Phage Therapy vs. the Use of Antibiotics in the Treatment of Salmonella-Infected Chickens: Comparison of Effects on Hematological Parameters and Selected Biochemical Markers
Abstract
1. Introduction
2. Results
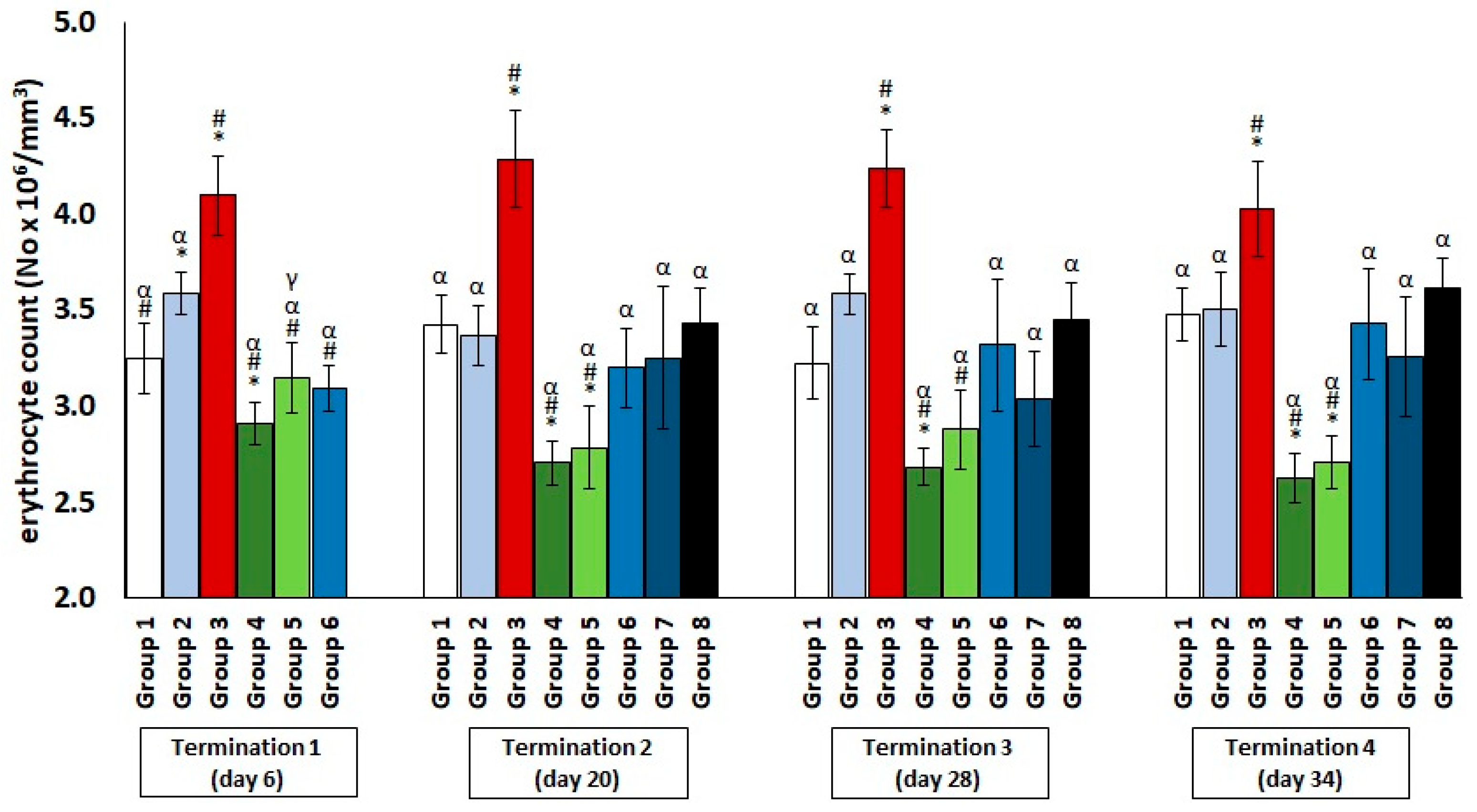
2.1. Changes in the Counts of Blood Morphotic Elements of Chickens Receiving Phage Cocktail or Antibiotics
2.2. Blood Biochemical Parameters in Plasma of Chickens Subjected to Phage Therapy and Antibiotic Therapy
3. Discussion
Conclusions
4. Materials and Methods
4.1. Animals
4.2. Bacteriophages and Bacterial Strain
4.3. The Preparation of Phage Pocktail
4.4. Animal Groups and the Schedule of the Treatment
4.5. Blood Collection
4.6. Analysis of Selected Blood Morphological Parameters
4.7. Determination of Alanine Transaminase (ALT) and Aspartate Aminotransferase (AST) Concentrations in Peripheral Blood Plasma
4.8. Determination of C-Reactive Protein (CRP) Concentration (Using ELISA) in Peripheral Blood Plasma
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Ahmad, S.M.; Bhat, S.A.; Ahmed, R.; Urwat, U.; Mumtaz, P.T.; Dar, T.A.; Shah, R.A.; Ganai, N.A. Salmonella Typhimurium in poultry: A review. Poult. Sci. J. 2017, 73, 345–354. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; et al.; EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel) Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-antibiotics strategies to control Salmonella infection in poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Pławińska-Czarnak, J.; Wódz, K.; Kizerwetter-Świda, M.; Bogdan, J.; Kwieciński, P.; Nowak, T.; Strzałkowska, Z.; Anusz, K. Multi-Drug Resistance to Salmonella spp. When Isolated from Raw Meat Products. Antibiotics 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, E.P.; de Melo, R.T.; Nalevaiko, P.C.; Monteiro, G.P.; Fonseca, B.B.; Galvão, N.N.; Giombelli, A.; Rossi, D.A. Spread of the serotypes and antimicrobial resistance in strains of Salmonella spp. isolated from broiler. Braz. J. Microbiol. 2019, 50, 515–522. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Perez, E.; Gonzalez-Ronquillo, M. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: A systematic review. Animals 2021, 12, 60. [Google Scholar] [CrossRef]
- Jerab, J.; Jansen, W.; Blackwell, J.; van Hout, J.; Palzer, A.; Lister, S.; Chantziaras, I.; Dewulf, J.; De Briyne, N. Real-World Data on Antibiotic Group Treatment in European Livestock: Drivers, Conditions, and Alternatives. Antibiotics 2022, 11, 1046. [Google Scholar] [CrossRef]
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderón, I.; Fuentes, J.A. One for All: Functional Transfer of OMV-Mediated Polymyxin B Resistance From Salmonella enterica sv. Typhi Δ tolR and Δ degS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8, 1539056. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Rahman, S.R. Use of Phages to Treat Antimicrobial-Resistant Salmonella Infections in Poultry. Vet. Sci. 2022, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Int. Food Res. J. 2019, 119, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Kosznik-Kwaśnicka, K.; Stasiłojć, M.; Grabowski, Ł.; Zdrojewska, K.; Wegrzyn, G.; Wegrzyn, A. Efficacy and safety of phage therapy against Salmonella enterica serovars Typhimurium and Enteritidis estimated by using a battery of in vitro tests and the Galleria mellonella animal model. Microbiol. Res. 2022, 261, 127052. [Google Scholar] [CrossRef]
- Kosznik-Kwaśnicka, K.; Podlacha, M.; Grabowski, Ł.; Stasiłojć, M.; Nowak-Zaleska, A.; Ciemińska, K.; Cyske, Z.; Dydecka, A.; Gaffke, L.; Mantej, J.; et al. Biological aspects of phage therapy versus antibiotics against Salmonella enterica serovar Typhimurium infection of chickens. Front. Cell. Infect. Microbiol. 2022, 12, 941867. [Google Scholar] [CrossRef]
- Grabowski, Ł.; Węgrzyn, G.; Wegrzyn, A.; Podlacha, M. Highly different effects of phage therapy and antibiotic therapy on immunological responses of chickens infected with Salmonella enterica serovar Typhimurium. Front. Immunol. 2022, 13, 956833. [Google Scholar] [CrossRef]
- Saleem, H.M.; Muhammed, T.M.; Al-Hetty, H.R.A.K.; Salman, D.A. Physiological, hematological and some biochemical alterations during pregnancy. Int. J. Health Sci. 2022, 6, 7156–7169. [Google Scholar] [CrossRef]
- Yap, K.N.; Dick, M.F.; Guglielmo, C.G.; Williams, T.D. Effects of experimental manipulation of hematocrit on avian flight performance in high- and low-altitude conditions. J. Exp. Biol. 2018, 221, jeb191056. [Google Scholar] [CrossRef]
- Buta, A.; Spătariu, S.; Ovidiu, O.; Daradics, Z.; Tamas-Krumpe, O.; Ognean, L. Current data regarding the evolution of hematological profile in Broiler chickens: A review. Lucr. Știnţifice 2019, 62, 172–179. [Google Scholar]
- Ribeiro, M.N.; Ribeiro, N.L.; Bozzi, R.; Costa, R.G. Physiological and biochemical blood variables of goats subjected to heat stress—A review. J. Appl. Anim. Res. 2018, 46, 1036–1041. [Google Scholar] [CrossRef]
- Alkhalf, A.; Alhaj, M.; Al-Homidan, I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J. Biol. Sci. 2010, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wessels, K.; Rip, D.; Gouws, P. Salmonella in chicken meat: Consumption, outbreaks, characteristics, current control methods and the potential of bacteriophage use. Foods 2021, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.A.; Shawrang, P.; Shakorzadeh, S. Immune Response of Salmonella Challenged Broiler Chickens Fed Diets Containing Gallipro®, a Bacillus subtilis Probiotic. Probiotics Antimicrob. Proteins 2015, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, Ł.; Gaffke, L.; Pierzynowska, K.; Cyske, Z.; Choszcz, M.; Węgrzyn, G.; Węgrzyn, A. Enrofloxacin–the ruthless killer of eukaryotic cells or the last hope in the fight against bacterial infections? Int. J. Mol. Sci. 2022, 23, 3648. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- Górski, A.; Borysowski, J.; Międzybrodzki, R. The contribution of phage therapy to medical knowledge. J. Glob. Antimicrob. Resist. 2022, 28, 238–240. [Google Scholar] [CrossRef]
- Dufour, N.; Delattre, R.; Chevallereau, A.; Ricard, J.D.; Debarbieux, L. Phage therapy of pneumonia is not associated with an overstimulation of the inflammatory response compared to antibiotic treatment in mice. Antimicrob. Agents Chemother. 2019, 63, e00379-19. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The safety and toxicity of phage therapy: A review of animal and clinical studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Villa, G.T.; Sieiro, C. Phage therapy, lysin therapy, and antibiotics: A trio due to come. Antibiotics 2020, 9, 604. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Animal models in the evaluation of the effectiveness of phage therapy for infections caused by gram-negative bacteria from the ESKAPE group and the reliability of its use in humans. Microorganisms 2021, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Bibi, F.; Archer, A.C. Bacteriophage therapy: Recent developments and applications of a renaissant weapon. Res. Microbiol. 2021, 172, 103863. [Google Scholar] [CrossRef]
- Saraf, S.; Jafra, B.S.; Ray, P.; Rawat, A.; Verma, S. Multidrug-Resistant Nontyphoidal Salmonella Associated with Invasive Disease in an Immunocompetent Child. Indian J. Pediat. 2021, 88, 1266. [Google Scholar] [CrossRef] [PubMed]
- Kosznik-Kwaśnicka, K.; Ciemińska, K.; Grabski, M.; Grabowski, Ł.; Górniak, M.; Jurczak-Kurek, A.; Węgrzyn, G.; Węgrzyn, A. Characteristics of a series of three bacteriophages infecting Salmonella enterica strains. Int. J. Mol. Sci. 2020, 21, 6152. [Google Scholar] [CrossRef]
- Kosznik-Kwaśnicka, K.; Grabowski, Ł.; Grabski, M.; Kaszubski, M.; Górniak, M.; Jurczak-Kurek, A.; Węgrzyn, G.; Węgrzyn, A. Bacteriophages vB_Sen-TO17 and vB_Sen-E22, Newly Isolated Viruses from Chicken Feces, Specific for Several Salmonella enterica Strains. Int. J. Mol. Sci. 2020, 21, 8821. [Google Scholar] [CrossRef]
- Neut, D.; Dijkstra, R.J.B.; Thompson, J.I.; Kavanagh, C.; van der Mei, H.C.; Busscher, H.J. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur. Cells Mater. 2015, 29, 42–56. [Google Scholar] [CrossRef]
- Roch-Lefèvre, S.; Martin-Bodiot, C.; Grégoire, E.; Desbrée, A.; Roy, L.; Barquinero, J.F. A mouse model of cytogenetic analysis to evaluate caesium137 radiation dose exposure and contamination level in lymphocytes. Radiat. Environ. Biophys. 2016, 55, 61–70. [Google Scholar] [CrossRef]
- Aouni, R.; Ben Attia, M.; Jaafoura, M.H.; Bibi-Derbel, A.; Haouari, M. Effects of the hydro-ethanolic extract of Marrubium vulgare in female rats. Asian Pac. J. Trop. Med. 2017, 10, 160–164. [Google Scholar] [CrossRef]





| Absolute Monocyte Count | Absolute Eosinophil Count | MCV | MCH | MCHC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Termination (day) | Group | (No × 109/L) | p | Absolute Eosinophil Count (No × 109/L) | p | (fL) | p | (pg) | p | (g/L) | p |
| Termination 1 (day 6) | Group 1 | 645.0 ± 84.1 | α | 449.0 ± 41.9 | α | 140.8 ± 2.3 | α | 51.5 ± 1.3 | α | 354.2 ± 2.0 | α |
| Group 2 | 601.4 ± 66.9 | α | 414.0 ± 48.8 | α | 139.8 ± 5.6 | α | 53.6 ± 1.5 | α | 352.4 ± 4.5 | α | |
| Group 3 | 1527.0 ± 70.5 | *, # | 983.0 ± 88.5 | *, # | 156.0 ± 4.9 | *, # | 44.6 ± 2.2 | *, # | 328.4 ± 2.2 | *, # | |
| Group 4 | 203.8 ± 25.0 | *, #, α | 120.6 ± 30.1 | *, #, α | 135.2 ± 3.5 | α | 60.6 ± 1.8 | *, #, α, γ | 388.5 ± 8.9 | *, #, α | |
| Group 5 | 331.4 ± 20.5 | *, #, α | 241.2 ± 14.4 | *, #, α | 139.5 ± 2.4 | α | 54.1 ± 1.3 | α | 372.8 ± 7.5 | *, #, α | |
| Group 6 | 647.8 ± 44.3 | α | 425.2 ± 51.2 | α | 146.6 ± 4.6 | α, γ | 51.2 ± 1.9 | α | 354.4 ± 11.4 | α | |
| Termination 2 (day 20) | Group 1 | 620.0 ± 66.6 | α | 392.2 ± 35.0 | α | 140.9 ± 3.8 | α | 50.7 ± 2.7 | α | 351.1 ± 1.9 | α |
| Group 2 | 650.0 ± 51.2 | α | 408.8 ± 17.1 | α | 140.8 ± 6.3 | α | 54.1 ± 1.6 | α | 354.6 ± 7.6 | α | |
| Group 3 | 1534.4 ±126.2 | *, # | 1019.4 ± 80.5 | *, # | 155.6 ± 4.4 | *, # | 42.5 ± 2.3 | *, # | 328.0 ± 14.0 | *, # | |
| Group 4 | 195.6 ± 15.7 | *, #, α | 138.4 ± 19.3 | *, #, α | 133.5 ± 5.6 | α | 56.4 ± 1.3 | α | 380.0 ± 11.4 | *, #, α | |
| Group 5 | 321.6 ± 37.3 | *, #, α | 264.4 ± 27.7 | *, #, α | 136.5 ± 2.8 | α | 54.5 ± 2.7 | α | 372.8 ± 6.9 | *, #, α | |
| Group 6 | 566.2 ± 60.9 | α | 414.0 ± 48.6 | α | 138.9 ± 3.3 | α | 49.7 ± 4.6 | - | 343.0 ± 9.7 | - | |
| Group 7 | 632.9 ± 72.8 | α | 422.8 ± 51.9 | α | 144.7 ± 3.6 | α | 48.4 ± 3.0 | #, α | 356.0 ± 10.0 | α | |
| Group 8 | 974.2 ± 130.8 | *, #, α | 537.0 ± 67.0 | *, #, α | 149.0 ± 4.0 | *, # | 48.1 ± 2.0 | #, α | 346.3 ± 5.3 | α | |
| Termination 3 (day 28) | Group 1 | 652.4 ± 104.9 | α | 377.2 ± 20.8 | α | 141.6 ± 1.5 | α | 51.9 ± 2.5 | α | 350.9 ± 3.8 | - |
| Group 2 | 629.0 ± 48.1 | α | 394.0 ± 38.7 | α | 145.4 ± 2.2 | α | 53.8 ± 1.2 | α | 351.0 ± 4.7 | - | |
| Group 3 | 1763.8 ± 78.2 | *, #,γ | 1052.4 ± 123.9 | *, # | 153.6 ± 3.0 | *, # | 42.2 ± 3.1 | *, # | 334.9 ± 17.3 | - | |
| Group 4 | 209.6 ± 42.2 | *, #, α | 129.8 ± 9.5 | *, #, α | 135.1 ± 4.3 | *, #,α | 59.9 ± 2.6 | *, α | 380.9 ± 12.5 | - | |
| Group 5 | 300.6 ± 23.5 | *, #, α | 249.2 ± 39.1 | *, #, α | 130.1 ± 1.2 | #, α, γ | 57.7 ± 3.2 | α | 373.8 ± 7.4 | *, # | |
| Group 6 | 568.2 ± 104.8 | α | 416.0 ± 41.5 | α | 140.3 ± 2.5 | α | 54.4 ± 3.8 | α | 356.9 ± 6.4 | - | |
| Group 7 | 557.0 ± 68.8 | α | 418.0 ± 69.8 | α | 142.1 ± 5.7 | - | 53.6 ± 3.9 | α, γ | 356.7 ± 7.9 | - | |
| Group 8 | 928.2 ± 31.2 | *, #, α | 480.2 ± 24.4 | *, α | 153.1 ± 4.2 | * | 48.1 ± 3.5 | - | 332.3 ± 5.9 | *, #, γ | |
| Termination 4 (day 34) | Group 1 | 641.4 ± 39.5 | α | 410.2 ± 35.9 | α | 142.8 ± 3.4 | α | 52.5 ± 2.4 | α | 346.0 ± 4.8 | α |
| Group 2 | 651.4 ± 37.0 | α | 397.7 ± 52.5 | α | 142.1 ± 3.2 | α | 54.2 ± 2.4 | α | 352.1 ± 7.5 | α | |
| Group 3 | 1607.6 ±150.8 | *, # | 1119.8 ± 90.7 | *, # | 155.7 ± 5.0 | *, # | 43.7 ± 2.2 | *, # | 323.2 ± 11.4 | *, # | |
| Group 4 | 205.3 ± 21.0 | *, #, α | 126.3 ± 15.5 | *, #, α | 135.0 ± 5.5 | *, #, α | 62.1 ± 2.4 | *, #, α, γ | 381.3 ± 9.2 | *, #, α | |
| Group 5 | 323.3 ± 48.5 | *, #, α | 223.7 ± 26.2 | *, #, α | 133.9 ± 4.5 | *, #, α | 57.4 ± 3.5 | *, α | 359.6 ± 14.5 | α | |
| Group 6 | 589.8 ± 60.6 | α | 428.0 ± 36.8 | α | 141.8 ± 2.6 | α | 52.2 ± 3.4 | α | 354.4 ± 12.0 | α | |
| Group 7 | 643.7 ± 61.7 | α | 409.2 ± 50.9 | α | 142.0 ± 3.4 | α | 50.0 ± 2.6 | #, α | 355.2 ± 8.4 | α | |
| Group 8 | 967.5 ± 74.5 | *, #, α | 504.6 ± 57.6 | *, #, α | 150.4 ± 4.0 | *, #, α | 44.4 ± 2.5 | *, #, γ | 325.4 ± 10.3 | *, #, α, γ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, Ł.; Węgrzyn, G.; Węgrzyn, A.; Podlacha, M. Phage Therapy vs. the Use of Antibiotics in the Treatment of Salmonella-Infected Chickens: Comparison of Effects on Hematological Parameters and Selected Biochemical Markers. Antibiotics 2022, 11, 1787. https://doi.org/10.3390/antibiotics11121787
Grabowski Ł, Węgrzyn G, Węgrzyn A, Podlacha M. Phage Therapy vs. the Use of Antibiotics in the Treatment of Salmonella-Infected Chickens: Comparison of Effects on Hematological Parameters and Selected Biochemical Markers. Antibiotics. 2022; 11(12):1787. https://doi.org/10.3390/antibiotics11121787
Chicago/Turabian StyleGrabowski, Łukasz, Grzegorz Węgrzyn, Alicja Węgrzyn, and Magdalena Podlacha. 2022. "Phage Therapy vs. the Use of Antibiotics in the Treatment of Salmonella-Infected Chickens: Comparison of Effects on Hematological Parameters and Selected Biochemical Markers" Antibiotics 11, no. 12: 1787. https://doi.org/10.3390/antibiotics11121787
APA StyleGrabowski, Ł., Węgrzyn, G., Węgrzyn, A., & Podlacha, M. (2022). Phage Therapy vs. the Use of Antibiotics in the Treatment of Salmonella-Infected Chickens: Comparison of Effects on Hematological Parameters and Selected Biochemical Markers. Antibiotics, 11(12), 1787. https://doi.org/10.3390/antibiotics11121787









