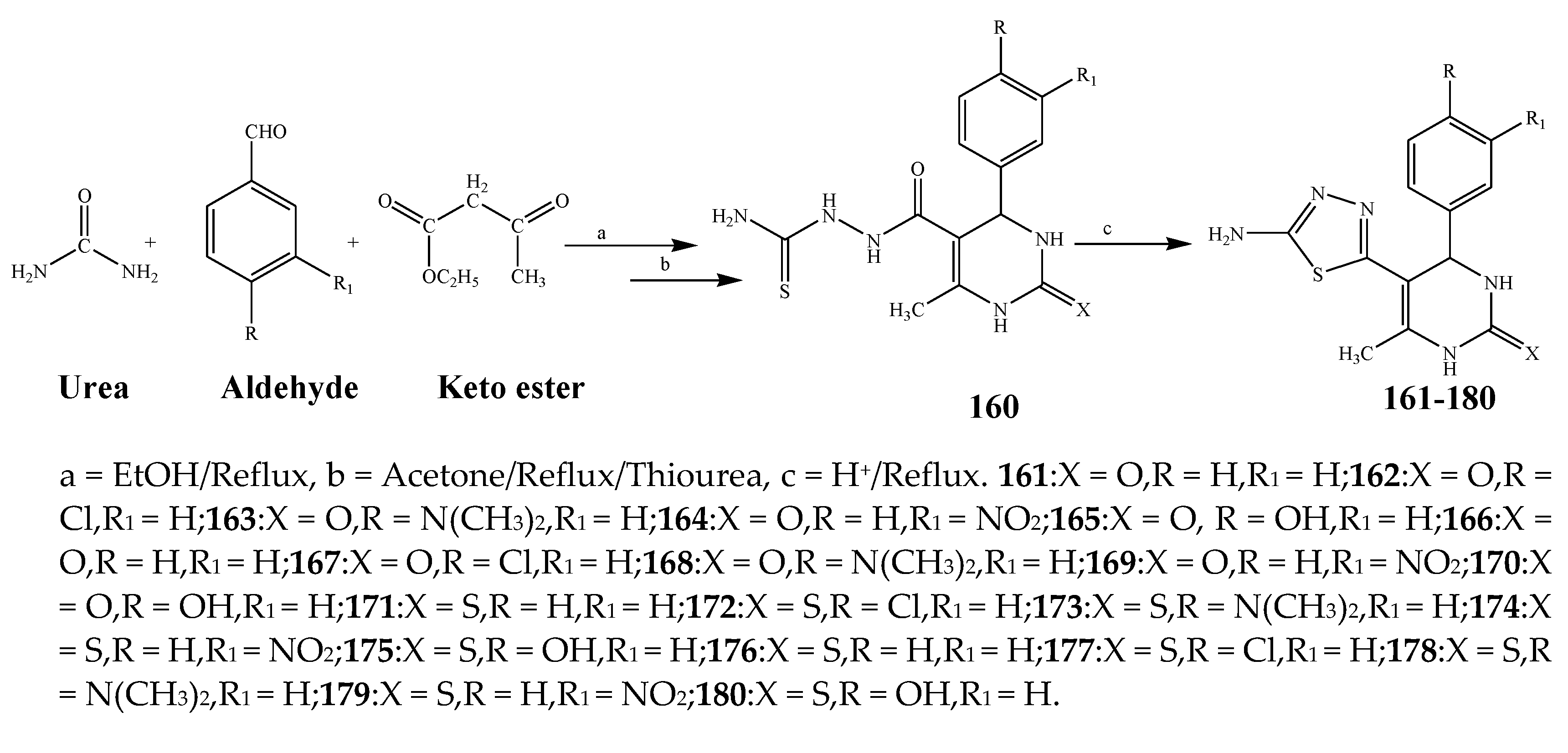
2.2. Synthesis and Antibacterial Activity of the Pyrimidine
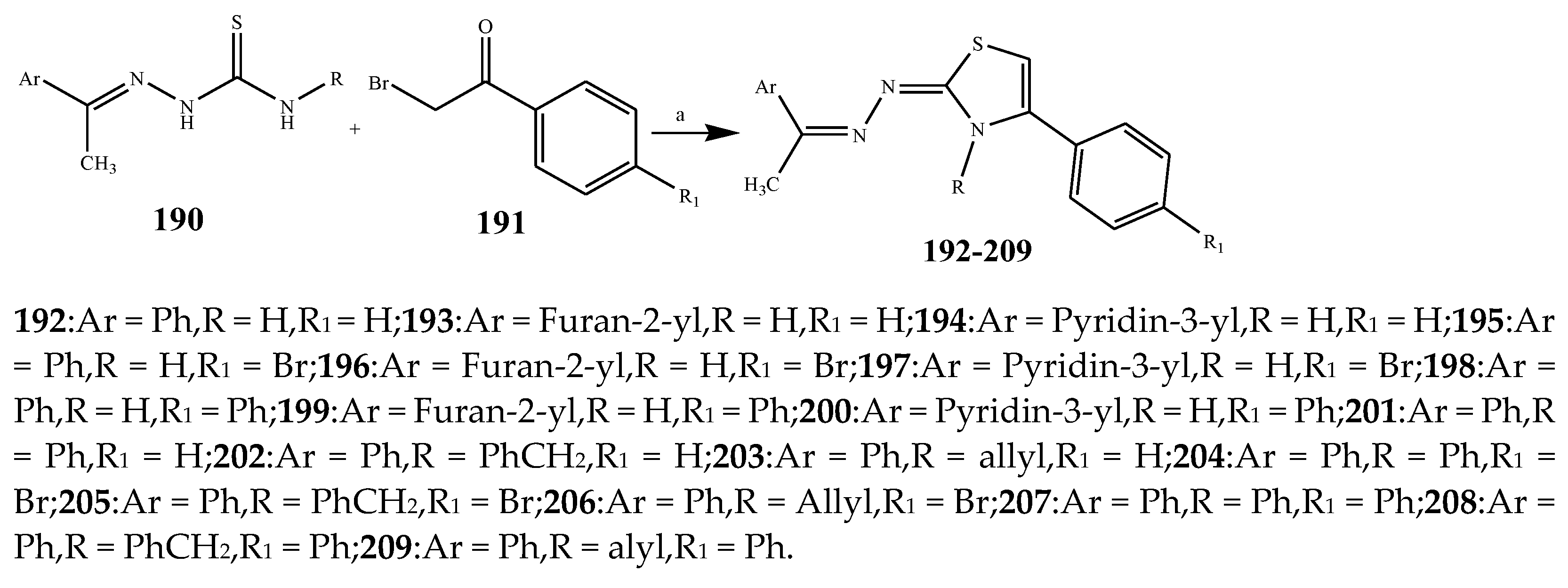
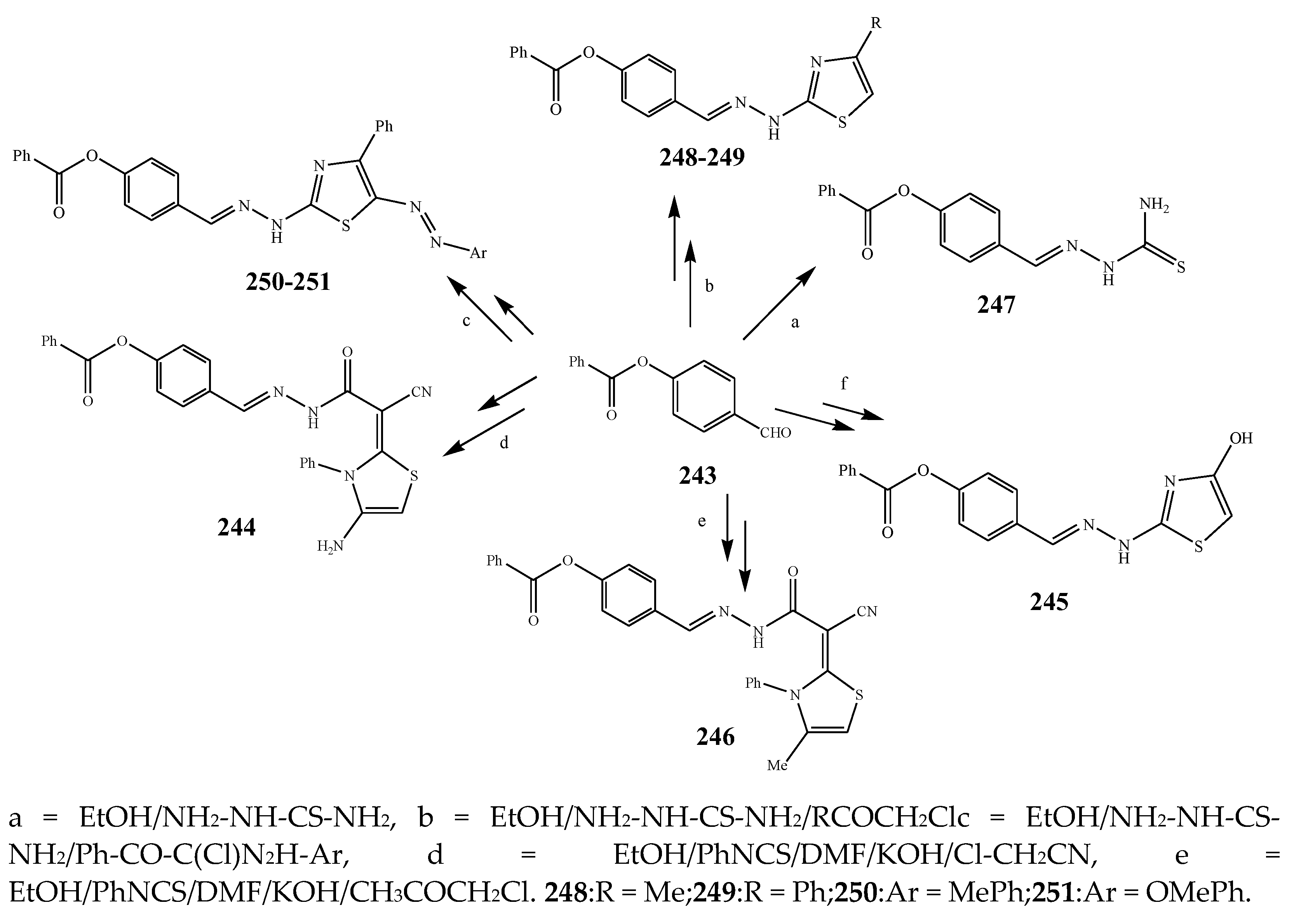
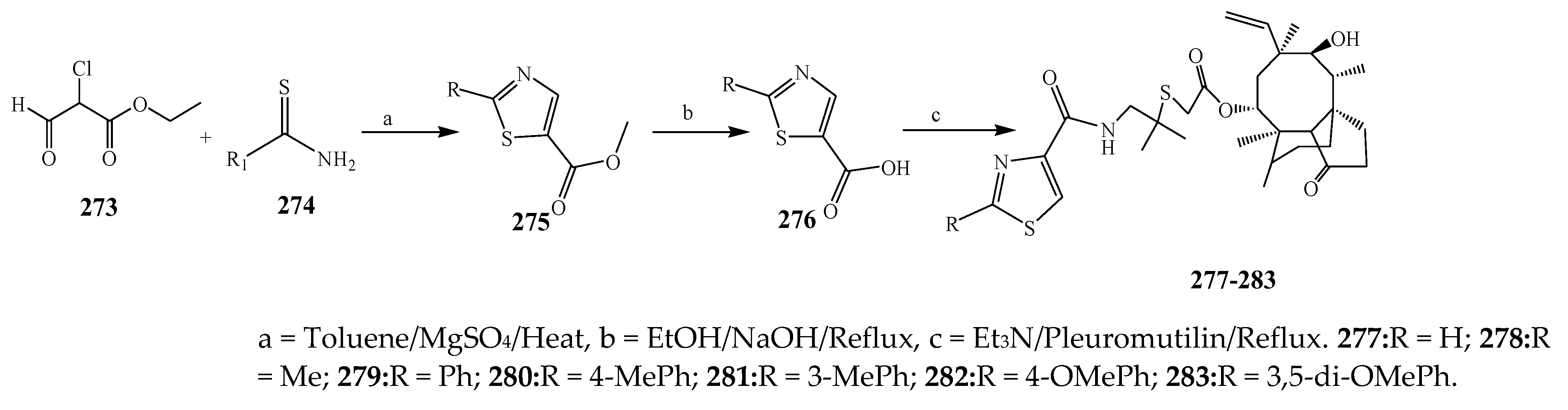
2.3. Synthesis and Antibacterial Activity of the Thiazole
2.4. Synthesis and Antibacterial Activity of the Pyridine
2.5. Synthesis and Antibacterial Activity of the Oxazole
2.6. Synthesis and Antibacterial Activity of the Thiazolidinone
2.7. Synthesis and Antibacterial Activity of the Imidazole
| Heterocyclic Compounds | Number/Code | Microbes | Reference |
|---|---|---|---|
| Pyrrole | 6, 10, 15 and 17 | B. subtilis | [16] |
| 16 | P. aeruginosa | [16] | |
| 18 | S. aureus | [16] | |
| 5 | E. coli | [16] | |
| 37 | E. faecalis, S. aureus, and E. coli | [19] | |
| 44 | S. aureus and E. coli | [21] | |
| 50, 51, and 52 | P. putida | [20] | |
| 65 and 71 | S. aureus and E. coli | [22] | |
| 49 | B. subtilis and S. aureus | [23] | |
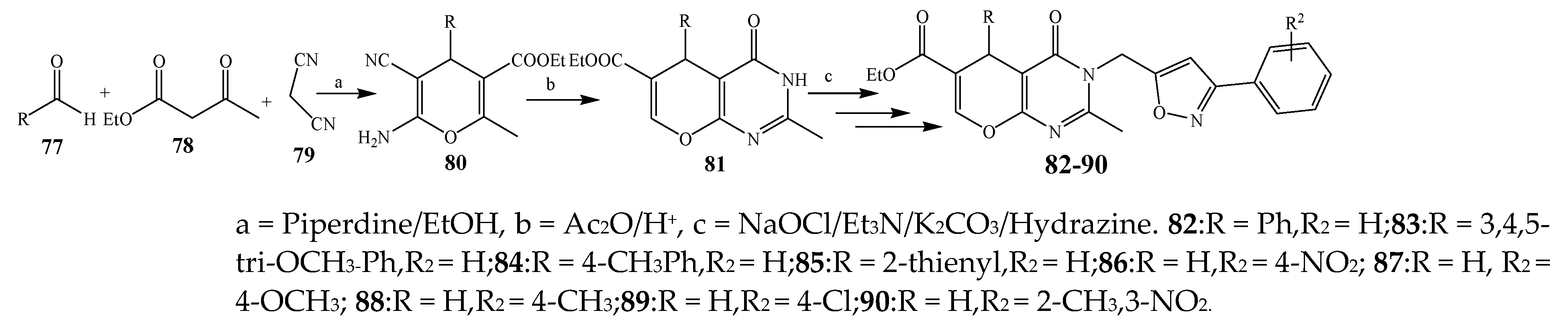
| Pyrimidine | 82, 86, and 88 | P. aeruginosa, S. aureus | [24] |
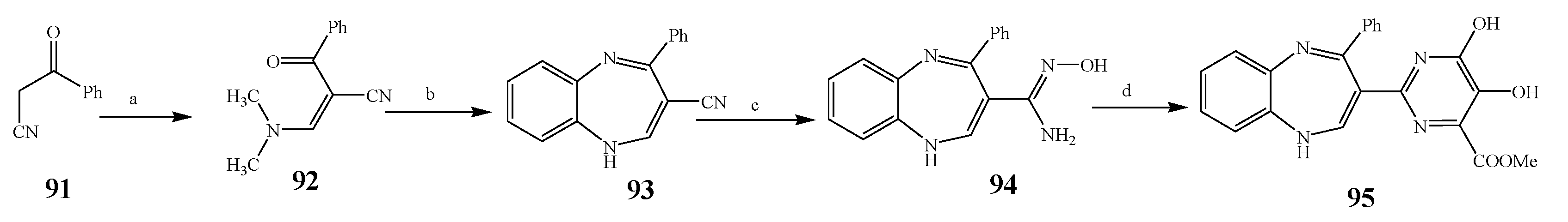
| 95 | E. coli and S. aureus | [25] | |
| 102, 101 and 106 | S. aureus, B. subtilis, E. coli, and S. typhimurium | [14] | |
| 118, 117, and 123 | E. coli | [26] | |
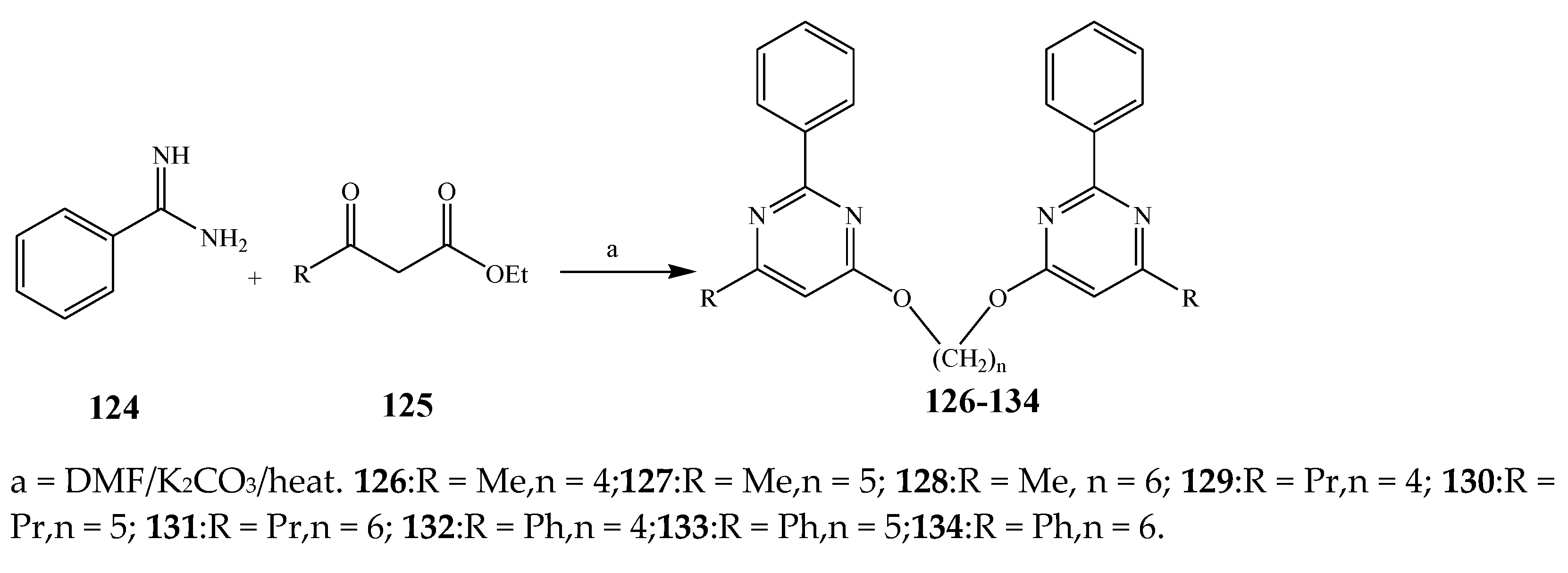
| 126–134 | B. subtilis, S. enterica, and M. luteus | [27] | |
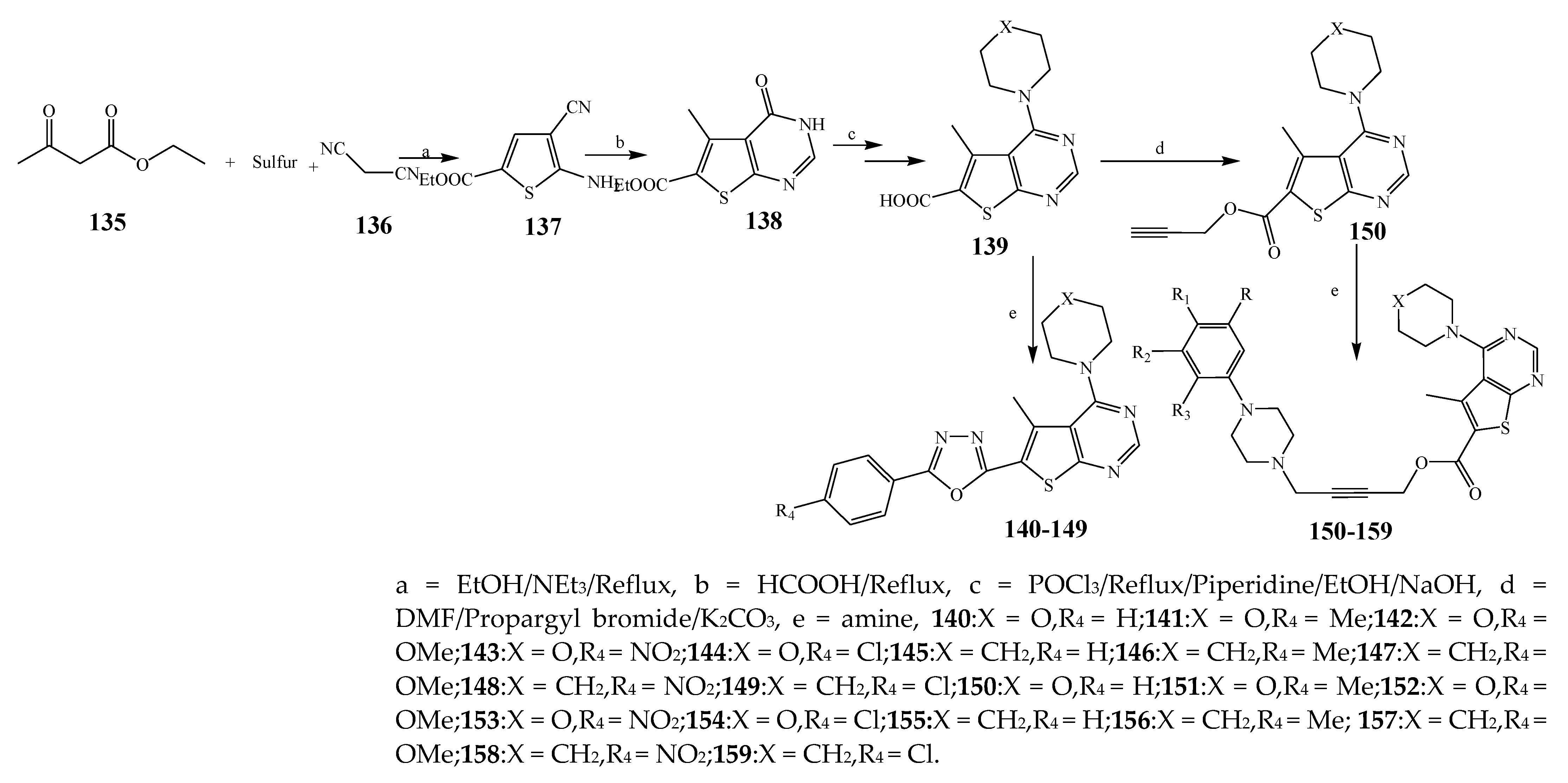
| 141 | P. aeruginosa, B. subtilis, S. aureus | [28] | |
| 161–170 | E. coli, S. aureus, and P. aeruginosa | [29] | |
| Thiazole | 192, 195, 194, 200 and 205 | E. coli | [30] |
| 195, 200, 192, 209, and 207 | K. pneumonia | [30] | |
| 194, 200, 192, 207, and 205 | P. aeruginosa higher | [30] | |
| 200, 205, 209, 207, and 196 | S. aureus | [30] | |
| 226 and 227 | E. coli, S. aureus, and B. subtilis | [31] | |
| 244, 247, 247, and 248 | S. aureus | [32] | |
| 247 | E. coli | [32] | |
| 248 | S. aureus and E. coli | [32] | |
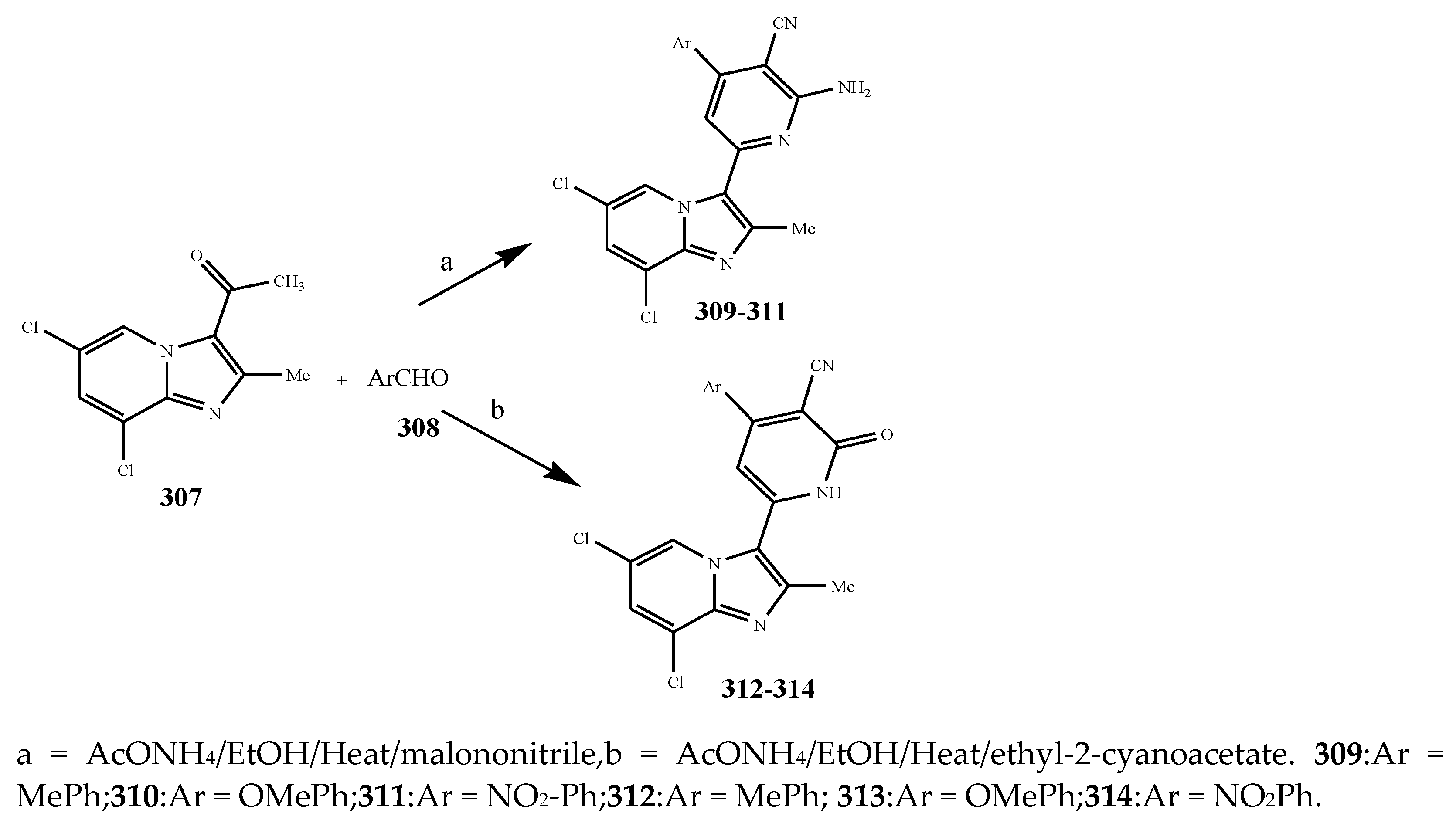
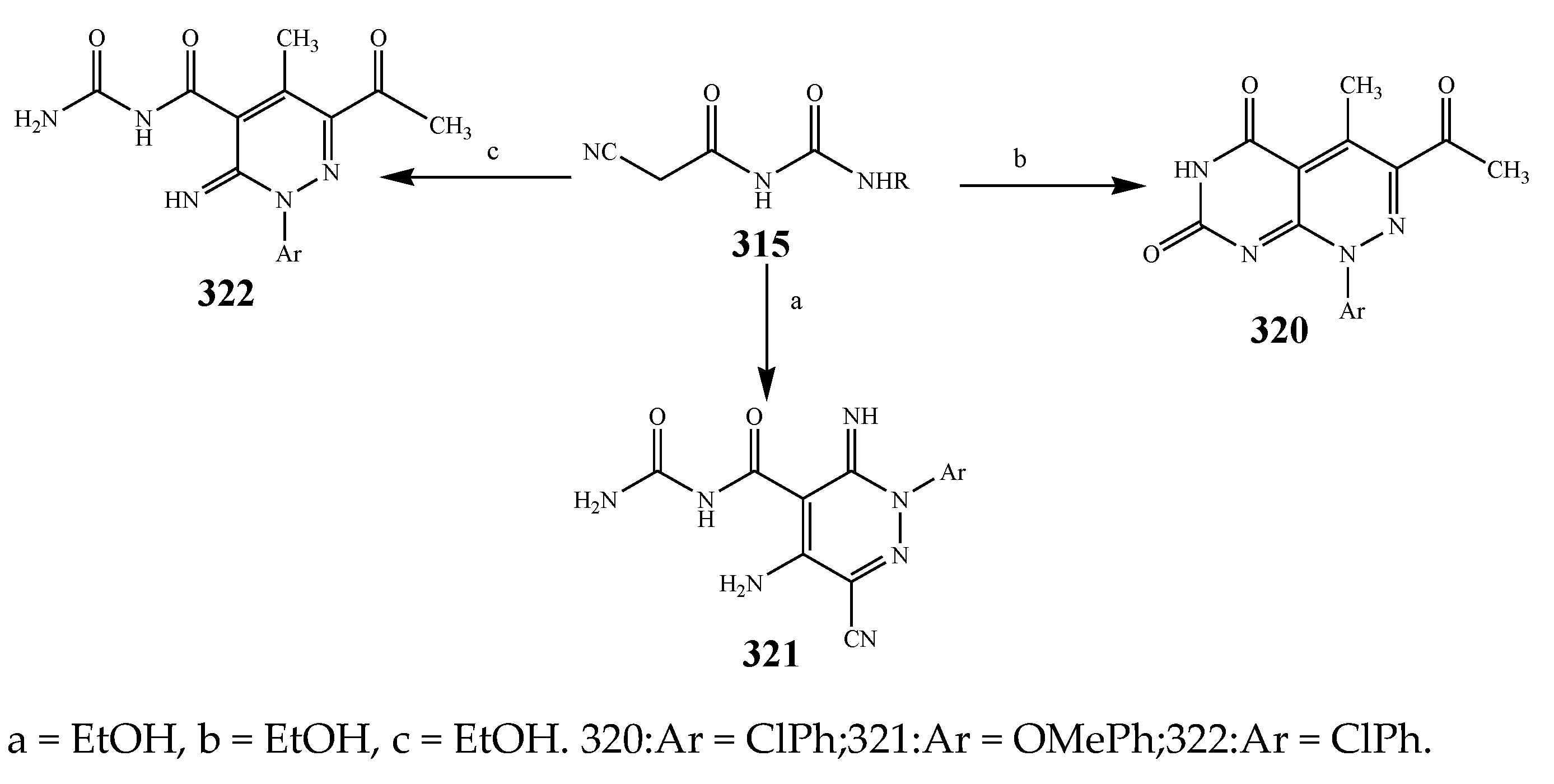
| Pyridine | 305 | B. subtilis | [37] |
| 291 | B. subtilis and S. aureus | [38] | |
| 317 and 316 | E. coli | [38] | |
| 318 | K. pneumonia | [38] | |
| 326–332 and 333–341 | Klebsiella planticola | [40] | |
| 344 | Vibrio parahaemolyticus | [41] | |
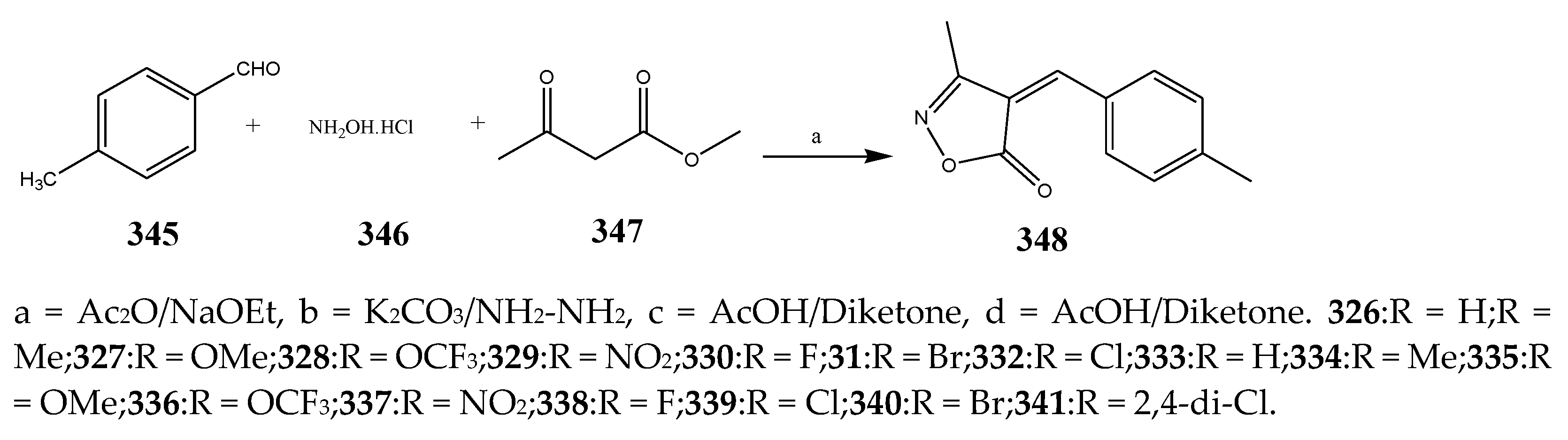
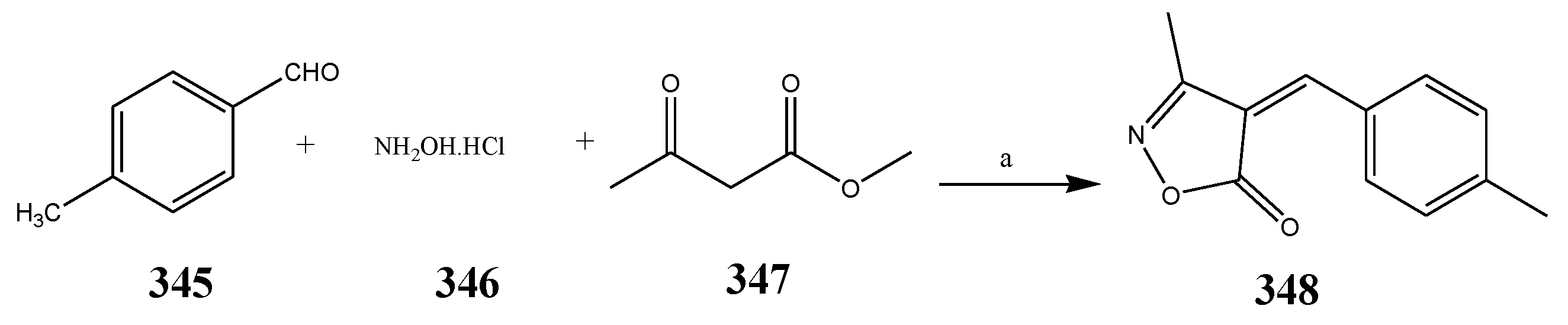
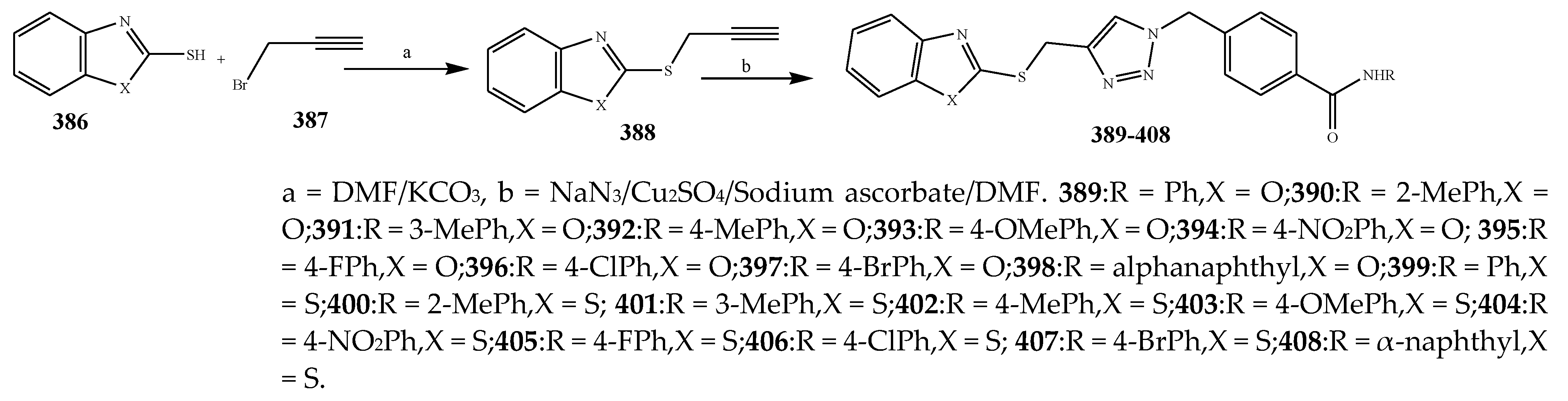
| Oxazole | 348 | E. faecium, and E. coli | [7] |
| 353 | P. aeruginosa | [42] | |
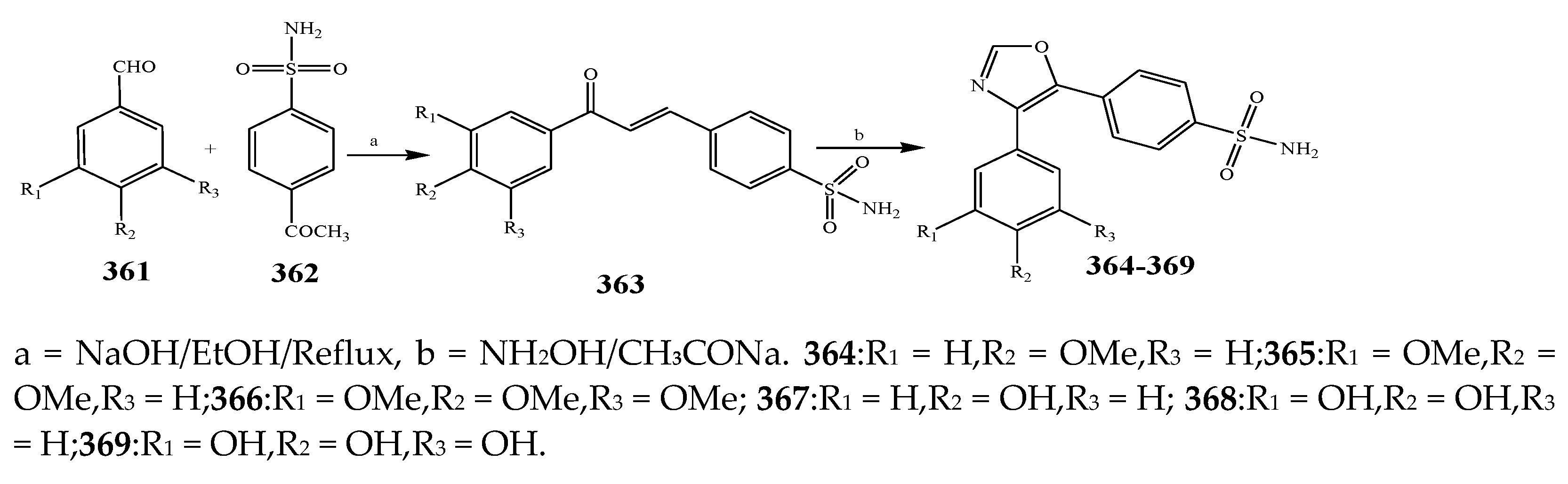
| 365, 367, 368, and 369 | S. epidermidis, E. coli, and P. mirabilis | [43] | |
| 385 | P. aerugenosa | [44] | |
| 384 | E. coli | [44] | |
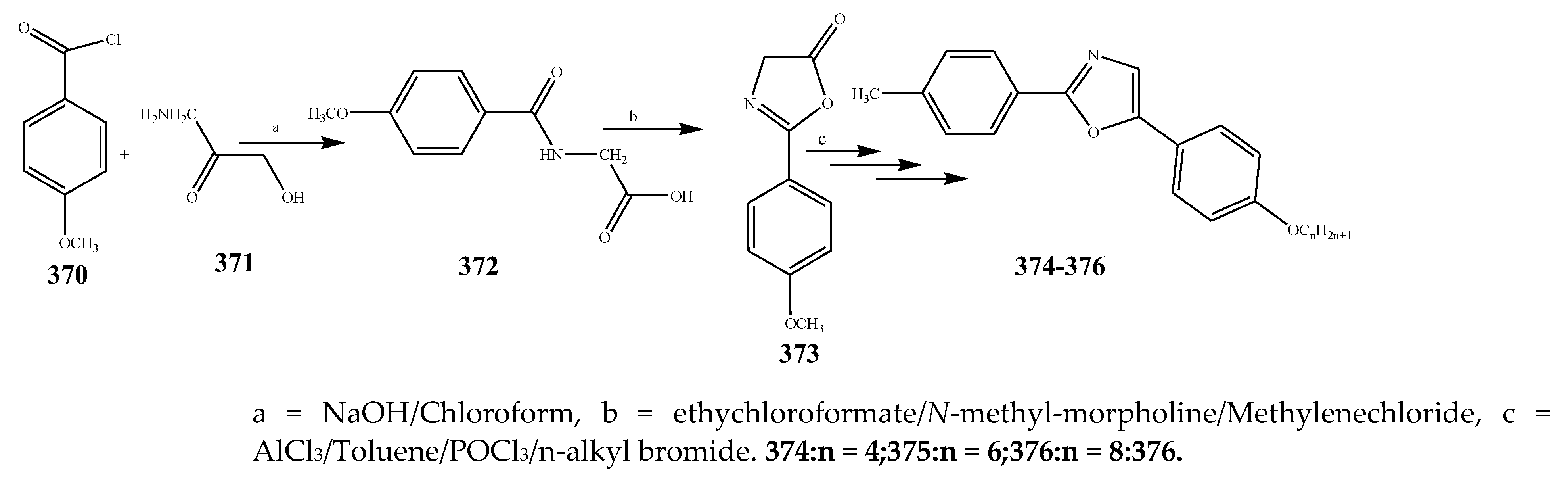
| 376 | P. aerugenosa | [44] | |
| 374 | E. coli | [44] | |
| 394 and 402 | E. coli and K. pneumonia | [45] | |
| 416 | S. aureus and E. coli | [46] | |
| 419 | S. enteric | [46] | |
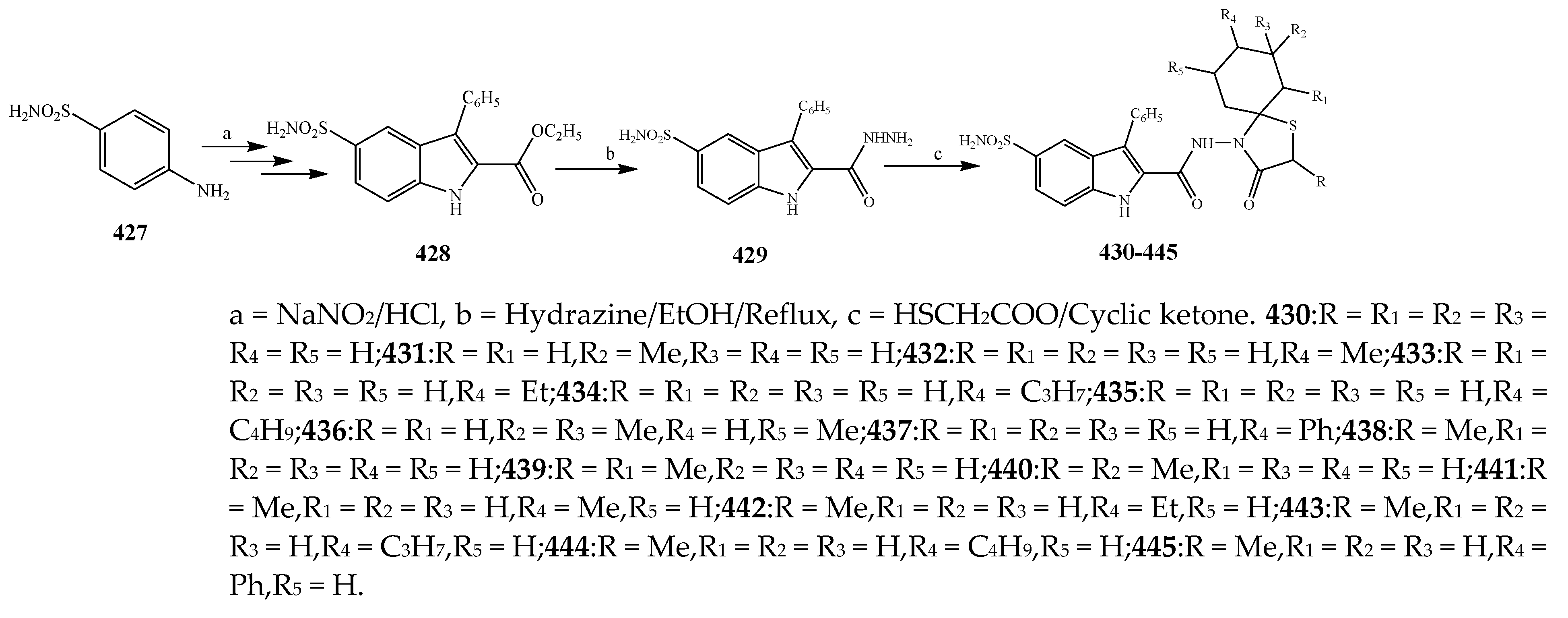
| Thiazolidinone | 434 | S. epidermidis | [47] |
| 458 | S. aureus | [29] | |
| 460 | E. coli | [29] | |
| 469 | B. subtilis | [48] | |
| 466 | P. aeruginosa | [48] | |
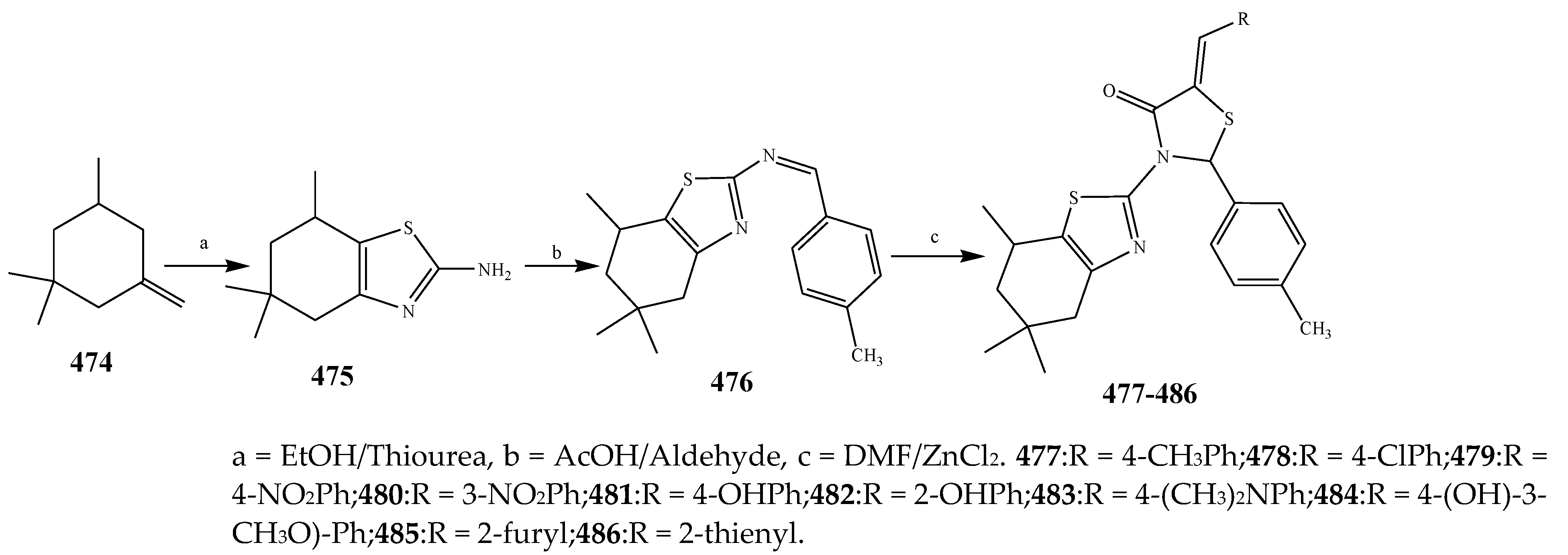
| 478 and 486 | C. violaceum | [49] | |
| 479 | E. Coli | [50] | |
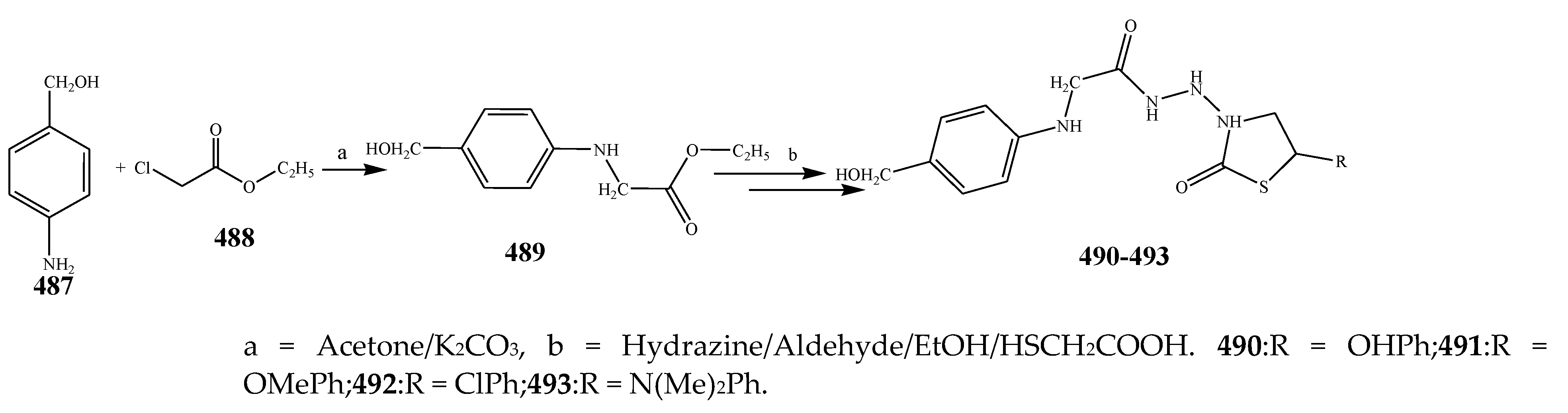
| 497 | E. coli and S. aureus | [51] | |
| 502 | S. aureus | [51] | |
| Imidazole | 518 | P. aeruginosa and E. coli | [53] |
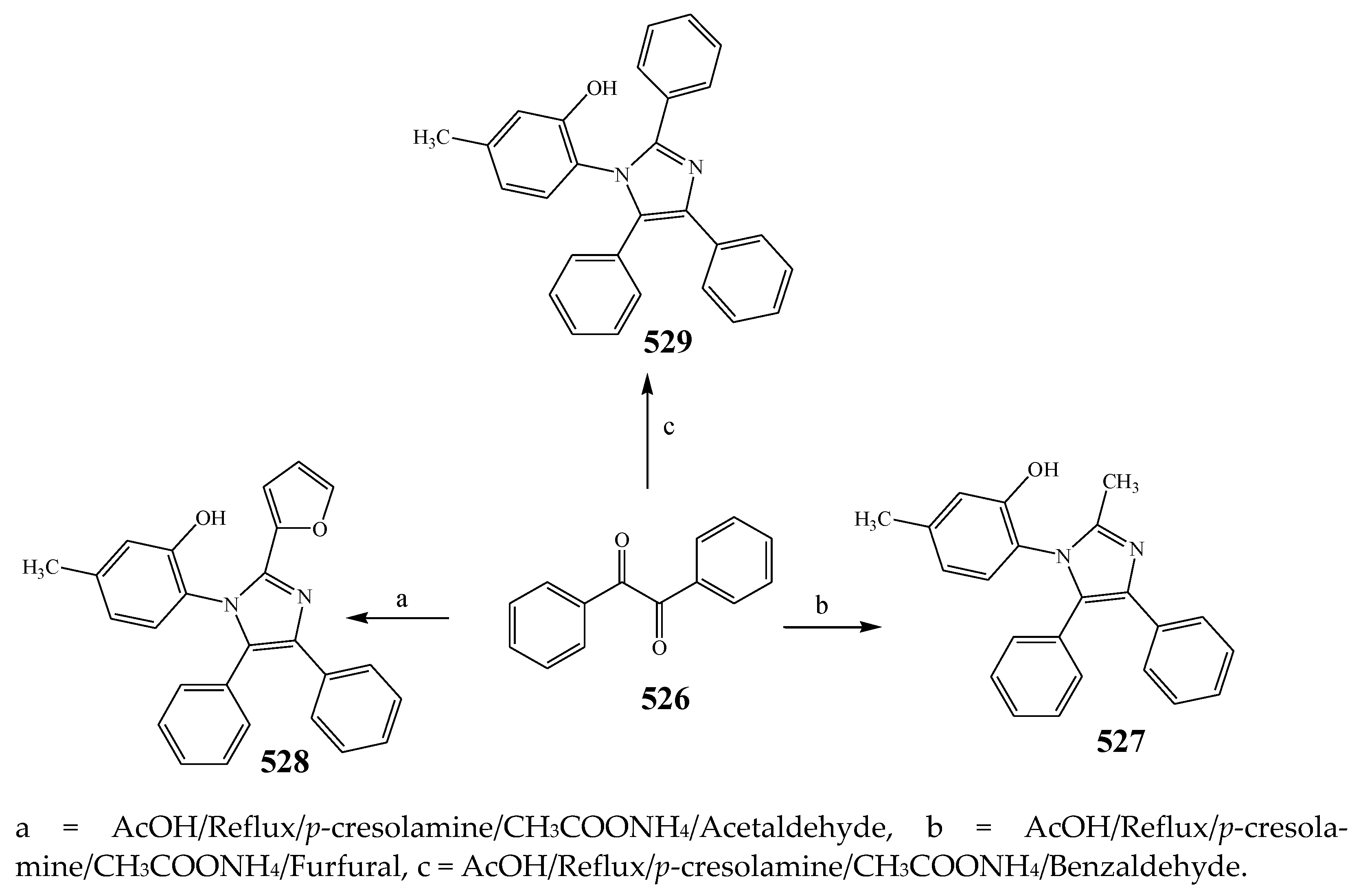
| 527 | B. subtilis | [54] |
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
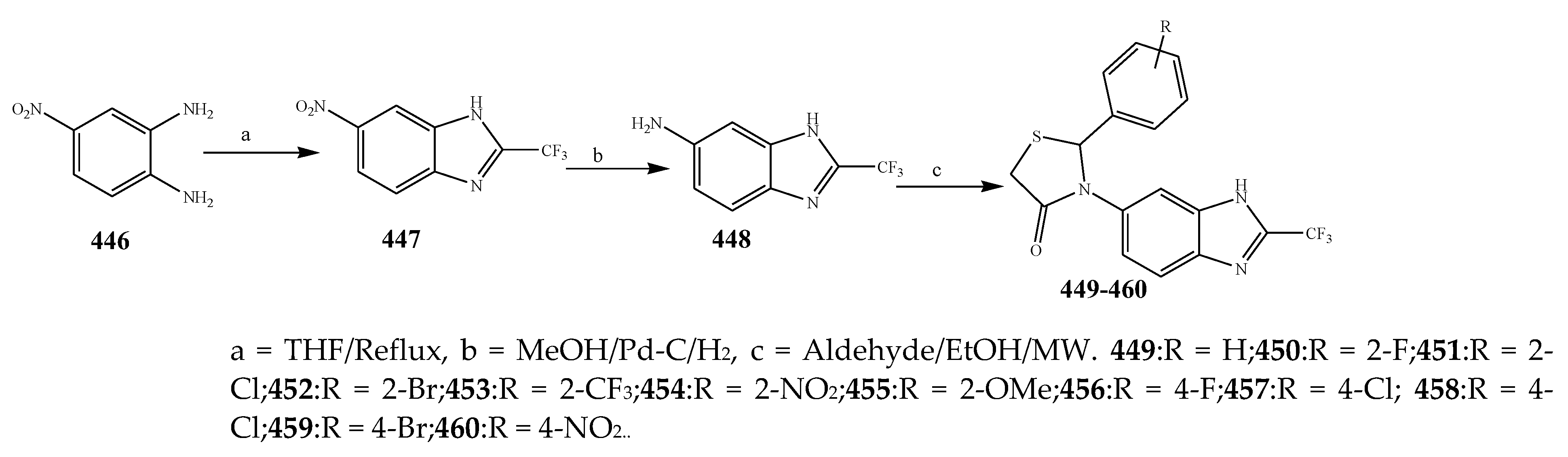
- Cheddie, A.; Shintre, S.A.; Bantho, A.; Mocktar, C.; Koorbanally, N.A. Synthesis and antibacterial activity of a series of 2-trifluoromethylbenzimidazole-thiazolidinone derivatives. J. Heterocycl. Chem. 2020, 57, 299–307. [Google Scholar] [CrossRef]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, I.B.; Macdonald, S.J.; Procopiou, P.A. Medicinal chemistry in drug discovery in big pharma: Past, present and future. Drug Discov. Today 2018, 23, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, R.; Saranya, S.; Anilkumar, G. Recent Trends in the Silver-Catalyzed Synthesis of Nitrogen Heterocycles. Adv. Synth. Catal. 2019, 361, 4625–4644. [Google Scholar] [CrossRef]
- Ghaemi, M.; Pordel, M. Isoxazolo [4, 3-e] indazole as a new heterocyclic system: Design, synthesis, spectroscopic characterization, and antibacterial activity. Chem. Heterocycl. Compd. 2016, 52, 52–57. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: Miniperspective. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Ferouani, G.; Nacer, A.; Ameur, N.; Bachir, R.; Ziani-Cherif, C. Facile Heterocyclic Synthesis and Antibacterial Activity of Substituted Isoxazol-5 (4H)-ones. J. Chin. Chem. Soc. 2018, 65, 459–464. [Google Scholar] [CrossRef]
- Nuchu, R.; Narayana, M. Synthesis and Characterization of Organo Sulphur Heterocyclic Derivates and Their Anti-Fungal Activity. World J. Pharm. Pharm. Sci. 2016, 5, 941–953. [Google Scholar]
- Balaji, K.; Bhatt, P.; Mallika, D.; Jha, A. Design, synthesis and antimicrobial evaluation of some mannich base derivative of 2 (2-substituted)-5-amino-thiadiazoles. Int. J. Pharm. Pharm. Sci. 2015, 7, 145–149. [Google Scholar]
- Al-Anazi, K.M.; Mahmoud, A.H.; AbulFarah, M.; Allam, A.A.; Fouda, M.M.; Gaffer, H.E. 2-Amino-5-arylazothiazole-Based Derivatives: In Vitro Cytotoxicity, Antioxidant Properties, and Bleomycin-Dependent DNA Damage. ChemistrySelect 2019, 4, 5570–5576. [Google Scholar] [CrossRef]
- Al-jubouri, A.A.; Qasir, A.J. Synthesis and Antibacterial Activity of bis Heterocyclic Derivatives of 1, 3, 4-thiadiazole. Iraqi J. Pharm. Sci. 2015, 24, 59–67. [Google Scholar] [CrossRef]
- Serban, G.; Stanasel, O.; Serban, E.; Bota, S. 2-Amino-1, 3, 4-thiadiazole as a potential scaffold for promising antimicrobial agents. Drug Des. Dev. Ther. 2018, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Ananthan, S. A new approach for the synthesis of some novel sulphur bridged pyrazoles and their characterization. J. Chem. Pharm. Res 2011, 3, 402–413. [Google Scholar]
- Ameli, S.; Pordel, M.; Davoodnia, A.; Jajarmi, M. Synthesis and antibacterial activity of some new benzo [5, 6] chromeno [2, 3-d] pyrimidines. Russ. J. Bioorganic Chem. 2017, 43, 429–434. [Google Scholar] [CrossRef]
- Aaglawe, M.; SS, D.; SS, B.; PS, W.; DB, S. Synthesis and antibacterial activity of some oxazolone derivatives. J. Korean Chem. Soc. 2003, 47, 133–136. [Google Scholar] [CrossRef] [Green Version]
- SAYED MOHAMED, M.; El-Domany, R.A.; Abd El-Hameed, R.H. Synthesis of certain pyrrole derivatives as antimicrobial agents. Acta Pharm. 2009, 59, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padwa, A.; Bur, S. Recent advances of 1, 3-dipolar cycloaddition chemistry for alkaloid synthesis. Adv. Heterocycl. Chem. 2016, 119, 241–305. [Google Scholar]
- Ferlin, F.; Luciani, L.; Viteritti, O.; Brunori, F.; Piermatti, O.; Santoro, S.; Vaccaro, L. Polarclean as a sustainable reaction medium for the waste minimized synthesis of heterocyclic compounds. Front. Chem. 2019, 6, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseyni Largani, T.; Imanzadeh, G.; Zahri, S.; Noroozi Pesyan, N.; Şahin, E. A facile synthesis and antibacterial activity of novel pyrrolo [3, 4-b] quinolin-2 (3H)-yl) benzamides. Green Chem. Lett. Rev. 2017, 10, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Mir, N.A.; Ramaraju, P.; Vanaparthi, S.; Choudhary, S.; Singh, R.P.; Sharma, P.; Kant, R.; Singh, R.; Sankaranarayanan, M.; Kumar, I. Sequential multicomponent catalytic synthesis of pyrrole-3-carboxaldehydes: Evaluation of antibacterial and antifungal activities along with docking studies. New J. Chem. 2020, 44, 16329–16339. [Google Scholar] [CrossRef]
- Idhayadhulla, A.; Kumar, R.S.; Nasser, A.J.A. Synthesis, characterization and antimicrobial activity of new pyrrole derivatives. J. Mex. Chem. Soc. 2011, 55, 218–223. [Google Scholar]
- Kheder, N.A. Hydrazonoyl chlorides as precursors for synthesis of novel bis-pyrrole derivatives. Molecules 2016, 21, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baral, N.; Mishra, D.R.; Mishra, N.P.; Mohapatra, S.; Raiguru, B.P.; Panda, P.; Nayak, S.; Nayak, M.; Kumar, P.S. Microwave-assisted rapid and efficient synthesis of chromene-fused pyrrole derivatives through multicomponent reaction and evaluation of antibacterial activity with molecular docking investigation. J. Heterocycl. Chem. 2020, 57, 575–589. [Google Scholar] [CrossRef]
- Kumar, B.; Lakshmi, P.; Veena, B.; Sujatha, E. Synthesis and antibacterial activity of novel pyrano [2, 3-d] pyrimidine-4-one–3-phenylisoxazole hybrids. Russ. J. Gen. Chem. 2017, 87, 829–836. [Google Scholar] [CrossRef]
- Misra, A.; Sharma, S.; Sharma, D.; Dubey, S.; Mishra, A.; Kishore, D.; Dwivedi, J. Synthesis and molecular docking of pyrimidine incorporated novel analogue of 1, 5-benzodiazepine as antibacterial agent. J. Chem. Sci. 2018, 130, 31. [Google Scholar] [CrossRef]
- Fang, Z.; Zheng, S.; Chan, K.-F.; Yuan, W.; Guo, Q.; Wu, W.; Lui, H.-K.; Lu, Y.; Leung, Y.-C.; Chan, T.-H. Design, synthesis and antibacterial evaluation of 2, 4-disubstituted-6-thiophenyl-pyrimidines. Eur. J. Med. Chem. 2019, 161, 141–153. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Shoja, S.; Sharifzadeh, B.; Rassa, M. Regioselective synthesis and antibacterial evaluation of novel bis-pyrimidine derivatives via a three-component reaction. Med. Chem. Res. 2014, 23, 1207–1213. [Google Scholar] [CrossRef]
- Triloknadh, S.; Rao, C.V.; Nagaraju, K.; Krishna, N.H.; Ramaiah, C.V.; Rajendra, W.; Trinath, D.; Suneetha, Y. Design, synthesis, neuroprotective, antibacterial activities and docking studies of novel thieno [2, 3-d] pyrimidine-alkyne Mannich base and oxadiazole hybrids. Bioorganic Med. Chem. Lett. 2018, 28, 1663–1669. [Google Scholar] [CrossRef]
- Andrews, B.; Komathi, K.; Mohan, S. Synthesis and comparing the antibacterial activities of pyrimidine derivatives. J. Chem. Sci. 2017, 129, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.A.; Ibrahim, Y.R.; El-Sheref, E.M.; Abdel-Aziz, M.; Bräse, S.; Nieger, M. Synthesis and Antibacterial Activity of 4-Aryl-2-(1-substituted ethylidene) thiazoles. Arch. Pharm. 2013, 346, 562–570. [Google Scholar] [CrossRef]
- Zha, G.-F.; Leng, J.; Darshini, N.; Shubhavathi, T.; Vivek, H.; Asiri, A.M.; Marwani, H.M.; Rakesh, K.; Mallesha, N.; Qin, H.-L. Synthesis, SAR and molecular docking studies of benzo [d] thiazole-hydrazones as potential antibacterial and antifungal agents. Bioorganic Med. Chem. Lett. 2017, 27, 3148–3155. [Google Scholar] [CrossRef]
- Abdel-Galil, E.; Moawad, E.B.; El-Mekabaty, A.; Said, G.E. Synthesis, characterization and antibacterial activity of some new thiazole and thiazolidinone derivatives containing phenyl benzoate moiety. Synth. Commun. 2018, 48, 2083–2092. [Google Scholar] [CrossRef]
- Khare, R.; Sharma, J.; Sharma, A. Synthesis, characterization, and antibacterial activity of some thiazoles derived from allyl thioureas. Russ. J. Gen. Chem. 2016, 86, 702–707. [Google Scholar] [CrossRef]
- Beyzaei, H.; Aryan, R.; Molashahi, H.; Zahedi, M.M.; Samzadeh-Kermani, A.; Ghasemi, B.; Moghaddam-Manesh, M. MgO nanoparticle-catalyzed, solvent-free Hantzsch synthesis and antibacterial evaluation of new substituted thiazoles. J. Iran. Chem. Soc. 2017, 14, 1023–1031. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Abd El-Megied, S.A.; Bashandy, M.S.; Ibrahim, H.M. Synthesis, application and antibacterial activity of new reactive dyes based on thiazole moiety. Pigment Resin Technol. 2018, 47, 246–254. [Google Scholar] [CrossRef]
- Wang, L.; Dai, F.-Y.; Zhu, J.; Dong, K.-K.; Wang, Y.-L.; Chen, T. Synthesis and antibacterial activities of pleuromutilin derivatives with thiazole-5-carboxamide and thioether moiety. J. Chem. Res. 2011, 35, 313–316. [Google Scholar] [CrossRef]
- Sirisha, K.; Achaiah, G.; Reddy, V.M. Facile Synthesis and Antibacterial, Antitubercular, and Anticancer Activities of Novel 1, 4-Dihydropyridines. Arch. Pharm. 2010, 343, 342–352. [Google Scholar] [CrossRef]
- Althagafi, I.; Abdel-Latif, E. Synthesis and Antibacterial Activity of New Imidazo [1, 2-a] pyridines Festooned with Pyridine, Thiazole or Pyrazole Moiety. Polycycl. Aromat. Compd. 2021, 42, 4487–4500. [Google Scholar] [CrossRef]
- Elagamey, A.G.; Sattar, S.A.; El-Taweel, F.; Said, S. An Efficient Synthesis and Antibacterial Activity of Pyrido [2, 3-d] Pyrimidine, Chromeno [3, 4-c] Pyridine, Pyridine, Pyrimido [2, 3-c] Pyridazine, Enediamines, and Pyridazine Derivatives. J. Heterocycl. Chem. 2016, 53, 1801–1806. [Google Scholar] [CrossRef]
- Pradeep, M.A.; Kumar, N.R.; Swaroop, D.K.; Reddy, N.S.; Sirisha, K.; Kumar, C.G.; Babu, N.J.; Ganapathi, T.; Narsaiah, B. Design and Synthesis of Novel Pyrimidine/Hexahydroquinazoline-Fused Pyrazolo [3, 4-b] Pyridine Derivatives, Their Biological Evaluation and Docking Studies#. ChemistrySelect 2019, 4, 138–144. [Google Scholar]
- Jemmezi, F.; Kether, F.B.H.; Amri, I.; Bassem, J.; Khiari, J. Synthesis and biological activity of novel benzothiazole pyridine derivatives. IOSR J. Appl. Chem. 2014, 7, 62–64. [Google Scholar] [CrossRef]
- Babu, H.R.; Ravinder, M.; Narsimha, S. Synthesis and Biological Evaluation of New 1, 2, 3-Triazole Based 2-Sulfonylbenzoxazoles as Potent Anti-inflammatory and Antibacterial Agents. Indian J. Heterocycl. Chem. 2019, 29, 389–395. [Google Scholar]
- Arshad, M. Synthesis, Characterization, and Antimicrobial Assessment of Some Computationally Bioactive 1, 2-Oxazole Derivatives. Russ. J. Gen. Chem. 2018, 88, 1886–1891. [Google Scholar] [CrossRef]
- Tomi, I.H.; Tomma, J.H.; Al-Daraji, A.H.; Al-Dujaili, A.H. Synthesis, characterization and comparative study the microbial activity of some heterocyclic compounds containing oxazole and benzothiazole moieties. J. Saudi Chem. Soc. 2015, 19, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, C.; Chahal, M. Synthesis and antibacterial activity of benzothiazole and benzoxazole-appended substituted 1, 2, 3-triazoles. J. Chem. Sci. 2020, 132, 142. [Google Scholar] [CrossRef]
- Kakkar, S.; Kumar, S.; Lim, S.M.; Ramasamy, K.; Mani, V.; Shah, S.A.A.; Narasimhan, B. Design, synthesis and biological evaluation of 3-(2-aminooxazol-5-yl)-2 H-chromen-2-one derivatives. Chem. Cent. J. 2018, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Güzel-Akdemir, Ö.; Trawally, M.; Özbek-Babuç, M.; Özbek-Çelik, B.; Ermut, G.; Özdemir, H. Synthesis and antibacterial activity of new hybrid derivatives of 5-sulfamoyl-1H-indole and 4-thiazolidinone groups. Mon. Chem.-Chem. Mon. 2020, 151, 1443–1452. [Google Scholar] [CrossRef]
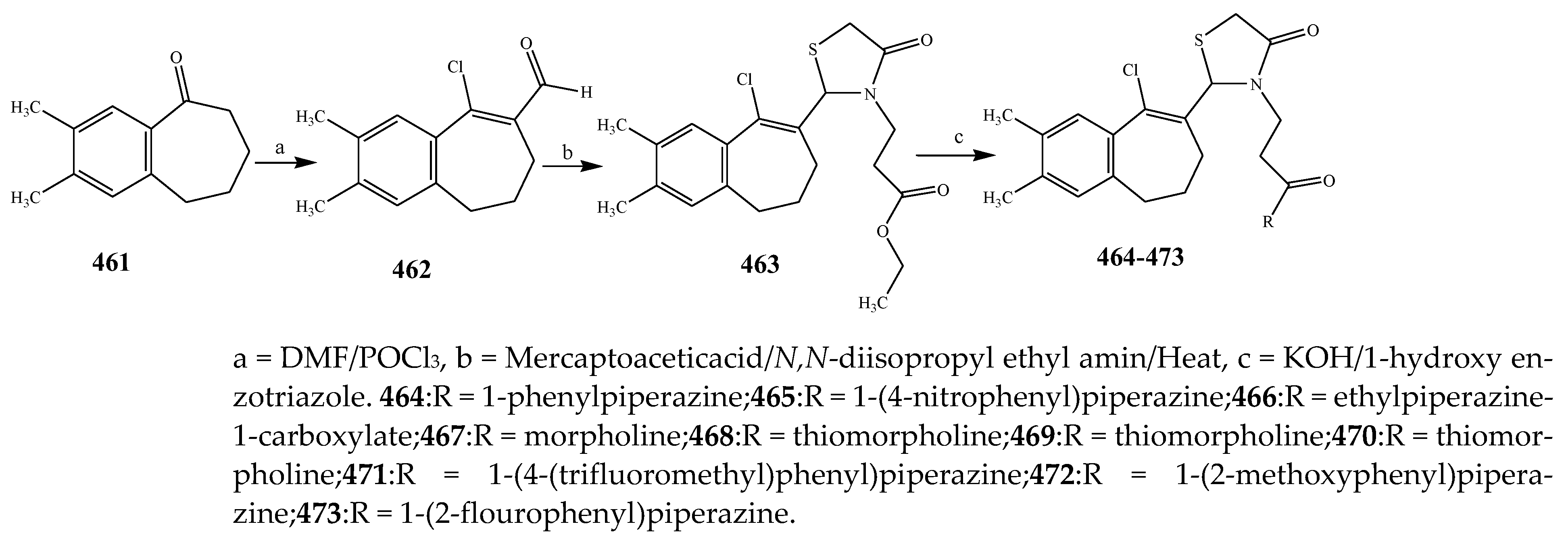
- Jilla, L.; Kolluri, P.K.; Bujji, S.; JP Naikal, S. Synthesis and antimicrobial agents of thiazolidinone derivatives from benzocyclohepetenone. J. Heterocycl. Chem. 2020, 57, 4078–4087. [Google Scholar] [CrossRef]
- Adki, N.; Ravi, G.; Kumar, S.S.; Rao, G.N. Synthesis of new biologically active compounds containing linked thiazolyl-thiazolidinone heterocycles. Org. Commun. 2012, 5, 160–170. [Google Scholar]
- Ismaeel, S.S.; Mahdi, M.F.; Abd Razik, B.M. Design, Synthesis and Antibacterial Study of New Agents Having 4-Thiazolidinone Pharmacophore. Egypt. J. Chem. 2020, 63, 2591–2603. [Google Scholar] [CrossRef]
- Ahmed, S.; Zayed, M.F.; El-Messery, S.M.; Al-Agamy, M.H.; Abdel-Rahman, H.M. Design, synthesis, antimicrobial evaluation and molecular modeling study of 1, 2, 4-triazole-based 4-thiazolidinones. Molecules 2016, 21, 568. [Google Scholar] [CrossRef] [PubMed]
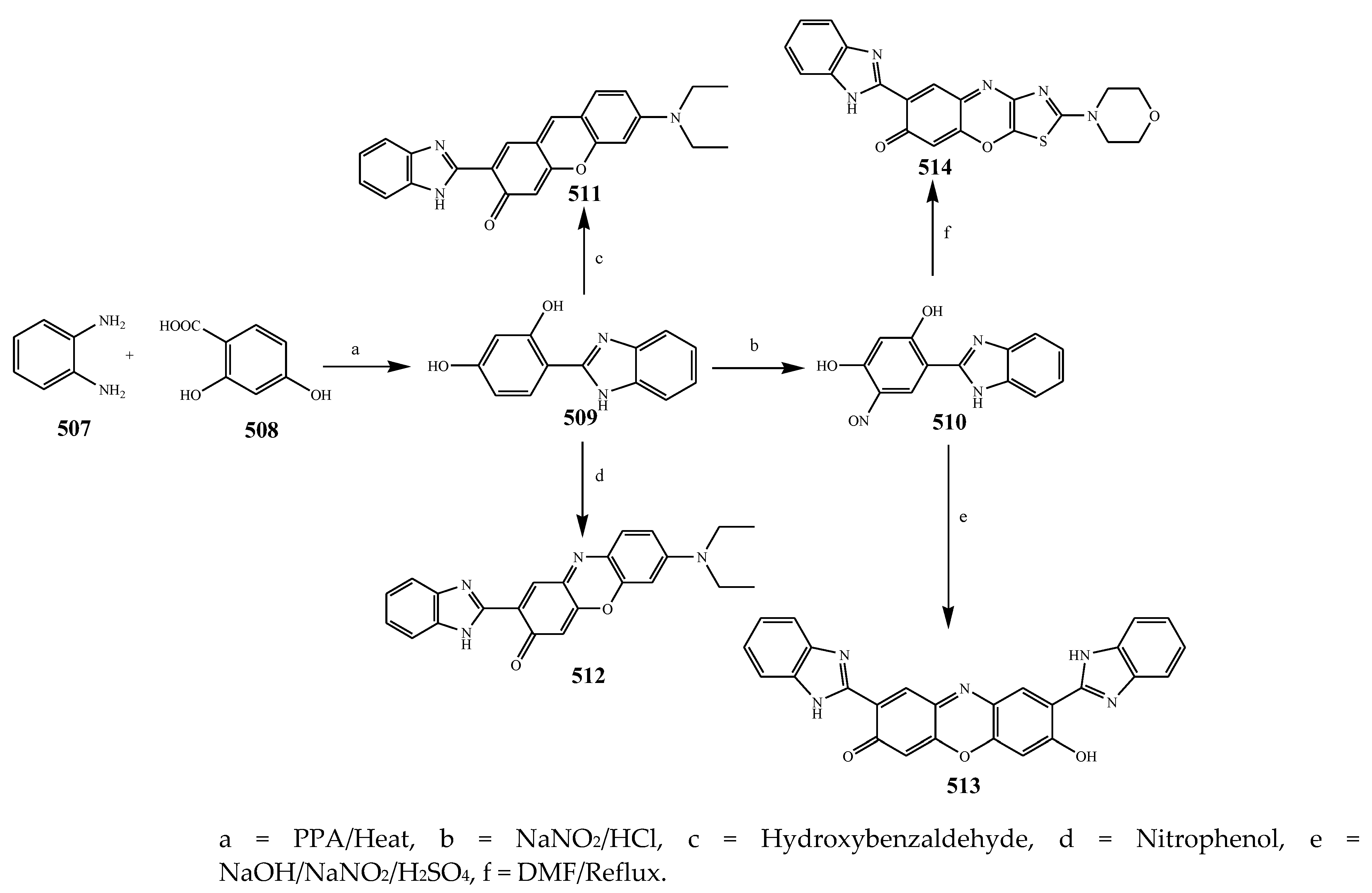
- Patil, V.S.; Padalkar, V.S.; Phatangare, K.R.; Umape, P.G.; Borase, B.N.; Sekar, N. Synthesis, Characterization, and Antibacterial Activity of Novel (1H-Benzo [d] imidazole-2-yl)-6-(diethylamino)-3H-one-xanthene, Phenoxazine, and Oxazine. J. Heterocycl. Chem. 2015, 52, 124–129. [Google Scholar] [CrossRef]
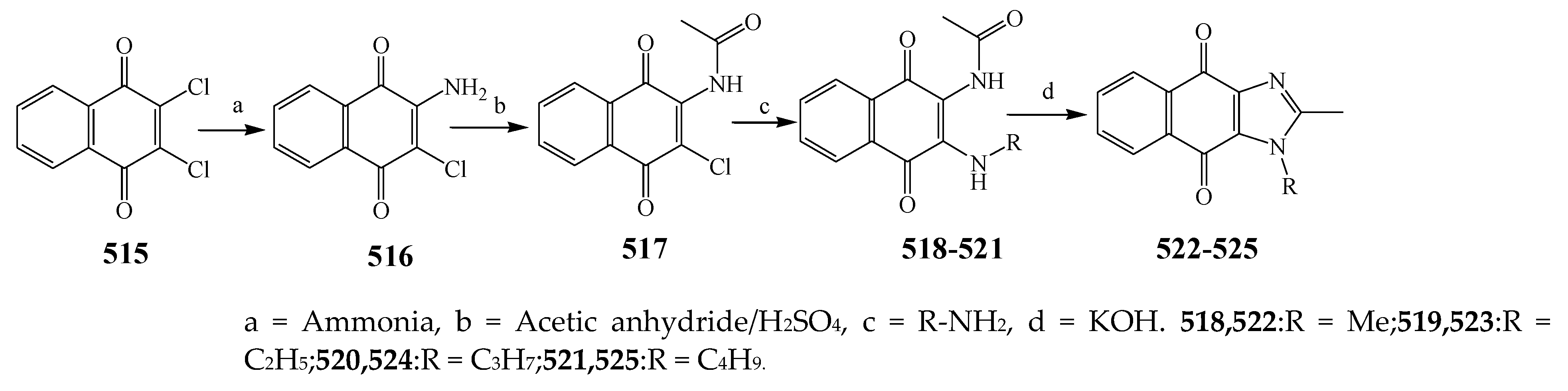
- Choudhari, D.; Salunke-Gawali, S.; Chakravarty, D.; Shaikh, S.R.; Lande, D.N.; Gejji, S.P.; Rao, P.K.; Satpute, S.; Puranik, V.G.; Gonnade, R. Synthesis and biological activity of imidazole based 1, 4-naphthoquinones. New J. Chem. 2020, 44, 6889–6901. [Google Scholar] [CrossRef]
- Shahzad, K.; Abbas, F.; Pandey, D.; Ajmal, S.; Khadim, M.; Tahir, M.U. Synthesis, Characterization and Biological Evaluation of Novel Tetrasubsituted Imidazole Compounds. 2020. Available online: https://www.preprints.org/manuscript/202006.0219/v1 (accessed on 30 November 2022).















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aatif, M.; Raza, M.A.; Javed, K.; Nashre-ul-Islam, S.M.; Farhan, M.; Alam, M.W. Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review. Antibiotics 2022, 11, 1750. https://doi.org/10.3390/antibiotics11121750
Aatif M, Raza MA, Javed K, Nashre-ul-Islam SM, Farhan M, Alam MW. Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review. Antibiotics. 2022; 11(12):1750. https://doi.org/10.3390/antibiotics11121750
Chicago/Turabian StyleAatif, Mohammad, Muhammad Asam Raza, Khadija Javed, Swah Mohd. Nashre-ul-Islam, Mohd Farhan, and Mir Waqas Alam. 2022. "Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review" Antibiotics 11, no. 12: 1750. https://doi.org/10.3390/antibiotics11121750
APA StyleAatif, M., Raza, M. A., Javed, K., Nashre-ul-Islam, S. M., Farhan, M., & Alam, M. W. (2022). Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review. Antibiotics, 11(12), 1750. https://doi.org/10.3390/antibiotics11121750










