Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine
Abstract
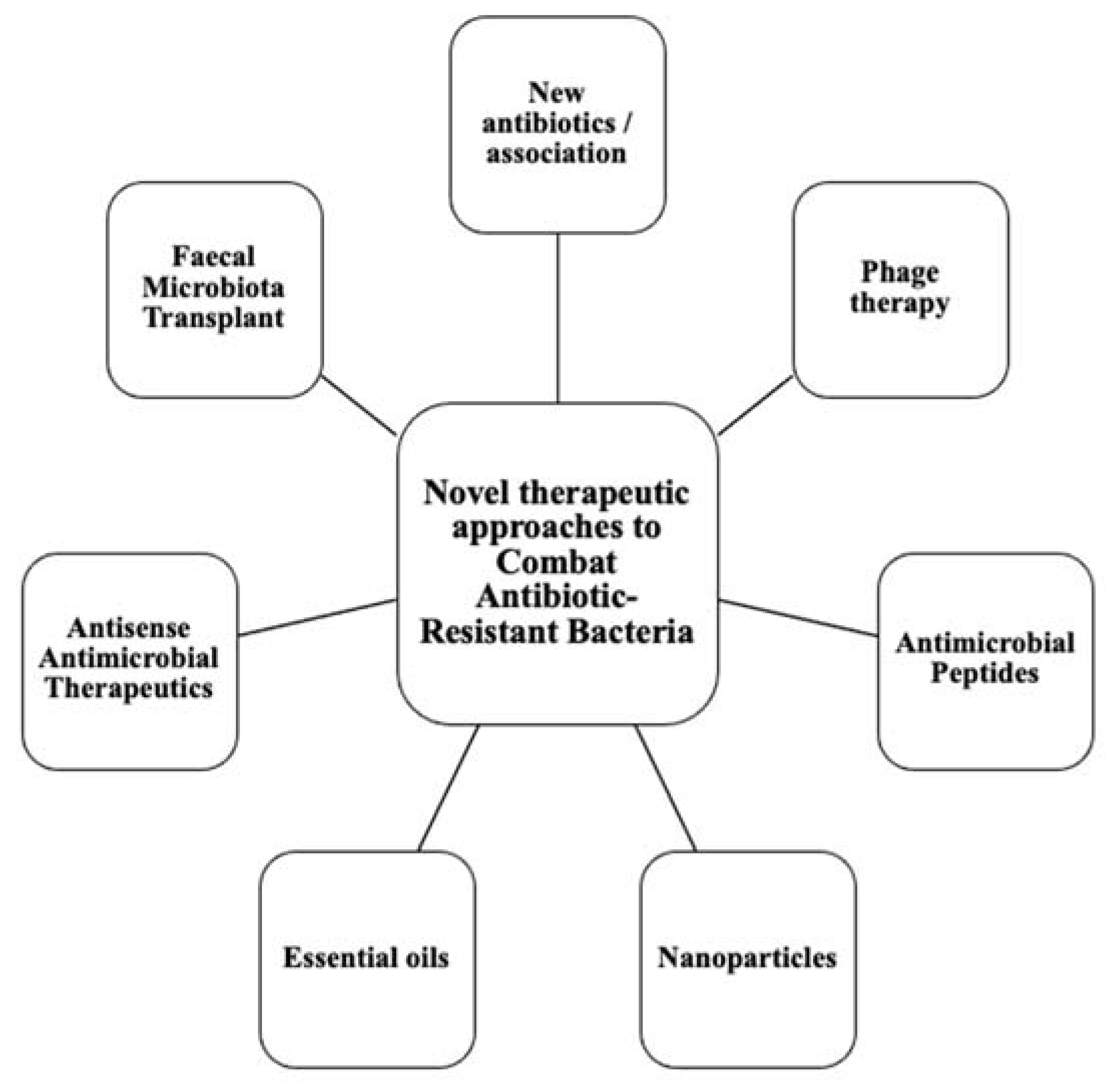
:1. Introduction
2. New Antibiotics Therapy
2.1. Plazomicin
2.2. Eravacycline
2.3. Cefiderocol
2.4. New Combination Antibiotic Therapy
2.4.1. Ceftazidime–Avibactam
2.4.2. Ceftolozane–Tazobactam
3. Phage Therapeutics
3.1. Applications in Medicine
3.2. Limitations
4. Antimicrobial Peptides
4.1. Advantages
4.2. Applications in Medicine
4.3. Limitations
5. Nanoparticles
5.1. Advantages
5.2. Applications in Medicine
5.3. Combination Therapy
5.4. Limitations
- Size, due to their rapid diffusion into human cells and their ability to pass across the blood-brain barrier (200 nm). NPs below 10 nm often exhibit substantial antibacterial activity but also high cytotoxicity;
- Agglomeration, which aids in the sedimentation process and slows NP diffusion which increases effective dosages;
- Surface charge, in order to control protein binding to NPs, cellular uptake, oxidative stress, autophagy, inflammation, and apoptosis, NPs’ charge is crucial (charged NPs have been shown to be more cytotoxic than neutral forms, and positively charged NPs were more cytotoxic than negative variants of similar size). Currently, MNPs can be designed to reduce their toxicity to humans [199]. The size can be tailored for optimal efficacy, and capping agents can be used to prevent agglomeration, avoid undesirable nanoparticles oxidation and enhance ion release. Commonly used capping agents are oleic acid, polyacrylic acid, polyethylene glycol (PEG), polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP) [200,201].
6. Essential Oils
6.1. Applications of EOs
6.2. Antimicrobial Effect of EOs
6.3. Limitations
7. Antisense Antimicrobial Therapeutics
7.1. Mechanism of Action
7.2. Efficacy
7.3. Applications in Medicine
8. Faecal Microbiota Transplant
8.1. Applications in Medicine
8.2. Limitations
9. Quorum-Sensing Inhibitors
9.1. Pseudomonas Aeruginosa as a Model Organism
9.2. Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawla, M.; Verma, J.; Gupta, R.; Das, B. Antibiotic Potentiators Against Multidrug-Resistant Bacteria: Discovery, Development, and Clinical Relevance. Front. Microbiol. 2022, 13, 887251. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E.; et al. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Du, F.; Long, M.; Li, P. Limitations of Phage Therapy and Corresponding Optimization Strategies: A Review. Molecules 2022, 27, 1857. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-infective Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Zyman, A.; Górski, A.; Międzybrodzki, R. Phage therapy of wound-associated infections. Folia Microbiol. 2022, 67, 193–201. [Google Scholar] [CrossRef]
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012–2017. New Engl. J. Med. 2020, 382, 1309–1319. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Alaran, A.J.; Okereke, M.; Oke, G.I.; Amos, O.A.; Olaoye, O.C.; Oladunjoye, I.; Olanrewaju, A.Y.; Ukor, N.A.; Lucero-Prisno, D.E. COVID-19 and Antimicrobial Resistance: A Review. Infect. Dis. Res. Treat. 2021, 14, 11786337211033870. [Google Scholar] [CrossRef]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983. [Google Scholar] [CrossRef]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Emerging and Zoonotic Infectious Diseases; Division of Healthcare Quality Promotion. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. 2022. Available online: https://stacks.cdc.gov/view/cdc/117915.2022 (accessed on 11 October 2022).
- Saravolatz, L.D.; Stein, G.E. Plazomicin: A New Aminoglycoside. Clin Infect Dis. 2020, 70, 704–709. [Google Scholar]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Ejim, L.; Stogios, P.J.; Koteva, K.; Bordeleau, E.; Evdokimova, E.; Sieron, A.O.; Savchenko, A.; Serio, A.W.; Krause, K.M.; et al. Plazomicin Retains Antibiotic Activity against Most Aminoglycoside Modifying Enzymes. ACS Infect. Dis. 2018, 4, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Davis, A.P.; Mendes, R.E.; Serio, A.W.; Krause, K.M.; Flamm, K. In Vitro Activity of Plazomicin against Gram-Negative and Gram-Positive Isolates Collected from U.S. Hospitals and Comparative Activities of Aminoglycosides against Carbapenem-Resistant Enterobacteriaceae and Isolates Carrying Carbapenemase Genes Mariana. Antimicrob Agents Chemother. 2018, 62, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. Medchemcomm 2016, 7, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, Y.; Wachino, J.-I.; Arakawa, Y. Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases. Infect. Dis. Clin. North. Am. 2016, 30, 523–537. [Google Scholar] [CrossRef] [Green Version]
- Walkty, A.; Karlowsky, J.A.; Baxter, M.R.; Adam, H.J.; Zhanel, G.G. In Vitro Activity of Plazomicin against Gram-Negative and Gram-Positive Bacterial Pathogens Isolated from Patients in Canadian Hospitals from 2013 to 2017 as Part of the CANWARD Surveillance Study. Antimicrob. Agents Chemother. 2019, 63, e02068-18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kashikar, A.; Bush, K. In vitro activity of plazomicin against β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE). J. Antimicrob Chemother. 2017, 72, 2792–2795. [Google Scholar] [CrossRef] [Green Version]
- Denervaud-Tendon, V.; Poirel, L.; E Connolly, L.; Krause, K.M.; Nordmann, P. Plazomicin activity against polymyxin-resistant Enterobacteriaceae, including MCR-1-producing isolates. J. Antimicrob. Chemother. 2017, 72, 2787–2791. [Google Scholar] [CrossRef]
- Thwaites, M.; Hall, D.; Shinabarger, D.; Serio, A.W.; Krause, K.M.; Marra, A.; Pillar, C. Evaluation of the Bactericidal Activity of Plazomicin and Comparators against Multidrug-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018, 62, 8–12. [Google Scholar] [CrossRef] [Green Version]
- McKinnell, J.A.; Dwyer, J.P.; Talbot, G.H.; Connolly, L.E.; Friedland, I.; Smith, A.; Jubb, A.M.; Serio, A.W.; Krause, K.M.; Daikos, G.L. Plazomicin for Infections Caused by Carbapenem-Resistant Enterobacteriaceae. N. Engl. J. Med. 2019, 380, 791–793. [Google Scholar] [CrossRef]
- Cattoir, V. Infections à bacilles à Gram négatif résistants: Nouvelles molécules, nouvelles associations. J. Des Anti-Infectieux 2013, 15, 159–165. [Google Scholar] [CrossRef]
- Clark, J.A.; Burgess, D.S. Plazomicin: A new aminoglycoside in the fight against antimicrobial resistance. Ther. Adv. Infect. Dis. 2020, 7, 2049936120952604. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, M.; Hall, D.; Stoneburner, A.; Shinabarger, D.; Serio, A.; Krause, K.; Marra, A.; Pillar, C. Activity of plazomicin in combination with other antibiotics against multidrug-resistant Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2018, 92, 338–345. [Google Scholar] [CrossRef] [PubMed]
- García-Salguero, C.; Rodríguez-Avial, I.; Picazo, J.J.; Culebras, E. Can Plazomicin Alone or in Combination Be a Therapeutic Option against Carbapenem-Resistant Acinetobacter baumannii? Antimicrob Agents Chemother. 2015, 59, 5959–5966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K pneumoniae: A case report and review of literature. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Kuti, J.L.; Kim, A.; Cloutier, D.J.; Nicolau, D.P. Evaluation of plazomicin, tigecycline, and meropenem pharmacodynamic exposure against carbapenem-resistant Enterobacteriaceae in patients with bloodstream infection or hospital-acquired/ventilator-associated pneumonia from the CARE study (ACHN-490-007). Infect. Dis. Ther. 2019, 8, 383–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaghrabi, R.; Clancy, C.J.; Doi, Y.; Hao, B.; Chen, L.; Shields, R.K.; Press, E.G.; Iovine, N.M.; Townsend, B.M.; Wagener, M.M.; et al. Carbapenem-Resistant Klebsiella pneumoniae Strains Exhibit Diversity in Aminoglycoside-Modifying Enzymes, Which Exert Differing Effects on Plazomicin and Other Agents. Antimicrob. Agents Chemother. 2014, 58, 4443–4451. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.-Y.; Hunt, D.K.; Zhou, J.; Clark, R.B.; Dunwoody, N.; Fyfe, C.; Grossman, T.H.; O’Brien, W.J.; Plamondon, L.; Rönn, M.; et al. Fluorocyclines. 1. 7-Fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: A Potent, Broad Spectrum Antibacterial Agent. J. Med. Chem. 2012, 55, 597–605. [Google Scholar] [CrossRef]
- Kwiatek, M.; Parasion, S.; Nakonieczna, A. Therapeutic bacteriophages as a rescue treatment for drug-resistant infections—an in vivo studies overview. J. Appl. Microbiol. 2020, 128, 985–1002. [Google Scholar] [CrossRef] [Green Version]
- Sutcliffe, J.A.; O’Brien, W.; Fyfe, C.; Grossman, T.H. Antibacterial Activity of Eravacycline (TP-434), a Novel Fluorocycline, against Hospital and Community Pathogens. Antimicrob. Agents Chemother. 2013, 57, 5548–5558. [Google Scholar] [CrossRef] [Green Version]
- Zhanel, G.G.; Baxter, M.R.; Adam, H.J.; Sutcliffe, J.; Karlowsky, J.A. In vitro activity of eravacycline against 2213 Gram-negative and 2424 Gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD surveillance study 2014–2015. Diagn. Microbiol. Infect. Dis. 2018, 91, 55–62. [Google Scholar] [CrossRef]
- Snydman, D.R.; McDermott, L.A.; Jacobus, N.V.; Kerstein, K.; Grossman, T.H.; Sutcliffe, J.A. Evaluation of the In Vitro Activity of Eravacycline against a Broad Spectrum of Recent Clinical Anaerobic Isolates. Antimicrob. Agents Chemother. 2018, 62, e02206-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citron, D.; Tyrrell, K.; Goldstein, E. In Vitro Activity of Eravacycline and Comparator Antimicrobials Against 143 Strains of Bacteroides Species. Open Forum Infect. Dis. 2017, 4, S369–S370. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, W.; Zhu, X.; Zhang, J.; Zhang, Y.; Han, Y.; Chen, K.; Yin, Y. Evaluation of Neisseria gonorrhoeae Isolates Susceptibility to Tetracycline Antibiotics from 9 Provinces in China Since 2020. Infect. Drug. Resist. 2022, 15, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Lagacé-Wiens, P.R.S.; Adam, H.J.; Laing, N.M.; Baxter, M.R.; Martin, I.; Mulvey, M.R.; Karlowsky, J.A.; Hoban, D.J.; Zhanel, G.G. Antimicrobial susceptibility of clinical isolates of Neisseria gonorrhoeae to alternative antimicrobials with therapeutic potential. J. Antimicrob. Chemother. 2017, 72, 2273–2277. [Google Scholar] [CrossRef] [PubMed]
- Heaney, M.; Mahoney, M.V.; Gallagher, J.C. Eravacycline: The Tetracyclines Strike Back. Ann. Pharmacother. 2019, 53, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Sway, A.; Lawrence, K.; Olesky, M.; Izmailyan, S.; Tsai, L. Eravacycline: A new treatment option for complicated intra-abdominal infections in the age of multidrug resistance. Futur. Microbiol. 2019, 14, 1293–1308. [Google Scholar] [CrossRef]
- Eljaaly, K.; Ortwine, J.K.; Shaikhomer, M.; Almangour, T.A.; Bassetti, M. Efficacy and safety of eravacycline: A meta-analysis. J. Glob. Antimicrob. Resist. 2021, 24, 424–428. [Google Scholar] [CrossRef]
- Hanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.; Walkty, A.; Gin, A.S.; et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 2016, 76, 567–588. [Google Scholar]
- Alosaimy, S.; Abdul-Mutakabbir, J.C.; Kebriaei, R.; Jorgensen, S.C.J.; Rybak, M.J. Evaluation of Eravacycline: A Novel Fluorocycline. Pharmacotherapy 2020, 40, 221–238. [Google Scholar] [CrossRef]
- Syed, Y.Y. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69 (Suppl. S7), S538–S543. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Streit, J.M.; Mendes, R.; Castanheira, M. In Vitro Activity of Cefiderocol against U.S. and European Gram-Negative Clinical Isolates Collected in 2020 as Part of the SENTRY Antimicrobial Surveillance Program. Microbiol. Spectr. 2022, 10, e0271221. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Skalidis, T.; Vardakas, K.Z.; Legakis, N.J.; on behalf of the Hellenic Cefiderocol Study Group. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J. Antimicrob. Chemother. 2017, 72, 1704–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e0217120. [Google Scholar] [CrossRef]
- Parsels, K.A.; Mastro, K.A.; Steele, J.M.; Thomas, S.J.; Kufel, W.D. Cefiderocol: A novel siderophore cephalosporin for multidrug-resistant Gram-negative bacterial infections. J. Antimicrob. Chemother. 2021, 76, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Portsmouth, S.; van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Tenke, P.; Den Nagata, T. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: A phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2018, 18, 1319–1328. [Google Scholar] [CrossRef]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam-β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef]
- Soriano, A.; Carmeli, Y.; Omrani, A.S.; Moore, L.S.P.; Tawadrous, M.; Irani, P. Ceftazidime-Avibactam for the Treatment of Serious Gram-Negative Infections with Limited Treatment Options: A Systematic Literature Review. Infect. Dis. Ther. 2021, 10, 1989–2034. [Google Scholar] [CrossRef]
- Thompson, C.A. Ceftazidime with β-lactamase inhibitor approved for complicated infections. Am. J. Health Syst. Pharm. 2015, 72, 511. [Google Scholar] [CrossRef] [PubMed]
- Herrero, F.S. Ceftazidime-avibactam. Rev. Española Quimioter. 2022, 35 (Suppl. S1), 40. [Google Scholar]
- Sader, H.S.; Castanheira, M.; Shortridge, D.; Mendes, R.E.; Flamm, R.K. Antimicrobial Activity of Ceftazidime-Avibactam Tested against Multidrug-Resistant Enterobacteriaceae and Pseudomonas aeruginosa Isolates from U.S. Medical Centers, 2013 to 2016. Antimicrob. Agents Chemother. 2017, 61, e01045-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsolaki, V.; Mantzarlis, K.; Mpakalis, A.; Malli, E.; Tsimpoukas, F.; Tsirogianni, A.; Papagiannitsis, C.; Zygoulis, P.; Papadonta, M.-E.; Petinaki, E.; et al. Ceftazidime-Avibactam To Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically Ill Mechanically Ventilated Patients. Antimicrob. Agents Chemother. 2020, 64, e02320-19. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients With Bloodstream Infections Caused by Metallo-β-lactamase–Producing Enterobacterales. Clin. Infect. Dis. 2020, 72, 1871–1878. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Bassetti, M.; De Rosa, F.G.; Del Bono, V.; Grossi, P.A.; Menichetti, F.; Pea, F.; Rossolini, G.M.; Tumbarello, M.; Viale, P.; et al. Ceftolozane/tazobactam: Place in therapy. Expert Rev. Anti-infective Ther. 2018, 16, 307–320. [Google Scholar] [CrossRef]
- Van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Bounthavong, M.; Hsu, D.; Hong, M.-C. Ceftolozane/tazobactam: A novel antipseudomonal cephalosporin and β-lactamase-inhibitor combination. Infect. Drug Resist. 2013, 6, 215. [Google Scholar] [CrossRef] [Green Version]
- Thabit, A.K.; Hamada, Y.; Nicolau, D.P. Physical compatibility of ceftolozane–tazobactam with selected i.v. drugs during simulated Y-site administration. Am. J. Heal. Pharm. 2017, 74, e47–e54. [Google Scholar] [CrossRef]
- Weber, C.; Schultze, T.; Göttig, S.; Kessel, J.; Schröder, A.; Tietgen, M.; Besier, S.; Burbach, T.; Häussler, S.; Wichelhaus, T.A.; et al. Antimicrobial Activity of Ceftolozane-Tazobactam, Ceftazidime-Avibactam, and Cefiderocol against Multidrug-Resistant Pseudomonas aeruginosa Recovered at a German University Hospital. Microbiol. Spectr. 2022, 10, e01697-22. [Google Scholar] [CrossRef]
- Kuo, S.-C.; Liu, C.-E.; Lu, P.-L.; Chen, Y.-S.; Lu, M.-C.; Ko, W.-C.; Hsueh, P.R.; Chuang, Y.C.; Wang, F.D. Activity of ceftolozane-tazobactam against Gram-negative pathogens isolated from lower respiratory tract infections in the Asia-Pacific region: SMART 2015–2016. Int. J. Antimicrob. Agents. 2020, 55, 105883. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.B.; Brigman, H.V.; Zucchi, P.C.; Chen, A.; Anderson, J.C.; Eliopoulos, G.M.; Cheung, N.; Gilbertsen, A.; Hunter, R.C.; Emery, C.L.; et al. Ceftolozane-tazobactam and ceftazidime-avibactam activity against β-lactam-resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Enterobacterales clinical isolates from US medical centres. J. Glob. Antimicrob. Resist. 2020, 22, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Grupper, M.; Sutherland, C.; Nicolau, D.P. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible Pseudomonas aeruginosa from blood, respiratory tract, and wounds. Antimicrob. Agents. Chemother. 2017, 61, e00875-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Hu, X.; Wang, Y. Alternatives to Conventional Antibiotic Therapy: Potential Therapeutic Strategies of Combating Antimicrobial-Resistance and Biofilm-Related Infections. Mol. Biotechnol. 2021, 63, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barceló, C.; Hochberg, M.E. Evolutionary Rationale for Phages as Complements of Antibiotics. Trends Microbiol. 2016, 24, 249–256. [Google Scholar] [CrossRef]
- Venturini, C.; Fabijan, A.P.; Lubian, A.F.; Barbirz, S.; Iredell, J. Biological foundations of successful bacteriophage therapy. EMBO Mol. Med. 2022, 14, e12435. [Google Scholar] [CrossRef]
- Azam, A.H.; Tan, X.-E.; Veeranarayanan, S.; Kiga, K.; Cui, L. Bacteriophage Technology and Modern Medicine. Antibiotics 2021, 10, 999. [Google Scholar] [CrossRef]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.-P.; Costechareyre, D.; Molle, V. Bacteriophage Therapy for Staphylococcus Aureus Infections: A Review of Animal Models, Treatments, and Clinical Trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314. [Google Scholar] [CrossRef]
- Venhorst, J.; van der Vossen, J.M.B.M.; Agamennone, V. Battling Enteropathogenic Clostridia: Phage Therapy for Clostridioides difficile and Clostridium perfringens. Front. Microbiol. 2022, 13, 891790. [Google Scholar] [CrossRef]
- White, H.E. Bacteriophages: Their Structural Organisation and Function. In Savva EVOE-R; Rijeka, Ed.; IntechOpen: London, UK, 2019; p. 2. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, V.; Suresh, A. Next-Generation Approaches Needed to Tackle Antimicrobial Resistance for the Development of Novel Therapies Against the Deadly Pathogens. Front. Pharmacol. 2022, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heuler, J.; Fortier, L.-C.; Sun, X. Clostridioides difficile phage biology and application. FEMS Microbiol. Rev. 2021, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rao, T.S.; Joshi, H.M. Phage therapy in the Covid-19 era: Advantages over antibiotics. Curr. Res. Microb. Sci. 2022, 3, 100115. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Nath, G.; Bhargava, A.; Aseri, G.K.; Jain, N. Phage therapeutics: From promises to practices and prospectives. Appl. Microbiol. Biotechnol. 2021, 105, 9047–9067. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef] [Green Version]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Negi, A.K.; Galav, V.; Thakur, S.; Agrawal, M.; Sharma, V. Nanotechnology Based Approaches in Phage Therapy: Overcoming the Pharmacological Barriers. Front. Pharmacol. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke. Off. J. Int. Stroke. Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Aranaga, C.; Pantoja, L.D.; Martínez, E.A.; Falco, A. Phage Therapy in the Era of Multidrug Resistance in Bacteria: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4577. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Agarwal, R.; Sharma, R.K. Bacteriophage Therapy for Critical and High-Priority Antibiotic-Resistant Bacteria and Phage Cocktail-Antibiotic Formulation Perspective. Food Environ. Virol. 2021, 13, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Cebriá-Mendoza, M.; Sanjuán, R.; Domingo-Calap, P. Directed Evolution of a Mycobacteriophage. Antibiotics 2019, 8, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanishvili, N. Bacteriophages as Therapeutic and Prophylactic Means: Summary of the Soviet and Post Soviet Experiences. Curr. Drug Deliv. 2016, 13, 309–323. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [Green Version]
- Caflisch, K.; A Suh, G.; Patel, R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert Rev. Anti-Infect. Ther. 2019, 17, 1011–1041. [Google Scholar] [CrossRef]
- Kutter, E.; Sulakvelidze, A. Bacteriophages: Biology and Applications; CRC Press: Boca Raton, FL, USA, 2004; Volume 1. [Google Scholar]
- Fruciano, D.E.; Bourne, S. Phage as an antimicrobial agent: D’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 19–26. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2019, 40, 459–463. [Google Scholar] [CrossRef]
- Altamirano, F.L.G.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Luo, T.; Yang, Y.; Dong, D.; Wang, R.; Wang, Y.; Xu, M.; Guo, X.; Hu, F.; He, P. Phage Therapy as a Promising New Treatment for Lung Infection Caused by Carbapenem-Resistant Acinetobacter baumannii in Mice. Front. Microbiol. 2018, 8, 2659. [Google Scholar] [CrossRef] [Green Version]
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. In Open forum infectious diseases; Oxford University Press: Oxford, UK, 2018; p. ofy064. [Google Scholar]
- Wu, N.; Chen, L.K.; Zhu, T. Phage therapy for secondary bacterial infections with COVID-19. Curr. Opin. Virol. 2022, 52, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical experience of personalized phage therapy against carbapenem-resistant Acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2021, 11, 631585. [Google Scholar] [CrossRef] [PubMed]
- Bagińska, N.; Cieślik, M.; Górski, A.; Jończyk-Matysiak, E. The role of antibiotic resistant A. baumannii in the pathogenesis of urinary tract infection and the potential of its treatment with the use of bacteriophage therapy. Antibiotics 2021, 10, 281. [Google Scholar] [PubMed]
- Anomaly, J. The future of phage: Ethical challenges of using phage therapy to treat bacterial infections. Public Health Ethics. 2020, 13, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Strathdee, S.; Patterson, T. The Perfect Predator: A Scientist’s Race to Save Her Husband from a Deadly Superbug: A Memoir; Hachette Books: New York, NY, USA, 2019. [Google Scholar]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Latz, S.; Krüttgen, A.; Häfner, H.; Buhl, E.M.; Ritter, K.; Horz, H.-P. Differential effect of newly isolated phages belonging to PB1-like, phiKZ-like and LUZ24-like viruses against multi-drug resistant Pseudomonas aeruginosa under varying growth conditions. Viruses 2017, 9, 315. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Liu, Z.; Tan, X.; Wang, H.; Liang, Y.; Kong, Y.; Sun, W.; Sun, L.; Ma, Y.; Lu, H. Bacteriophage therapy for empyema caused by carbapenem-resistant Pseudomonas aeruginosa. Biosci. Trends 2022, 16, 158–162. [Google Scholar] [CrossRef]
- Terwilliger, A.; Clark, J.; Karris, M.; Hernandez-Santos, H.; Green, S.; Aslam, S.; Maresso, A. Phage Therapy Related Microbial Succession Associated with Successful Clinical Outcome for a Recurrent Urinary Tract Infection. Viruses 2021, 13, 2049. [Google Scholar] [CrossRef]
- Easwaran, M.; De Zoysa, M.; Shin, H. Application of phage therapy: Synergistic effect of phage EcSw (ΦEcSw) and antibiotic combination towards antibiotic-resistant Escherichia coli. Transbound. Emerg. Dis. 2020, 67, 2809–2817. [Google Scholar] [CrossRef]
- Amarillas, L.; Rubí-Rangel, L.; Chaidez, C.; González-Robles, A.; Lightbourn-Rojas, L.; León-Félix, J. Isolation and characterization of phiLLS, a novel phage with potential biocontrol agent against multidrug-resistant Escherichia coli. Front. Microbiol. 2017, 8, 1355. [Google Scholar] [CrossRef]
- Taha, O.A.; Connerton, P.L.; Connerton, I.; El-Shibiny, A. Bacteriophage ZCKP1: A Potential Treatment for Klebsiella pneumoniae Isolated From Diabetic Foot Patients. Front. Microbiol. 2018, 9, 2127. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Shafique, M.; Khawaja, K.A.; Alvi, I.A.; Rehman, Y.; Sheik, C.; Abbas, Z.; Rehman, S.U. Complete genome analysis of a Siphoviridae phage TSK1 showing biofilm removal potential against Klebsiella pneumoniae. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Wu, N.; Bao, J.; Shi, X.; Ou, H.; Ye, S.; Zhao, W.; Wei, Z.; Cai, J.; Li, L.; et al. Heterogeneous Klebsiella pneumoniae Co-infections Complicate Personalized Bacteriophage Therapy. Front. Cell. Infect. Microbiol. 2021, 10, 608402. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Chen, L.; Guo, G.; Li, P.; Ma, J.; Chen, R.; Du, H.; Liu, Y.; Zhang, W. Identification of a phage-derived depolymerase specific for KL47 capsule of Klebsiella pneumoniae and its therapeutic potential in mice. Virol. Sin. 2022, 37, 538–546. [Google Scholar] [CrossRef]
- Tompkins, K.; van Duin, D. Treatment for carbapenem-resistant Enterobacterales infections: Recent advances and future directions. Eur. J. Clin. Microbiol. 2021, 40, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.; Tian, C.; Chen, X.; Hu, L.; Wei, X.; Li, H.; Lin, W.; Jiang, A.; Feng, R.; et al. Characterizing the Biology of Lytic Bacteriophage vB_EaeM_φEap-3 Infecting Multidrug-Resistant Enterobacter aerogenes. Front. Microbiol. 2019, 10, 420. [Google Scholar] [CrossRef]
- Jamal, M.; Bukhari, S.M.A.U.S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef]
- Paul, K.; Merabishvili, M.; Hazan, R.; Christner, M.; Herden, U.; Gelman, D.; Khalifa, L.; Yerushalmy, O.; Coppenhagen-Glazer, S.; Harbauer, T.; et al. Bacteriophage Rescue Therapy of a Vancomycin-Resistant Enterococcus faecium Infection in a One-Year-Old Child following a Third Liver Transplantation. Viruses 2021, 13, 1785. [Google Scholar] [CrossRef]
- Soleimani-Delfan, A.; Bouzari, M.; Wang, R. vB_EfaS-DELF1, a novel Siphoviridae bacteriophage with highly effective lytic activity against vancomycin-resistant Enterococcus faecalis. Virus Res. 2021, 298, 198391. [Google Scholar] [CrossRef]
- Topka-Bielecka, G.; Nejman-Faleńczyk, B.; Bloch, S.; Necel, A.; Węgrzyn, A.; Węgrzyn, G. Phage-Bacteria Interactions in Potential Applications of Bacteriophage vB_EfaS-271 against Enterococcus faecalis. Viruses 2021, 13, 318. [Google Scholar] [CrossRef]
- Barros, J.; Melo, L.D.; Poeta, P.; Igrejas, G.; Ferraz, M.P.; Azeredo, J.; Monteiro, F.J. Lytic bacteriophages against multidrug-resistant Staphylococcus aureus, Enterococcus faecalis and Escherichia coli isolates from orthopaedic implant-associated infections. Int. J. Antimicrob. Agents 2019, 54, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Fernández, L.; Gutiérrez, D.; Iglesias, B.; Rodríguez, A.; García, P. Methicillin-Resistant Staphylococcus aureus in Hospitals: Latest Trends and Treatments Based on Bacteriophages. J. Clin. Microbiol. 2019, 57, e01006-19. [Google Scholar] [CrossRef] [PubMed]
- Speck, P.G.; Warner, M.S.; Bihari, S.; Bersten, A.D.; Mitchell, J.G.; Tucci, J.; Gordon, D.L. Potential for bacteriophage therapy for Staphylococcus aureus pneumonia with influenza A coinfection. Futur. Microbiol. 2021, 16, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhuyse, B.; Galant, C.; Brichard, B.; Docquier, P.-L.; Djebara, S.; Pirnay, J.-P.; Van der Linden, D.; Merabishvili, M.; Chatzis. A Case of In Situ Phage Therapy against Staphylococcus aureus in a Bone Allograft Polymicrobial Biofilm Infection: Outcomes and Phage-Antibiotic Interactions. Viruses 2021, 13, 1898. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Ferreira, R.; Azevedo, N.F.; Oleastro, M.; Azeredo, J.; Figueiredo, C.; Melo, L.D.R. Helicobacter pylori infection: From standard to alternative treatment strategies. Crit. Rev. Microbiol. 2021, 48, 376–396. [Google Scholar] [CrossRef]
- Singh, A.; Mahajan, S. Advancements in Diagnostics and Treatments of Helicobacter Pylori. J. Pharm. Res. Int. 2021, 435, 444. [Google Scholar] [CrossRef]
- Hafez, R.; El-Didamony, G.; Wagih, A.E.E.; Said, E.A.; Mohammed, B.O.; Mohamed, A.M.; Mohammed, H.A. Anti-Helicobacter pylori activity of Egyptian medicinal plants and bacteriophages. Microbes. Infect. Dis. 2020, 1, 168–181. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Lis, L.; Lasagabaster, A.; Nafarrate, I.; Ferrocino, I.; Cocolin, L.; Rantsiou, K. Campylobacter spp. prevalence and mitigation strategies in the broiler production chain. Food Microbiol. 2022, 104, 103998. Available online: https://www.sciencedirect.com/science/article/pii/S0740002022000223 (accessed on 11 October 2022). [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Makalatia, K.; Kakabadze, E.; Wagemans, J.; Grdzelishvili, N.; Bakuradze, N.; Natroshvili, G.; Macharashvili, N.; Sedrakyan, A.; Arakelova, K.; Ktsoyan, Z.; et al. Characterization of Salmonella Isolates from Various Geographical Regions of the Caucasus and Their Susceptibility to Bacteriophages. Viruses 2020, 12, 1418. [Google Scholar] [CrossRef]
- Gambino, M.; Nørgaard, S.A.; Ahern, S.; Smyrlis, G.; Gencay, Y.E.; Hendrix, H.; Neve, H.; Noben, J.P.; Lavigne, R.; Brøndsted, D. Phage S144, a new polyvalent phage infecting Salmonella spp. and Cronobacter sakazakii. Int. J. Mol. Sci. 2020, 21, 5196. [Google Scholar] [CrossRef] [PubMed]
- Makalatia, K.; Kakabadze, E.; Bakuradze, N.; Grdzelishvili, N.; Natroshvili, G.; Kusradze, I.; Goderdzishvili, M.; Sedrakyan, A.; Arakelova, K.; Mkrtchyan, M.; et al. Activity of bacteriophages to multiply resistant strains of salmonella and their various serotypes. Bull. Veterinary Biotechnol. 2018, 32, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Suay-García, B.; Pérez-Gracia, M.T. Future Prospects for Neisseria gonorrhoeae Treatment. Antibiot 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cater, K.; Międzybrodzki, R.; Morozova, V.; Letkiewicz, S.; Łusiak-Szelachowska, M.; Rękas, J.; Weber-Dąbrowska, B.; Górski, A. Potential for Phages in the Treatment of Bacterial Sexually Transmitted Infections. Antibiotics 2021, 10, 1030. [Google Scholar] [CrossRef]
- Qadir, M.I.; Sajjad, S. Phage Therapy against Streptococcus pneumoniae: Modern Tool to Control Pneumonia. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 289–295. [Google Scholar] [CrossRef]
- Fernández, L.; Cima-Cabal, M.D.; Duarte, A.C.; Rodríguez, A.; García-Suárez, M.D.M.; García, P. Gram-positive pneumonia: Possibilities offered by phage therapy. Antibiotics 2021, 10, 100. [Google Scholar] [CrossRef]
- Jones, J.D.; Varghese, D.; Pabary, R.; Langley, R.J. The potential of bacteriophage therapy in the treatment of paediatric respiratory infections. Paediatr Respir Rev. 2022. ahead of print. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Oliveira, H.; Pires, D.P.; Dabrowska, K.; Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 2020, 46, 78–99. [Google Scholar] [CrossRef]
- Szaleniec, J.; Gibała, A.; Pobiega, M.; Parasion, S.; Składzień, J.; Stręk, P.; Gosiewski, T.; Szaleniec, M. Exacerbations of chronic rhinosinusitis—Microbiology and perspectives of phage therapy. Antibiotics 2019, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, R.; Farahani, A. Shigella: Antibiotic-Resistance Mechanisms And New Horizons For Treatment. Infect. Drug Resist. 2019, 12, 3137–3167. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.-S.; Biswas, S.K.; Tan, W.S.; Saha, A.K.; Leo, B.-F. Efficacy and potential of phage therapy against multidrug resistant Shigella spp. Peer J. 2019, 7, e6225. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Chaudhry, W.N.; Hussain, T.; Das, C.R.; Andleeb, S. Characterization of new Myoviridae bacteriophage WZ1 against multi-drug resistant (MDR) Shigella dysenteriae. J. Basic Microbiol. 2015, 55, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Borysowski, J.; Międzybrodzki, R. Phage Therapy: Towards a Successful Clinical Trial. Antibiotics 2020, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2013, 5, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Nale, J.Y.; Clokie, M.R. Preclinical data and safety assessment of phage therapy in humans. Curr. Opin. Biotechnol. 2021, 68, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.-X.; Zhang, X.-Z. Bacteriophage-mediated modulation of microbiota for diseases treatment. Adv. Drug Deliv. Rev. 2021, 176, 113856. [Google Scholar] [CrossRef]
- Abedon, S.T. Ecology of Anti-Biofilm Agents I: Antibiotics versus Bacteriophages. Pharmaceuticals 2015, 8, 525–558. [Google Scholar] [CrossRef]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the Host Defense Peptide Landscape. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Mook-Kanamori, B.B.; Geldhoff, M.; van der Poll, T.; van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 2011, 24, 557–591. [Google Scholar] [CrossRef] [PubMed]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.G. Phagocytin: A Bactericidal Substance from Polymorphonuclear Leucocytes. J. Exp. Med. 1956, 103, 589–611. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Deal, B.E.; Grove, A.S. General Relationship for the Thermal Oxidation of Silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef] [Green Version]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides – Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Büyükkiraz, M.E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2021, 132, 1573–1596. [Google Scholar] [CrossRef]
- Hazam, P.K.; Goyal, R.; Ramakrishnan, V. Peptide based antimicrobials: Design strategies and therapeutic potential. Prog. Biophys. Mol. Biol. 2019, 142, 10–22. [Google Scholar] [CrossRef]
- García-Bayona, L.; Comstock, L.E. Bacterial antagonism in host-associated microbial communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [Green Version]
- Lewis, B.B.; Pamer, E.G. Microbiota-based therapies for Clostridium difficile and antibiotic-resistant enteric infections. Annu. Rev. Microbiol. 2017, 71, 157–178. [Google Scholar] [CrossRef]
- Martínez, B.; Rodríguez, A.; Suárez, E. Antimicrobial Peptides Produced by Bacteria: The Bacteriocins. In New Weapons to Control Bacterial Growth; Villa, T.G., Vinas, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 15–38. ISBN 978-3-319-28368-5. [Google Scholar] [CrossRef]
- Lubelski, J.; Rink, R.; Khusainov, R.; Moll, G.N.; Kuipers, O.P. Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell. Mol. Life Sci. 2008, 65, 455–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.W.; Ray, P.; Steinmetz, T.; Hanekamp, T.; Ray, B. Gene organization and sequences of pediocin AcH/PA-1 production operons in Pediococcus and Lactobacillus plasmids. Lett. Appl. Microbiol. 2005, 40, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. 2013, 59, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Hormoz, S. Amino acid composition of proteins reduces deleterious impact of mutations. Sci. Rep. 2013, 3, srep02919. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2016, 55, 1–12. [Google Scholar] [CrossRef]
- Fernández, L.; Delgado, S.; Herrero, H.; Maldonado, A.; Rodríguez, J.M. The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. J. Hum. Lact. Off J. Int. Lact Consult Assoc. 2008, 24, 311–316. [Google Scholar] [CrossRef]
- Foxman, B.; D’Arcy, H.; Gillespie, B.; Bobo, J.K.; Schwartz, K. Lactation Mastitis: Occurrence and Medical Management among 946 Breastfeeding Women in the United States. Am. J. Epidemiology 2002, 155, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.; Sandhu, P.; Ror, P.; Dash, E.; Sharma, S.; Arakha, M.; Jha, S.; Akhter, Y.; Saleem, M. Lipid-II Independent Antimicrobial Mechanism of Nisin Depends On Its Crowding And Degree Of Oligomerization. Sci. Rep. 2016, 6, 37908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, J.M.; Rajasekaran, A.K. Gramicidin A: A New Mission for an Old Antibiotic. J. Kidney Cancer VHL 2015, 2, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yousef, A.E. Antimicrobial peptides produced by Brevibacillus spp.: Structure, classification and bioactivity: A mini review. World J. Microbiol. Biotechnol. 2018, 34, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Hu, J.T.; Zhang, C.; Zhou, W.; Chen, X.F.; Jiang, L.Y.; Tang, Z.H. The safety and efficacy of daptomycin versus other antibiotics for skin and soft-tissue infections: A meta-analysis of randomised controlled trials. BMJ Open 2014, 4, e004744. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Gao, R.; Liu, C.; Shan, B.; Gao, F.; He, J.; Yuan, M.; Xie, H.; Jin, S.; Ma, Y. Potential role of the antimicrobial peptide Tachyplesin III against multidrug-resistant P. aeruginosa and A. baumannii coinfection in an animal model. Infect. Drug Resist. 2019, 12, 2865–2874. [Google Scholar] [CrossRef] [Green Version]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Dale, G.E.; Halabi, A.; Petersen-Sylla, M.; Wach, A.; Zwingelstein, C. Pharmacokinetics, Tolerability, and Safety of Murepavadin, a Novel Antipseudomonal Antibiotic, in Subjects with Mild, Moderate, or Severe Renal Function Impairment. Antimicrob. Agents Chemother. 2018, 62, e00490-18. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pauer, A.C.; Gonzales, A.A.; Fenniri, H. Enhanced antibiotic activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int. J. Nanomedicine. 2019, 14, 281–7289. [Google Scholar] [CrossRef] [Green Version]
- Agreles, M.A.A.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Synergism between metallic nanoparticles and antibiotics. Appl. Microbiol. Biotechnol. 2022, 106, 3973–3984. Available online: http://europepmc.org/abstract/MED/35670851 (accessed on 11 October 2022). [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Lemaoui, C.-E.; Layaida, H.; Badi, A.; Foudi, N. Stratégies actuelles de lutte contre la résistance aux antibiotiques. J. Anti-Infectieux 2017, 5258, 1. Available online: http://www.sciencedirect.com/science/article/pii/S2210-6545(17)30003-0 (accessed on 11 October 2022). [CrossRef]
- Jelinkova, P.; Mazumdar, A.; Sur, V.P.; Kociova, S.; Dolezelikova, K.; Jimenez, A.M.J.; Koudelkova, Z.; Mishra, P.K.; Smerkova, K.; Heger, Z.; et al. Nanoparticle-drug conjugates treating bacterial infections. J. Control. Release 2019, 307, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wu, H.; Yin, T.; Wang, L.; Shan, A. Nanostructured Antimicrobial Peptides: Crucial Steps of Overcoming the Bottleneck for Clinics. Front. Microbiol. 2021, 12, 710199. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Masson, J.-L. Le cuivre sur les surfaces en milieux de santé pour lutter contre les infections nosocomiales. Univ. Lorraine. 2015, 1999, 1–6. Available online: http://docnum.univ-lorraine.fr/public/BUPHA_T_2015_MASSON_JULIEN-LUC.pdf (accessed on 11 October 2022).
- Dar, M.A.; Gul, R.; Karuppiah, P.; Al-Dhabi, N.A.; Alfadda, A.A. Antibacterial Activity of Cerium Oxide Nanoparticles against ESKAPE Pathogens. Crystals 2022, 12, 179. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. 2021, 61, 557–568. J. Basic Microbiol. 2021, 61, 557–588. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 1–30. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. Available online: https://www.sciencedirect.com/science/article/pii/S1549963411001791 (accessed on 11 October 2022). [CrossRef] [PubMed]
- Sheng, Y.; Narayanan, M.; Basha, S.; Elfasakhany, A.; Brindhadevi, K.; Xia, C.; Pugazhendhi, A. In vitro and in vivo efficacy of green synthesized AgNPs against Gram negative and Gram-positive bacterial pathogens. Process Biochem. 2021, 112, 241–247. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Altun, E.; Aydogdu, M.O.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Edirisinghe, M. Metal-based nanoparticles for combating antibiotic resistance. Appl. Phys. Rev. 2021, 8, 041303. [Google Scholar] [CrossRef]
- Lopez-Carrizales, M.; Velasco, K.I.; Castillo, C.; Flores, A.; Magaña, M.; Martinez-Castanon, G.A.; Martinez-Gutierrez, F. In Vitro Synergism of Silver Nanoparticles with Antibiotics as an Alternative Treatment in Multiresistant Uropathogens. Antibiotics 2018, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Lane, L.A. Physics in nanomedicine: Phenomena governing the in vivo performance of nanoparticles. Appl. Phys. Rev. 2020, 7, 011316. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against antimicrobial resistance: Harnessing the power of nanoscale materials and technologies. J. Nanobiotechnol. 2022, 20, 1–25. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef] [Green Version]
- Chakansin, C.; Yostaworakul, J.; Warin, C.; Kulthong, K.; Boonrungsiman, S. Resazurin rapid screening for antibacterial activities of organic and inorganic nanoparticles: Potential, limitations and precautions. Anal. Biochem. 2021, 637, 114449. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Sun, B.; Xia, T.; Hu, S. Predictive Metabolomic Signatures for Safety Assessment of Metal Oxide Nanoparticles. ACS Nano 2019, 13, 13065–13082. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Shah, A.M.; Shah, A.; Lone, S.A.; Hussain, A.; Hassan, Q.P.; Ali, M.N. Bovine mastitis: An appraisal of its alternative herbal cure. Microb. Pathog. 2018, 114, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef]
- Zouari, N. Essential Oils Chemotypes: A Less Known Side. Med. Aromat. Plants 2013, 2, 4172. [Google Scholar]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. Available online: https://www.sciencedirect.com/science/article/pii/S0377840110000775 (accessed on 11 October 2022). [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [Green Version]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and Potential Use of Citrus Essential Oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Herman, R.A.; Ayepa, E.; Shittu, S.; Fometu, S.S.; Wang, J. Essential Oils and Their Applications—A Mini Review. Adv. Nutr. Food Sci. 2019, 4, 1–13. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, A.; Singh, V. Composition and pharmacological activity of essential oils from two imported Amomum subulatum fruit samples. J. Taibah Univ. Med Sci. 2020, 16, 231–239. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. Available online: https://www.sciencedirect.com/science/article/pii/S259025981930007X (accessed on 11 October 2022). [CrossRef]
- Talbaoui, A.; Jamaly, N.; Aneb, M.; Idrissi, A., II; Bouksaim, M.; Gmouh, S.; Amzazi, S.; El Moussaouiti, M.; Benjouad, A.; Youssef, B. Chemical composition and antibacterial activity of essential oils from six Moroccan plants. J. Med. Plant Res. 2012, 15, 4593–4600. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Mourey, A.; Canillac, N. Anti-Listeria monocytogenes activity of essential oils components of conifers. Food Control 2002, 13, 289–292. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crop. Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Trosko, J.E. Evolution of microbial quorum sensing to human global quorum sensing: An insight into how gap junctional intercellular communication might be linked to the global metabolic disease crisis. Biology 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukaina, O.; Ibrahimi, M.; Loqman, S.; Benrazzouk, K.; Ouhdouch, Y.; Markouk, M.; Bekkouche, K.; Larhsini, M. Essential Oil from Aerial Parts of Andryala pinnatifida subsp. mogadorensis: Chemical Composition, Antioxidant and Aantimicrobial Synergistic Effect Against Multidrug-Resistant Bacteria. J. Essent. Oil Bear. Plants 2022, 25, 147–159. [Google Scholar]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Cheyadmi, S.; Ouzakar, S.; Senhaji, N.S.; Abrini, J. Evaluation of the combined effect of antibiotics and essential oils against Campylobacter multidrug resistant strains and their biofilm formation. S. Afr. J. Bot. 2022, 150, 451–465. [Google Scholar] [CrossRef]
- Abed, A.H.; Hegazy, E.F.; Omar, S.A.; El-Baky, R.M.; El-Beih, A.A.; Al-Emam, A.; Menshawy, A.M.; Khalifa, E. Carvacrol Essential Oil: A Natural Antibiotic against Zoonotic Multidrug-Resistant Staphylococcus Species Isolated from Diseased Livestock and Humans. Antibiotics 2021, 10, 1328. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Moghaddam, M. Essential oils: Biological activity and therapeutic potential. In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 167–179. [Google Scholar]
- Suschke, U.; Sporer, F.; Schneele, J.; Geiss, H.K.; Reichling, J. Antibacterial and cytotoxic activity of Nepeta cataria L., N. cataria var. citriodora (Beck.) Balb. and Melissa officinalis L. essential oils. Nat. Prod. Commun. 2007, 2, 1934578X0700201218. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. "Essential oils’ chemical characterization and investigation of some biological activities: A critical review". Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Sully, E.K.; Geller, B.L. Antisense antimicrobial therapeutics. Curr. Opin. Microbiol. 2016, 33, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Eller, K.A.; Aunins, T.R.; Courtney, C.M.; Campos, J.K.; Otoupal, P.B.; Erickson, K.E.; Madinger, N.E.; Chatterjee, A. Facile accelerated specific therapeutic (FAST) platform develops antisense therapies to counter multidrug-resistant bacteria. Commun. Biol. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Streicher, L.M. Exploring the future of infectious disease treatment in a post-antibiotic era: A comparative review of alternative therapeutics. J. Glob. Antimicrob. Resist. 2021, 24, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Nakano-Narusawa, Y.; Hashimoto, N.; Yokohira, M.; Matsuda, Y. Development and Clinical Trials of Nucleic Acid Medicines for Pancreatic Cancer Treatment. Int. J. Mol. Sci. 2019, 20, 4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, X.; Gatti, P.; Papoian, T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov. Today 2017, 22, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Good, L.; Awasthi, S.K.; Dryselius, R.; Larsson, O.; Nielsen, P.E. Bactericidal antisense effects of peptide–PNA conjugates. Nat. Biotechnol. 2001, 19, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2014, 22, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Hegarty, J.P.; Stewart, D.B. Advances in therapeutic bacterial antisense biotechnology. Appl. Microbiol. Biotechnol. 2018, 102, 1055–1065. [Google Scholar] [CrossRef]
- Beha, M.J.; Ryu, J.S.; Kim, Y.S.; Chung, H.J. Delivery of antisense oligonucleotides using multi-layer coated gold nanoparticles to methicillin-resistant S. aureus for combinatorial treatment. Mater. Sci. Eng. C 2021, 126, 112167. [Google Scholar] [CrossRef]
- Subhan, A.; Attia, S.A.; Torchilin, V.P. Advances in siRNA delivery strategies for the treatment of MDR cancer. Life Sci. 2021, 274, 119337. [Google Scholar] [CrossRef]
- Valsamatzi-Panagiotou, A.; Popova, K.B.; Penchovsky, R. Methods for prevention and constraint of antimicrobial resistance: A review. Environ. Chem. Lett. 2021, 19, 2005–2012. [Google Scholar] [CrossRef]
- Fatima, H.; Goel, N.; Sinha, R.; Khare, S.K. Recent strategies for inhibiting multidrug-resistant and β-lactamase producing bacteria: A review. Colloids Surf. B Biointerfaces 2021, 205, 111901. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.V.; Aubry, C.; Boudaoud, N.; Gaubert, A.; Langlois, M.-H.; Marchivie, M.; Gaudin, K.; Arpin, C.; Barthélémy, P.; Kauss, T. Oligonucleotide solid nucleolipid nanoparticles against antibiotic resistance of ESBL-producing bacteria. Pharmaceutics 2022, 14, 299. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, D.; Alonso, D.; Altman, S. Combined effect of a peptide–morpholino oligonucleotide conjugate and a cell-penetrating peptide as an antibiotic. Proc. Natl. Acad. Sci. USA 2013, 110, 8686–8689. [Google Scholar] [CrossRef] [Green Version]
- Orr, R.M. Technology evaluation: Fomivirsen, Isis Pharmaceuticals Inc/CIBA vision. Curr. Opin. Mol. Ther. 2001, 3, 288–294. [Google Scholar]
- Ruppé, É.; Andremont, A. Le microbiote intestinal est l’avenir de la multirésistance bactérienne. J. Des Anti-Infect. 2013, 15, 166–177. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Lu, N.; Zhu, B. The abundance of antibiotic resistance genes in human guts has correlation to the consumption of antibiotics in animal. Gut Microbes 2014, 5, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Wellington, E.M.H.; Boxall, A.B.A.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Ther. Adv. Gastroenterol. 2015, 9, 229–239. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, W.; Shi, Y.; Fan, Z.; Ji, G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1755. [Google Scholar] [CrossRef]
- Monteiro, H.F.; Faciola, A.P. Ruminal acidosis, bacterial changes, and lipopolysaccharides. J. Anim. Sci. 2020, 98, skaa248. [Google Scholar] [CrossRef]
- Stojek, M.; Jabłońska, A.; Adrych, K. The Role of Fecal Microbiota Transplantation in the Treatment of Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 4055. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, S14459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodworth, M.H.; Hayden, M.K.; Young, V.B.; Kwon, J.H. The Role of Fecal Microbiota Transplantation in Reducing Intestinal Colonization With Antibiotic-Resistant Organisms: The Current Landscape and Future Directions. Open Forum Infect. Dis. 2019, 6, ofz288. [Google Scholar]
- Bar-Yoseph, H.; Carasso, S.; Shklar, S.; Korytny, A.; Dar, R.E.; Daoud, H.; Nassar, R.; Maharshak, N.; Hussein, K.; Geffen, Y.; et al. Oral Capsulized Fecal Microbiota Transplantation for Eradication of Carbapenemase-producing Enterobacteriaceae Colonization with a Metagenomic Perspective. Clin. Infect. Dis. 2021, 73, e166–e175. [Google Scholar] [CrossRef]
- Stripling, J.; Kumar, R.; Baddley, J.W.; Nellore, A.; Dixon, P.B.; Howard, D.; Ptacek, T.; Lefkowitz, E.; Tallaj, J.A.; Benjamin, W.H., Jr.; et al. Loss of Vancomycin-Resistant Enterococcus Fecal Dominance in an Organ Transplant Patient With Clostridium difficile Colitis After Fecal Microbiota Transplant. Open Forum Infect. Dis. 2015, 2, ofv078. [Google Scholar] [CrossRef] [Green Version]
- Liubakka, A.; Vaughn, B.P. Clostridium difficile Infection and Fecal Microbiota Transplant. AACN Adv. Crit. Care. 2016, 27, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Gong, J.; Zhu, W.; Guo, D.; Gu, L.; Li, N.; Li, J. Fecal microbiota transplantation restores dysbiosis in patients with methicillin resistant Staphylococcus aureus enterocolitis. BMC Infect. Dis. 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Manges, A.R.; Steiner, T.S.; Wright, A.J. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: A review. Infect. Dis. 2016, 48, 587–592. [Google Scholar] [CrossRef]
- Choi, H.H.; Cho, Y.-S. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin. Endosc. 2016, 49, 257–265. [Google Scholar] [CrossRef]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coquant, G.; Grill, J.-P.; Seksik, P. Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity. Front. Immunol. 2020, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Rai, R.; Venkatesh, K.V. Quorum sensing biosensors. In Quorum Sensing vs Quorum Quenching: A Battle with no End in Sight; Springer: Berlin/Heidelberg, Germany, 2015; pp. 173–183. [Google Scholar]
- Zhang, Q.; Li, S.; Hachicha, M.; Boukraa, M.; Soulère, L.; Efrit, M.L.; Queneau, Y. Heterocyclic Chemistry Applied to the Design of N-Acyl Homoserine Lactone Analogues as Bacterial Quorum Sensing Signals Mimics. Molecules 2021, 26, 5135. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, K.; Li, H.; Liu, K.; Li, J.; Chu, Y.; Prithiviraj, B.; Yue, B.; Zhang, X. Antibacterial and anti-virulence effects of furazolidone on Trueperella pyogenes and Pseudomonas aeruginosa. BMC Veter Res. 2022, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Genet. 2012, 10, 841–851. [Google Scholar] [CrossRef]
- Imperi, F.; Massai, F.; Pillai, C.R.; Longo, F.; Zennaro, E.; Rampioni, G.; Visca, P.; Leoni, L. New Life for an Old Drug: The Anthelmintic Drug Niclosamide Inhibits Pseudomonas aeruginosa Quorum Sensing. Antimicrob. Agents Chemother. 2013, 57, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Costabile, G.; d’Angelo, I.; Rampioni, G.; Bondì, R.; Pompili, B.; Ascenzioni, F.; Mitidieri, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Miro, A.; et al. Toward repositioning niclosamide for antivirulence therapy of Pseudomonas aeruginosa lung infections: Development of inhalable formulations through nanosuspension technology. Mol. Pharm. 2015, 12, 2604–2617. [Google Scholar] [CrossRef]
- Shaaban, M.; Elgaml, A.; Habib, E.-S.E. Biotechnological applications of quorum sensing inhibition as novel therapeutic strategies for multidrug resistant pathogens. Microb. Pathog. 2018, 127, 138–143. [Google Scholar] [CrossRef]
- Wei, G.; Lo, C.; Walsh, C.; Hiller, N.L.; Marculescu, R. In Silico Evaluation of the Impacts of Quorum Sensing Inhibition (QSI) on Strain Competition and Development of QSI Resistance. Sci. Rep. 2016, 6, 35136. [Google Scholar] [CrossRef] [Green Version]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losa, D.; Köhler, T.; Bacchetta, M.; Saab, J.B.; Frieden, M.; van Delden, C.; Chanson, M. Airway Epithelial Cell Integrity Protects from Cytotoxicity of Pseudomonas aeruginosa Quorum-Sensing Signals. Am. J. Respir. Cell. Mol. Biol. 2015, 53, 265–275. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, R.; Nuñez-López, L.; Jasso-Chávez, R.; Kwan, B.W.; Belmont, J.A.; Rangel-Vega, A.; Maeda, T.; Wood, T.K. Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating. ISME J. 2015, 9, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Criteria | Advantages |
|---|---|
| Specificity to bacteria | Highly specific to bacteria (by specific and targeted endolysin mode of action) [79,80] Have no effect on the human host microbiota [80,81] |
| Effect on the immune system | Circumvent the dysbiosis and subsequent overgrowth of pathogenic species often associated with antibiotic treatment [80] |
| Resistance | Phage mixture minimizes the likelihood that bacteria will acquire phage resistance and kill their bacterial host quickly [82] |
| Effectivity on bacterial biofilms | Eradicate biofilms due to the presence of EPS-degrading enzymes like endolysins and depolymerase in their tails [79,83] |
| Dose | Harmless entities showing no ill effects to eukaryotic cells even at high titers (targeted therapy) [84,85,86] Capacity to naturally control bacterial populations (self-dosing property) [86,87] |
| Genetics | Genetic exchange between phages rarely happens [80,88] |
| Environmental impact | Rapid elimination from the environment [79] |
| Priority | Pathogen Species | Antibiotic-Resistant Bacterium | References |
|---|---|---|---|
| Critical | Acinetobacter baumannii, | Carbapenem-resistant | [96,97,98,99,100,101,102] |
| Pseudomonas aeruginosa, | Carbapenem-resistant | [103,104,105] | |
| Enterobacteriaceae: | |||
| Escherichia coli | ESBL-producing Carbapenem-resistant, | [106,107,108] | |
| Klebsiella pneumoniae, | Multidrug-resistant Carbapenem-resistant | [109,110,111,112] [112,113] | |
| Enterobacter spp., | Carbapenem-resistant | [114,115] | |
| High | Enterococcus faecium, | Vancomycin-resistant | [116,117,118] |
| Staphylococcus aureus, | Methicillin-resistant, vancomycin-resistant, | [119,120,121,122] | |
| Helicobacter pylori, | Clarithromycin-resistant, | [123,124,125] | |
| Campylobacter spp., | Fluoroquinolone-resistant, | [126,127] | |
| Salmonellae | Fluoroquinolone-resistant, | [128,129,130] | |
| Neisseria gonorrhoeae, | Cephalosporin-resistant, Fluoroquinolone-resistant, | [131,132] | |
| Medium | Streptococcus pneumoniae, | penicillin-non-susceptible, | [133,134] |
| Haemophilus influenzae, | Ampicillin-resistant, | [135,136,137] | |
| Shigella spp., | Fluoroquinolone-resistant, | [138,139,140] |
| Limits of Application of Phage Therapy | References | |
|---|---|---|
| 1 | Dosage of bacteriophages, duration of treatment and routes of administration, is poorly controlled, involving the safety and effectiveness of treatment. | [68] |
| 2 | Inability to replicate the in vitro results in the actual situations. | [141] |
| 3 | Results of experimentation in small animal models does not consistently translate into clinical success, just as in vitro phage activity often fails to correlate with in vivo efficacy. | [136,142,143] |
| 4 | As same as antibiotics, bacteria also develop resistance to phages by specific defense mechanisms. | [80,144] |
| 5 | Phages display a short circulation time due to clearance by the spleen. | [145] |
| 6 | Bacterial remnants in the lysate produced from mass production of phages are difficult to be completely eliminated, leading to health risks. | [144,145] |
| 7 | Strain specificity of phages hinders mass production. | [76] |
| 8 | Possibility to contribute to the antimicrobial resistance development through transduction “phage conversion”. | [81] |
| 9 | Key mechanisms that may allow the prediction of in vivo pharmacokinetics and dynamics linked to therapeutic outcome have not yet been fully elucidated. | [68] |
| 10 | Physicochemical properties of phages in vivo are not fully understood. | [68] |
| 11 | Lack of regulatory approval for human use. | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaoui Mdarhri, H.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Guennouni Assimi, H.; Hamam, M.; Boussettine, R.; Filali-Ansari, N.; Lahlou, F.A.; Diawara, I.; et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics 2022, 11, 1826. https://doi.org/10.3390/antibiotics11121826
Alaoui Mdarhri H, Benmessaoud R, Yacoubi H, Seffar L, Guennouni Assimi H, Hamam M, Boussettine R, Filali-Ansari N, Lahlou FA, Diawara I, et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics. 2022; 11(12):1826. https://doi.org/10.3390/antibiotics11121826
Chicago/Turabian StyleAlaoui Mdarhri, Hiba, Rachid Benmessaoud, Houda Yacoubi, Lina Seffar, Houda Guennouni Assimi, Mouhsine Hamam, Rihabe Boussettine, Najoie Filali-Ansari, Fatima Azzahra Lahlou, Idrissa Diawara, and et al. 2022. "Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine" Antibiotics 11, no. 12: 1826. https://doi.org/10.3390/antibiotics11121826
APA StyleAlaoui Mdarhri, H., Benmessaoud, R., Yacoubi, H., Seffar, L., Guennouni Assimi, H., Hamam, M., Boussettine, R., Filali-Ansari, N., Lahlou, F. A., Diawara, I., Ennaji, M. M., & Kettani-Halabi, M. (2022). Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics, 11(12), 1826. https://doi.org/10.3390/antibiotics11121826






