β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity
Abstract
1. Introduction
2. Dose of β-Lactams in Critical Illness
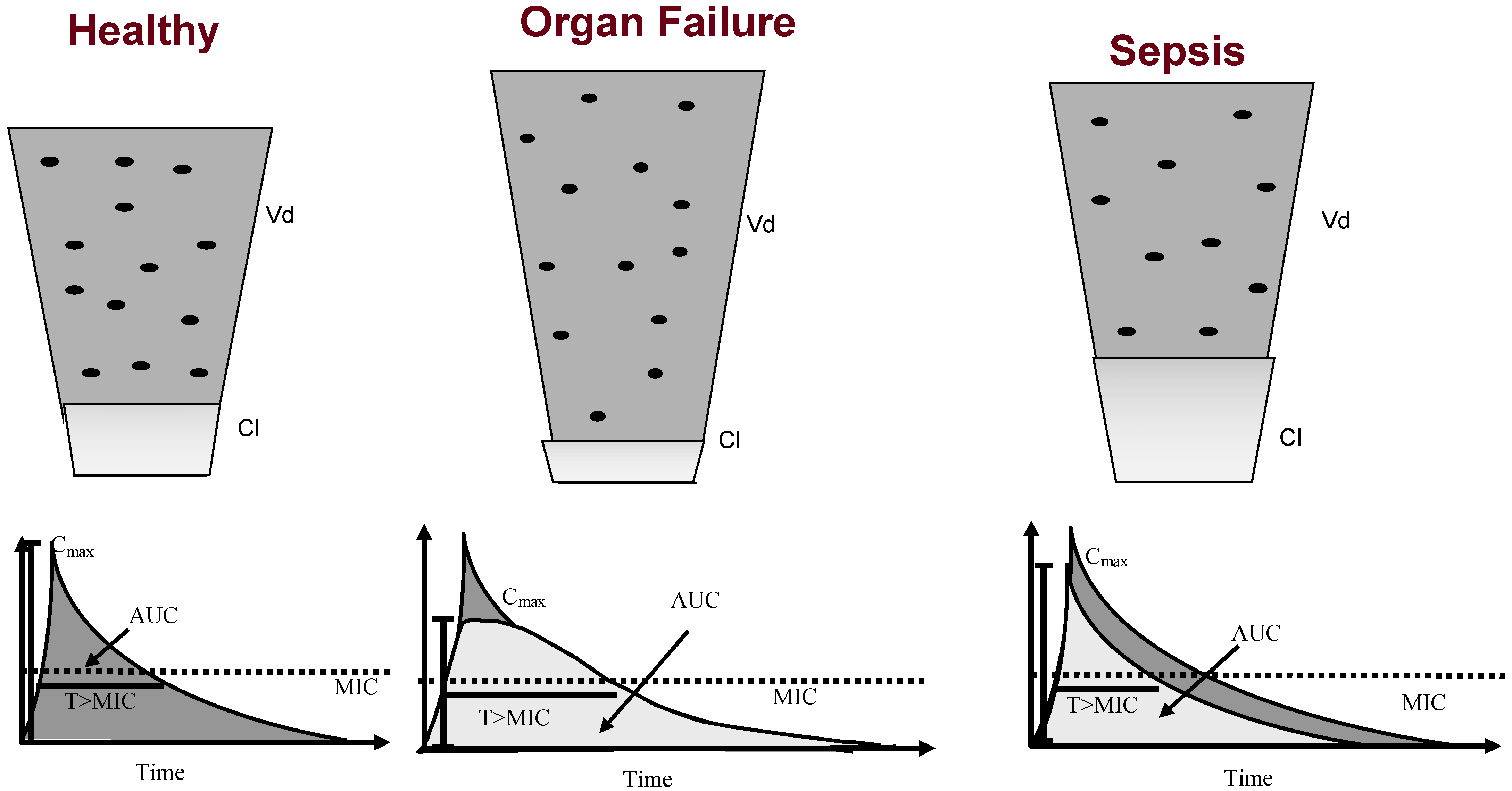
2.1. Pharmacokinetics of Sepsis
2.1.1. Changes in Antibiotic Pharmacokinetics in Critically Ill Patients
2.1.2. β-Lactam Antibiotic Mechanisms of Action
2.1.3. Dose Modulation
2.1.4. Therapeutic Drug Monitoring
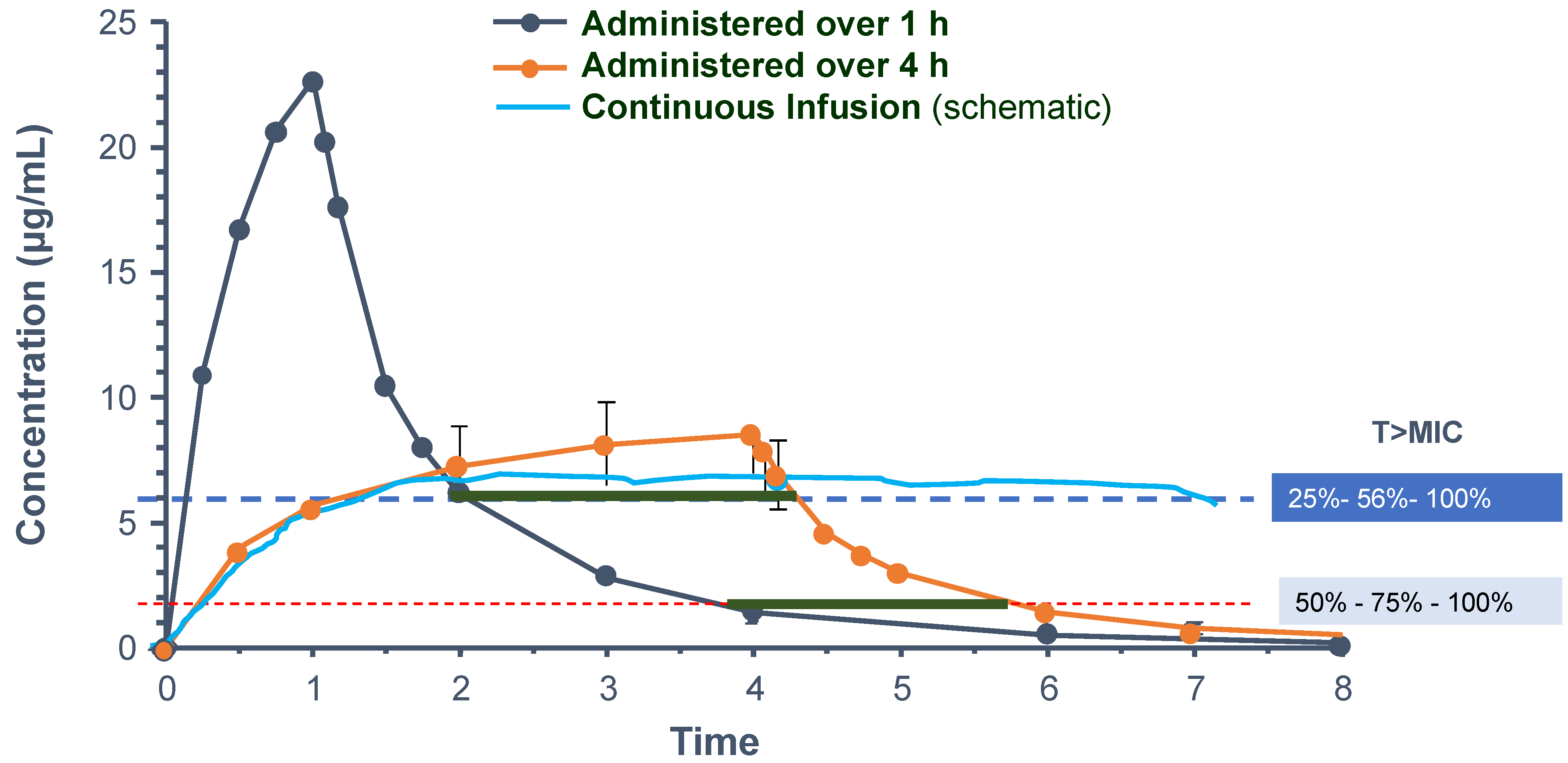
2.1.5. Continuous Infusion of β-Lactams
2.2. Antibiotic Pharmacokinetics in Organ Failure and Extracorporeal Support
2.2.1. Renal Failure
2.2.2. Renal Replacement Therapy
2.2.3. Hepatic Failure
2.2.4. Extracorporeal Membrane Oxygenation
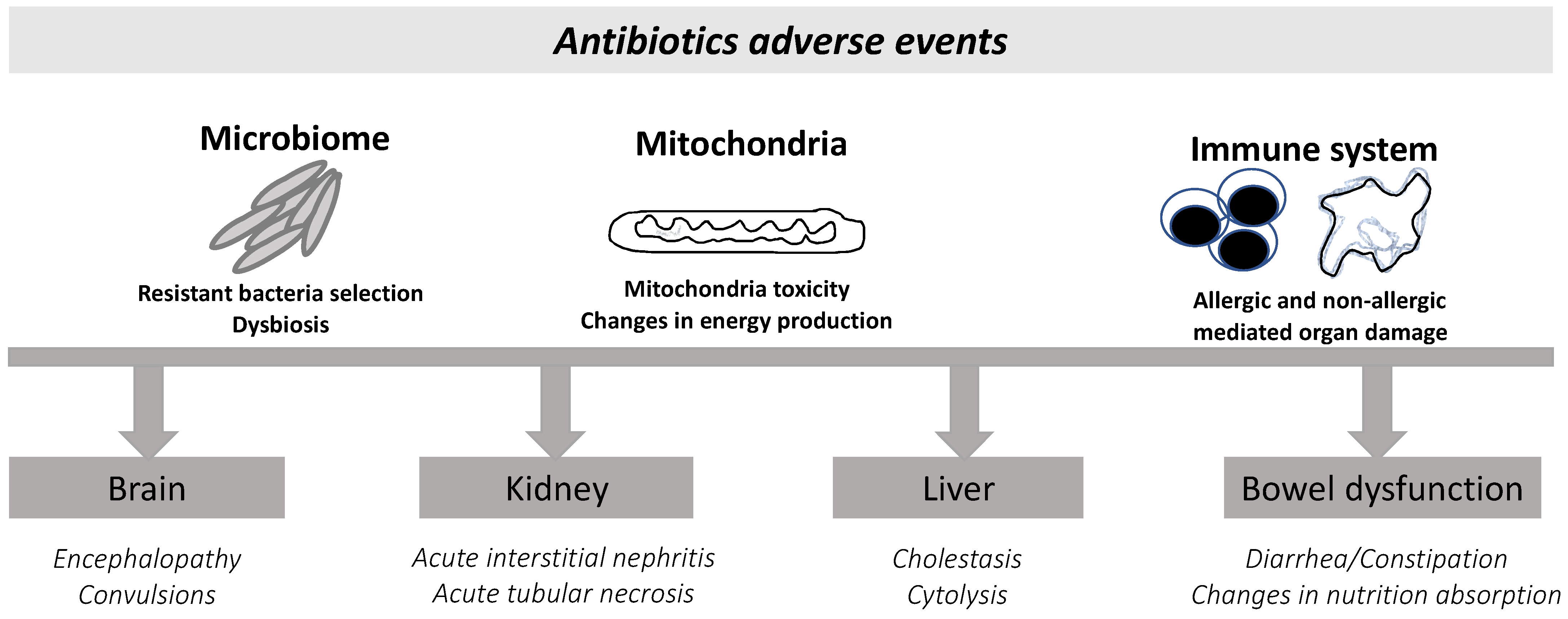
2.3. β-Lactam-Related Adverse Events
2.3.1. Kidney Toxicity
2.3.2. Neurological Toxicity of β-Lactams
2.3.3. The Impact of β-Lactams on Mitochondria
2.3.4. Microbiome Changes and Dysbiosis
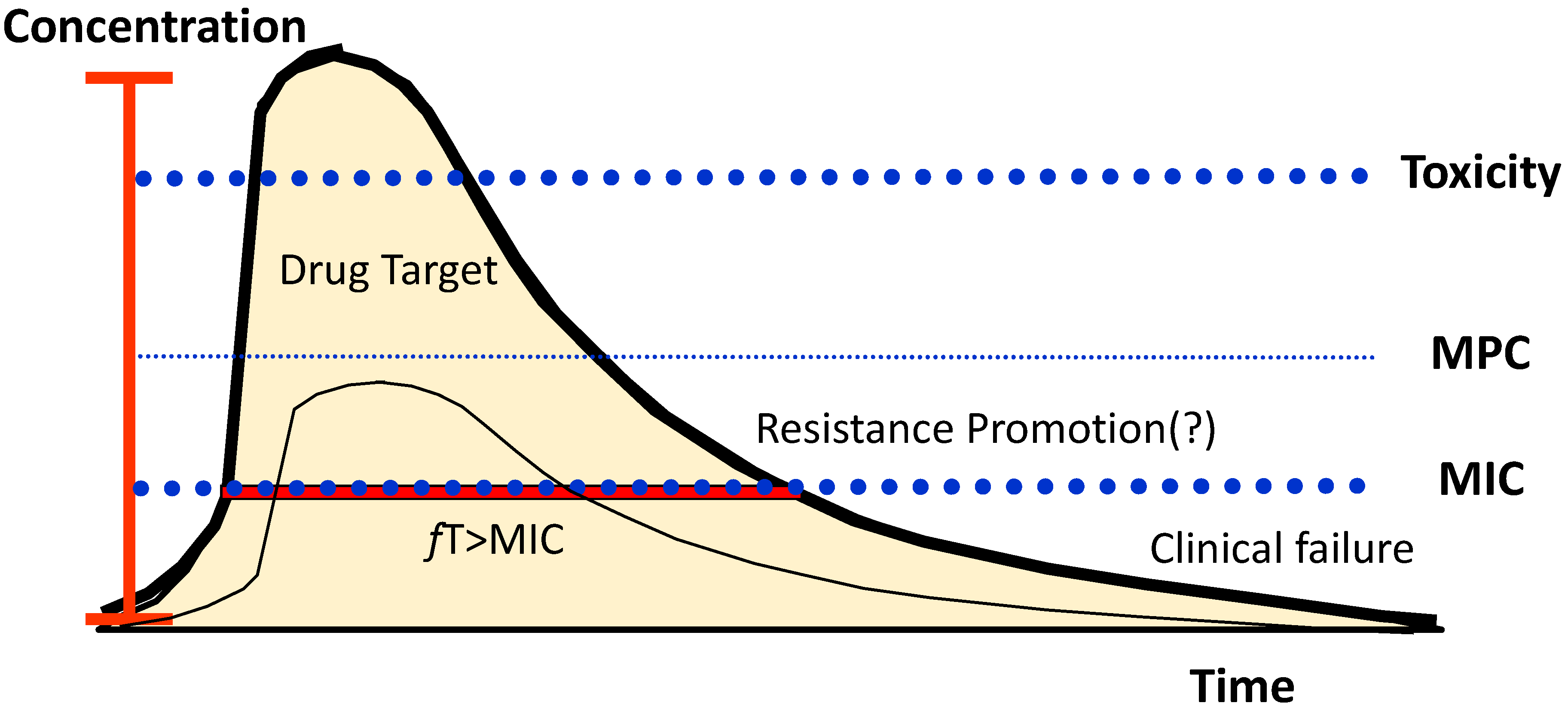
2.4. Resistance
Antibiotic Dosage to Suppress Resistance
2.5. Challenges, Biosafety, and Clinical Translation
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Power, B.M.; Forbes, A.M.; van Heerden, P.V.; Ilett, K.F. Pharmacokinetics of drugs used in critically ill adults. Clin. Pharmacokinet. 1998, 34, 25–56. [Google Scholar] [CrossRef] [PubMed]
- Turnidge, J.D. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 1998, 27, 10–22. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/9789241514880 (accessed on 11 December 2022).
- European Centre for Disease Prevention and Control. ESAC-NET AER 2020—Antimicrobial Consumption in the EU EEA; ECDC: Stockolm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/ESAC-Net%20AER-2020-Antimicrobial-consumption-in-the-EU-EEA.pdf (accessed on 11 December 2022).
- Kumar, A. An alternate pathophysiologic paradigm of sepsis and septic shock: Implications for optimizing antimicrobial therapy. Virulence 2014, 5, 80–97. [Google Scholar] [CrossRef]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of “bug and drug”. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; Kullar, R.; Gilchrist, M.; File, T.M. Antibiotics and adverse events: The role of antimicrobial stewardship programs in “doing no harm”. Curr. Opin. Infect. Dis. 2019, 32, 553–558. [Google Scholar] [CrossRef]
- Chant, C.; Leung, A.; Friedrich, J.O. Optimal dosing of antibiotics in critically ill patients by using continuous/extended infusions: A systematic review and meta-analysis. Crit. Care 2013, 17, R279. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; de Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P. DALI: Defining antibiotic levels in intensive care unit patients: Are current ß-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Lipman, J.; Rello, J. Antibiotic dosing in multiple organ dysfunction syndrome. Chest 2011, 139, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741.e9–741.e18. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.F.; Maganti, R.K. Neurotoxic effects associated with antibiotic use: Management considerations. Br. J. Clin. Pharmacol. 2011, 72, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. Mitochondria and antibiotics: For good or for evil? Biomolecules 2021, 11, 1050. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Li, X.; Cao, Y.; Chan, A.T.; Rimm, E.B.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Duration and Life-Stage of Antibiotic Use and Risks of All-Cause and Cause-Specific Mortality: Prospective Cohort Study. Circ. Res. 2020, 126, 364–373. [Google Scholar] [CrossRef]
- Drusano, G.L. Prevention of Resistance: A Goal for Dose Selection for Antimicrobial Agents. Clin. Infect. Dis. 2003, 36 (Suppl. S1), S42–S50. [Google Scholar] [CrossRef]
- Sinnollareddy, M.G.; Roberts, M.S.; Lipman, J.; Roberts, J.A. β-Lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: A structured review. Clin. Exp. Pharmacol. Physiol. 2012, 39, 489–496. [Google Scholar] [CrossRef]
- Póvoa, P.; Moniz, P.; Pereira, J.G.; Coelho, L. Optimizing antimicrobial drug dosing in critically ill patients. Microorganisms 2021, 9, 1401. [Google Scholar] [CrossRef]
- Cotta, M.O.; Roberts, J.A.; Lipman, J. Antibiotic dose optimization in critically ill patients. Med. Intensiva 2015, 39, 563–572. [Google Scholar] [CrossRef]
- Roberts, J.A.; Roberts, M.S.; Semark, A.; Udy, A.A.; Kirkpatrick, C.M.; Paterson, D.L.; Roberts, M.J.; Kruger, P.; Lipman, J. Antibiotic dosing in the “at risk” critically ill patient: Linking pathophysiology with pharmacokinetics/pharmacodynamics in sepsis and trauma patients. BMC Anesthesiol. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Pea, F. Plasma pharmacokinetics of antimicrobial agents in critically ill patients. Curr. Clin. Pharmacol. 2013, 8, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pereira, J.; Póvoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of β-lactams. Crit. Care 2011, 15, R206. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of β-lactam antibiotics in critically Ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef]
- Kovacevic, T.; Miljkovic, B.; Mikov, M.; Stojisavljevic Satara, S.; Dragic, S.; Momcicevic, D.; Kovacevic, P. The Effect of Hypoalbuminemia on the Therapeutic Concentration and Dosage of Vancomycin in Critically Ill Septic Patients in Low-Resource Countries. Dose-Response 2019, 17. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Sime, F.B.; Lipman, J.; Roberts, J.A. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect. Dis. 2014, 14, 288. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Póvoa, P. Impact of Pharmacokinetics of Antibiotics in ICU clinical practice. ICU Manag. Pract. 2013, 11, 30–33. [Google Scholar]
- Casu, G.S.; Hites, M.; Jacobs, F.; Cotton, F.; Wolff, F.; Beumier, M.; de Backer, D.; Vincent, J.-L.; Taccone, F.S. Can changes in renal function predict variations in β-lactam concentrations in septic patients? Int. J. Antimicrob. Agents 2013, 42, 422–428. [Google Scholar] [CrossRef]
- Udy, A.A.; Baptista, J.P.; Lim, N.L.; Joynt, G.M.; Jarrett, P.; Wockner, L.; Boots, R.J.; Lipman, J. Augmented renal clearance in the ICU: Results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit. Care Med. 2014, 42, 520–527. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D. β-Lactamases and β-lactamase inhibitors. Int. J. Antimicrob. Agents 1999, 12 (Suppl. S1), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Anon, J.; Jacobs, M.R.; Craig, W.A. Application of pharmacokinetics and pharmacodynamics to antimicrobial therapy of respiratory tract infections. Clin. Lab. Med. 2004, 24, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A.; Middleton, W.S. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Srimani, J.K.; Huang, S.; Lopatkin, A.J.; You, L. Drug detoxification dynamics explain the postantibiotic effect. Mol. Syst. Biol. 2017, 13, 948. [Google Scholar] [CrossRef]
- Goncalves-Pereira, J.; Paiva, J.-A. Dose modulation: A new concept of antibiotic therapy in the critically ill patient? J. Crit. Care 2013, 28, 341–346. [Google Scholar] [CrossRef]
- Montravers, P.; Tubach, F.; Lescot, T.; Veber, B.; Esposito-Farèse, M.; Seguin, P.; Paugam, C.; Lepape, A.; Meistelman, C.; Cousson, J. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: The DURAPOP randomised clinical trial. Intensive Care Med. 2018, 44, 300–310. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.-Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P. Comparison of 8 versus 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef]
- LaPlante, K.L.; Rybak, M.J. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 2004, 48, 4665–4672. [Google Scholar] [CrossRef]
- Nicasio, A.M.; Eagye, K.J.; Nicolau, D.P.; Shore, E.; Palter, M.; Pepe, J.; Kuti, J.L. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J. Crit. Care 2010, 25, 69–77. [Google Scholar] [CrossRef]
- Sjövall, F.; Alobaid, A.S.; Wallis, S.C.; Perner, A.; Lipman, J.; Roberts, J.A. Maximally effective dosing regimens of meropenem in patients with septic shock. J. Antimicrob. Chemother. 2018, 73, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Joynt, G.M.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S. The Effect of Renal Replacement Therapy and Antibiotic Dose on Antibiotic Concentrations in Critically Ill Patients: Data from the Multinational Sampling Antibiotics in Renal Replacement Therapy Study. Clin. Infect. Dis. 2021, 72, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Stove, V.; Wallis, S.C.; de Waele, J.J.; Verstraete, A.G.; Lipman, J.; Roberts, J.A. Assays for therapeutic drug monitoring of β-lactam antibiotics: A structured review. Int. J. Antimicrob. Agents 2015, 46, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.E.; Andrews, L.J.; Abbey, T.C.; Dahlquist, A.E.; Wenzler, E. The importance of pharmacokinetics and pharmacodynamics in antimicrobial drug development and their influence on the success of agents developed to combat resistant gram negative pathogens: A review. Front. Pharmacol. 2022, 13, 888079. [Google Scholar] [CrossRef]
- Seyler, L.; Cotton, F.; Taccone, F.S.; de Backer, D.; Macours, P.; Vincent, J.-L.; Jacobs, F. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care 2011, 15, R137. [Google Scholar] [CrossRef]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of bolus dosing versus continuous infusion of piperacillin and tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; de Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef]
- Kollef, M.H. Antibiotics for the critically ill: More than just selecting appropriate initial therapy. Crit. Care 2013, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Lipman, J.; Roberts, J. Does Appropriate Antibiotic Therapy Mean Only Adequate Spectrum and Timing? Crit. Care Med. 2015, 43, 1773–1774. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, I.; Vaccaro, N.; Turner, K.; Solanki, B.; Natarajan, J.; Redman, R. Pharmacokinetics, safety, and tolerability of doripenem after 0.5-, 1-, and 4-hour infusions in healthy volunteers. J. Clin. Pharmacol. 2009, 49, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Lomaestro, B.; Drusano, G.L. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 2007, 44, 357–363. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Oliveira, B.; Janeiro, S.; Estilita, J.; Monteiro, C.; Salgueiro, A.; Vieira, A.; Gouveia, J.; Paulino, C.; Bento, L. Continuous infusion of piperacillin/tazobactam in septic critically ill patients—A multicenter propensity matched analysis. PLoS ONE 2012, 7, e49845. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L. A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L. Continuous Infusion of Beta-Lactam Antibiotics in Severe Sepsis: A Multicenter Double-Blind, Randomized Controlled Trial. Clin. Infect. Dis. 2013, 56, 236–244. [Google Scholar] [CrossRef]
- Shiu, J.; Wang, E.; Tejani, A.M.; Wasdell, M. Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Database Syst. Rev. 2013, 2013, CD008481. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Webb, S.; Paterson, D.; Ho, K.M.; Lipman, J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit. Care Med. 2009, 37, 2071–2078. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus intermittent β-lactam infusion in severe sepsis: A meta-analysis of individual patient data from randomized trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lipman, J.; Brett, S.; de Waele, J.; Cotta, M.O.; Davis, J.S.; Finfer, S.; Glass, P.; Knowles, S.; McGuinness, S.; Myburgh, J. A protocol for a phase 3 multicentre randomised controlled trial of continuous versus intermittent β-lactam antibiotic infusion in critically ill patients with sepsis: BLING III. Crit. Care Resusc. 2019, 21, 63–68. [Google Scholar]
- Scheetz, M.H.; Lodise, T.P.; Downes, K.J.; Drusano, G.; Neely, M. The case for precision dosing: Medical conservatism does not justify inaction. J. Antimicrob. Chemother. 2021, 76, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, T.; Seligman, R.; Diaz, E.; Rodriguez, A.; Teixeira, P.J.Z.; Rello, J. C-reactive protein correlates with bacterial load and appropriate antibiotic therapy in suspected ventilator-associated pneumonia. Crit. Care Med. 2008, 36, 166–171. [Google Scholar] [CrossRef]
- Huang, D.T.; Yealy, D.M.; Filbin, M.R.; Brown, A.M.; Chang, C.-C.H.; Doi, Y.; Donnino, M.W.; Fine, J.; Fine, M.J.; Fischer, M.A. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N. Engl. J. Med. 2018, 379, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.; Chang, S.; Wang, W. Using the rate of bacterial clearance determined by real-time polymerase chain reaction as a timely surrogate marker to evaluate the appropriateness of antibiotic usage in critical patients with Acinetobacter baumannii bacteremia. Crit. Care Med. 2012, 40, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pereira, J.; Oliveira, B. Antibiotics and extracorporeal circulation: One size does not fit all. Crit. Care 2014, 18, 695. [Google Scholar] [CrossRef][Green Version]
- Choi, G.; Gomersall, C.D.; Tian, Q.; Joynt, G.M.; Li, A.M.M.Y.; Lipman, J. Principles of antibacterial dosing in continuous renal replacement therapy. Blood Purif. 2010, 30, 195–212. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Mueller, B.A. Antibiotic Dosing in Patients with Acute Kidney Injury: “Enough but Not Too Much”. J. Intensive Care Med. 2014, 31, 164–176. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M.; Schortgen, F. Ten shortcomings of the current definition of AKI. Intensive Care Med. 2017, 43, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Beumier, M.; Casu, G.S.; Hites, M.; Seyler, L.; Cotton, F.; Vincent, J.-L.; Jacobs, F.; Taccone, F.S. Beta-Lactam Antibiotic Concentrations During Continuous Renal Replacement Therapy. Crit. Care 2014, 18, R105. [Google Scholar] [CrossRef]
- Sime, F.B.; Roberts, M.S.; Peake, S.L.; Lipman, J.; Roberts, J.A. Does Beta-lactam Pharmacokinetic Variability in Critically Ill Patients Justify Therapeutic Drug Monitoring? A Systematic Review. Ann. Intensive Care 2012, 2, 35. [Google Scholar] [CrossRef]
- Morgan, D.J.; McLean, A.J. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease. An update. Clin. Pharmacokinet. 1995, 29, 370–391. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Wallis, S.C.; Rello, J.; Lipman, J. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: Special emphasis on unbound pharmacokinetics. J. Antimicrob. Chemother. 2010, 65, 1771–1778. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Fisquet, S.; Barnett, A.G.; Mullany, D.V. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, R194. [Google Scholar] [CrossRef]
- Shekar, K.; Fraser, J.F.; Taccone, F.S.; Welch, S.; Wallis, S.C.; Mullany, D.V. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: A matched cohort study. Crit. Care 2014, 18, 565. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Roberts, J.A. Antibiotic dosing during extracorporeal membrane oxygenation: Does the system matter? Curr. Opin. Anaesthesiol. 2020, 33, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, J.; Heath, T.; Watt, K. Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin. Ther. 2016, 38, 1976–1994. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, M.; Dreesen, E.; Annaert, P.; Nicolai, J.; Debaveye, Y.; Wauters, J.; Spriet, I. Meropenem pharmacokinetics and target attainment in critically ill patients are not affected by extracorporeal membrane oxygenation: A matched cohort analysis. Microorganisms 2021, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Arulkumaran, N.; Routledge, M.; Schlebusch, S.; Lipman, J.; Morris, A.C. Antimicrobial-associated harm in critical care: A narrative review. Intensive Care Med. 2020, 46, 225–235. [Google Scholar] [CrossRef]
- Lagacé-Wiens, P.; Rubinstein, E. Adverse reactions to β-lactam antimicrobials. Expert Opin. Drug Saf. 2012, 11, 381–399. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Darby, R.R.; Raibagkar, P.; Castro, L.N.G.; Berkowitz, A.L. Antibiotic-associated encephalopathy. Neurology 2016, 86, 963–971. [Google Scholar] [CrossRef]
- Jeimy, S.; Ben-Shoshan, M.; Abrams, E.M.; Ellis, A.K.; Connors, L.; Wong, T. Practical guide for evaluation and management of beta-lactam allergy: Position statement from the Canadian Society of Allergy and Clinical Immunol. Allergy Asthma Clin. Immunol. 2020, 16, 95. [Google Scholar] [CrossRef]
- Muriithi, A.K.; Nasr, S.H.; Leung, N. Utility of Urine Eosinophils in the Diagnosis of Acute Interstitial Nephritis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alamo, B.; Cases-Corona, C.; Fernandez-Juarez, G. Facing the Challenge of Drug-Induced Acute Interstitial Nephritis. Nephron 2022. on-line ahead of print:1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Fogazzi, G.B.; Garigali, G.; Messa, P. Acute interstitial nephritis after amoxycillin with hematuria, red blood cell casts and hematuria induced acute tubular injury. Clin. Nephrol. 2013, 80, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Cotner, S.E.; Rutter, W.C.; Burgess, D.R.; Wallace, K.L.; Martin, C.A.; Burgess, D.S. Influence of β-Lactam Infusion Strategy on Acute Kidney Injury. Antimicrob. Agents Chemother. 2017, 61, e00871-17. [Google Scholar] [CrossRef]
- Burgess, L.D.; Drew, R.H. Comparison of the Incidence of Vancomycin-Induced Nephrotoxicity in Hospitalized Patients with and without Concomitant Piperacillin-Tazobactam. Pharmacotherapy 2014, 34, 670–676. [Google Scholar] [CrossRef]
- Jiang, S.; Li, T.; Zhou, X.; Qin, W.; Wang, Z.; Liao, Y. Antibiotic drug piperacillin induces neuron cell death through mitochondrial dysfunction and oxidative damage. Can. J. Physiol. Pharmacol. 2018, 96, 562–568. [Google Scholar] [CrossRef]
- Barnhill, A.E.; Brewer, M.T.; Carlson, S.A. Adverse Effects of Antimicrobials via Predictable or Idiosyncratic Inhibition of Host Mitochondrial Components. Antimicrob. Agents Chemother. 2012, 56, 4046–4051. [Google Scholar] [CrossRef]
- Martínez-García, J.J.; Martínez-Banaclocha, H.; Angosto-Bazarra, D.; de Torre-Minguela, C.; Baroja-Mazo, A.; Alarcón-Vila, C.; Martínez-Alarcón, L.; Amores-Iniesta, J.; Martín-Sánchez, F.; Ercole, G.A. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat. Commun. 2019, 10, 2711. [Google Scholar] [CrossRef]
- Tune, B.M.; Hsu, C.Y. The renal mitochondrial toxicity of beta-lactam antibiotics: In Vitro effects of cephaloglycin and imipenem. J. Am. Soc. Nephrol. 1990, 1, 815–821. [Google Scholar] [CrossRef]
- Oyebode, O.T.; Adebiyi, O.R.; Olorunsogo, O.O. Toxicity of some broad-spectrum antibacterials in normal rat liver: The role of mitochondrial membrane permeability transition pore. Toxicol. Mech. Methods 2019, 29, 128–137. [Google Scholar] [CrossRef]
- Paech, F.; Messner, S.; Spickermann, J.; Wind, M.; Schmitt-Hoffmann, A.-H.; Witschi, A.T.; Howell, B.A.; Church, R.J.; Woodhead, J.; Engelhardt, M. Mechanisms of hepatotoxicity associated with the monocyclic β-lactam antibiotic BAL30072. Arch. Toxicol. 2017, 91, 3647–3662. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Huang, Y.; Yang, M.; Lu, Z.; Li, Y.; Zhan, F.; Lin, L.; Qin, Z. Antibiotic-induced alternations in gut microflora are associated with the suppression of immune-related pathways in grass carp (Ctenopharyngodon idellus). Front. Immunol. 2022, 13, 970125. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Pujolassos, M.; Burrello, C.; Giuffrè, M.R.; Lattanzi, G.; Caprioli, F.; Troisi, J.; Facciotti, F. Antibiotic-associated dysbiosis affects the ability of the gut microbiota to control intestinal inflammation upon fecal microbiota transplantation in experimental colitis models. Microbiome 2021, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Escudeiro, P.; Pothier, J.; Dionisio, F.; Nogueira, T. Antibiotic Resistance Gene Diversity and Virulence Gene Diversity Are Correlated in Human Gut and Environmental Microbiomes. mSphere 2019, 4, e00135-19. [Google Scholar] [CrossRef]
- Siwicka-Gieroba, D.; Barud, M.; Dabrowski, W. Why is it worth remembering the lung microbiome in ICU patients? Anaesthesiol. Intensive Ther. 2021, 53, 466–474. [Google Scholar] [CrossRef]
- Zakharkina, T.; Martin-Loeches, I.; Matamoros, S.; Povoa, P.; Torres, A.; Kastelijn, J.B.; Hofstra, J.J.; de Wever, B.; de Jong, M.; Schultz, M.J. The dynamics of the pulmonary microbiome during mechanical ventilation in the intensive care unit and the association with occurrence of pneumonia. Thorax 2017, 72, 803–810. [Google Scholar] [CrossRef]
- Kwon, J.; Kong, Y.; Wade, M.; Williams, D.J.; Creech, C.B.; Evans, S.; Walter, E.B.; Martin, J.M.; Gerber, J.S.; Newland, J.G. Gastrointestinal Microbiome Disruption and Antibiotic-Associated Diarrhea in Children Receiving Antibiotic Therapy for Community-Acquired Pneumonia. J. Infect. Dis. 2022, 226, 1109–1119. [Google Scholar] [CrossRef]
- Wardill, H.R.; van der Aa, S.A.R.; da Silva Ferreira, A.R.; Havinga, R.; Tissing, W.J.E.; Harmsen, H.J.M. Antibiotic-induced disruption of the microbiome exacerbates chemotherapy-induced diarrhoea and can be mitigated with autologous faecal microbiota transplantation. Eur. J. Cancer 2021, 153, 27–39. [Google Scholar] [CrossRef]
- Riou, M.M.; Carbonnelle, S.; Avrain, L.L.; Mesaros, N.; Pirnay, J.-P.P.; Bilocq, F.; de Vos, D.; Simon, A.; Piérard, D.; Jacobs, F.F. In vivo development of antimicrobial resistance in Pseudomonas aeruginosa strains isolated from the lower respiratory tract of Intensive Care Unit patients with nosocomial pneumonia and receiving antipseudomonal therapy. Int. J. Antimicrob. Agents 2010, 36, 513–522. [Google Scholar] [CrossRef]
- Morawska, L.P.; Hernandez-Valdes, J.A.; Kuipers, O.P. Diversity of bet-hedging strategies in microbial communities—Recent cases and insights. WIREs Mech. Dis. 2022, 14, e1544. [Google Scholar] [CrossRef] [PubMed]
- Rajer, F.; Sandegren, L. The Role of Antibiotic Resistance Genes in the Fitness Cost of Multiresistance Plasmids. mBio 2022, 13, e03552-21. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; van den Brink, M.R.M.; Kamboj, M. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Maisnier-Patin, S.; Andersson, D.I. Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res. Microbiol. 2004, 155, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Soothill, G.; Hu, Y.; Coates, A. Can we prevent antimicrobial resistance by using antimicrobials better? Pathogens 2013, 2, 422–435. [Google Scholar] [CrossRef]
- Cunha, B.A. Effective antibiotic-resistance control strategies. Lancet 2001, 357, 1307–1308. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Dhaese, S.; Heffernan, A.; Liu, D.; Abdul-Aziz, M.H.; Stove, V.; Tam, V.H.; Lipman, J.; Roberts, J.A.; de Waele, J.J. Prolonged Versus Intermittent Infusion of β-Lactam Antibiotics: A Systematic Review and Meta-Regression of Bacterial Killing in Preclinical Infection Models. Clin. Pharmacokinet. 2020, 59, 1237–1250. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Restricting the Selection of Antibiotic-Resistant Mutant Bacteria: Measurement and Potential Use of the Mutant Selection Window. J. Infect. Dis. 2002, 185, 561–565. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. A unified anti-mutant dosing strategy. J. Antimicrob. Chemother. 2008, 62, 434–436. [Google Scholar] [CrossRef]
- Firsov, A.A.; Smirnova, M.V.; Lubenko, I.Y.; Vostrov, S.N.; Portnoy, Y.A.; Zinner, S.H. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to daptomycin and vancomycin in an In Vitro dynamic model. J. Antimicrob. Chemother. 2006, 58, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Schilling, A.N.; Neshat, S.; Poole, K.; Melnick, D.A.; Coyle, E.A. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 4920–4927. [Google Scholar] [CrossRef] [PubMed]
- Day, T.; Read, A.F. Does High-Dose Antimicrobial Chemotherapy Prevent the Evolution of Resistance? PLoS Comput. Biol. 2016, 12, e1004689. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.K.; Forrest, A.; Bhavnani, S.M.; Hyatt, J.M.; Cheng, A.; Ballow, C.H.; Schentag, J.J. Pharmacodynamic Evaluation of Factors Associated with the Development of Bacterial Resistance in Acutely Ill Patients during Therapy. Antimicrob. Agents Chemother. 1998, 42, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M. Multi-drug resistant gram-negative bacterial pneumonia: Etiology, risk factors, and drug resistance patterns. Pneumonia 2022, 14, 4. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M. The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections. Expert Opin. Pharmacother. 2019, 20, 2169–2184. [Google Scholar] [CrossRef]
- Monogue, M.L.; Nicolau, D.P. Pharmacokinetics-pharmacodynamics of β-lactamase inhibitors: Are we missing the target? Expert Rev. Anti-Infect. Ther. 2019, 17, 571–582. [Google Scholar] [CrossRef]
- Barreto, E.F.; Webb, A.J.; Pais, G.M.; Rule, A.D.; Jannetto, P.J.; Scheetz, M.H. Setting the Beta-Lactam Therapeutic Range for Critically Ill Patients: Is There a Floor or Even a Ceiling? Crit. Care Explor. 2021, 3, e0446. [Google Scholar] [CrossRef]

| Antibiotic | Loading Dose * | Maintenance Dose | |||
|---|---|---|---|---|---|
| Acute Kidney Injury | Continuous RRT | Hepatic Failure | ECMO | ||
| Flucloxacillin | 2 g q4 h | 1 g q4 h | 1 g q4 h | 2 g q4 h | ND |
| Ceftriaxone | 1–2 g q12 h | 1 g q12 h | 1–2 g q12 h | 1 g q12 h | 1–2 g q12 h |
| Ceftazidime | 2 g q8 h | 1 g q8 h | 1 g q8 h | 2 g q8 h | ND |
| Cefepime | 1–2 g q8–12 h | 500 mg–1 g q12 h | 1–2 g q12 h | 1–2 g q8–12 h | ND |
| Piperacillin/tazobactam | 4.5 g q4–6 h | 4.5 q8 h or 2.25 g q6 h | 4.5 g q8 h | 4.5 g q6 h | 4.5 q6 h |
| Ertapenem | 1 g q12 h | 500 mg q12 h | 500 mg q8–12 h | 1 g q12 h | ND |
| Imipenem | 1 g q6 h | 500 mg q6–8 h | 500 mg–1 g q12 h | 500 mg q6–8 h | 750 mg–1 g q6 h |
| Meropenem | 2 g q8 h | 500 mg q12 h | 500 mg q8 h | 1 g q8 h | 1–2 g q8 h |
| Ceftazidime/avibactam | 2.5 g q8 h | 1.25 g q8 h | 1.25 g q8 h | 2.5 g q8 h | ND |
| Side Effect | β-Lactam More Frequently Implicated | Dose-Related | Risk Factors | Note |
|---|---|---|---|---|
| Encephalopathy | Cefepime; Piperacillin/tazobactam | Yes | High risk for neurotoxicity if patient has acute kidney injury, previous neurological disease, hepatic encephalopathy, or advanced age | Higher risk with cefepime—up to 48% of exposed patients |
| Convulsions | Cefazolin; Cefepime; Imipenem | Yes | Compared to imipenem, lower risk for ertapenem, meropenem, and doripenem | |
| Neuropsychiatric changes | Piperacillin/tazobactam | Yes | ||
| Acute kidney injury | Piperacillin/tazobactam | Yes | Particularly in association with other nephrotoxins | Might relate to direct mitochondrial impairment |
| Acute interstitial nephritis | Piperacillin/tazobactam; Cephalosporins | No | Older age, increased incidence but reason is unknown | Non-IgE-mediated hypersensitivity reaction Can occur days to weeks after AB exposure Corticosteroids are recommended if resolution does not occur after stopping the culprit. |
| Acute kidney injury secondary to hemolytic anemia | Piperacillin/tazobactam; Ceftriaxone (However, all β-lactams have been associated with hemolytic anemia.) | Both | Previous hemolytic anemia associated with β-lactams | Very rare event (1 per million per year of incidence). High mortality |
| Renal obstruction due to crystallization | Amoxicillin; Ceftriaxone | Yes | Dehydration, acidic urine pH | Precipitation in renal tubules, lithiasis, and consequent obstruction |
| Hepatic lesion | AAC; Pip/taz | Yes | Other | Might be related to direct mitochondrial impairment |
| Dysbiosis | All | Yes | Critical illness | One of the most known consequences: clostridium difficile infection related to diarrhea associated with AB |
| Allergic reaction | All | Both | Unknown | Overestimation in the clinic led to a higher use of broad-spectrum antibiotics or second-line antibiotics (adapted from [92]). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.G.; Fernandes, J.; Duarte, A.R.; Fernandes, S.M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics 2022, 11, 1839. https://doi.org/10.3390/antibiotics11121839
Pereira JG, Fernandes J, Duarte AR, Fernandes SM. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics. 2022; 11(12):1839. https://doi.org/10.3390/antibiotics11121839
Chicago/Turabian StylePereira, João Gonçalves, Joana Fernandes, Ana Rita Duarte, and Susana Mendes Fernandes. 2022. "β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity" Antibiotics 11, no. 12: 1839. https://doi.org/10.3390/antibiotics11121839
APA StylePereira, J. G., Fernandes, J., Duarte, A. R., & Fernandes, S. M. (2022). β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics, 11(12), 1839. https://doi.org/10.3390/antibiotics11121839





