Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics
Abstract
:1. Introduction
2. Results
2.1. Growth Performance
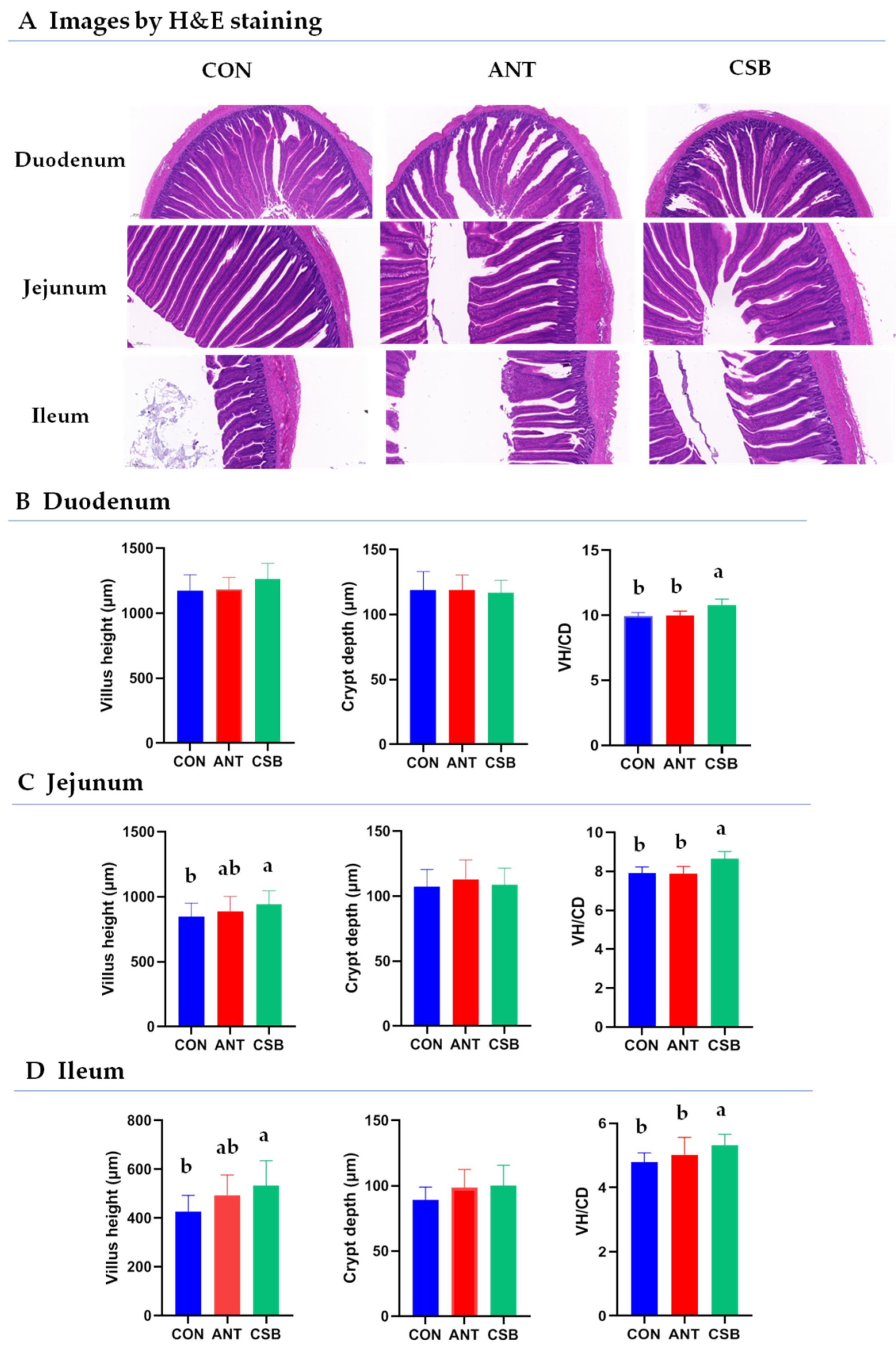
2.2. Intestinal Histomorphologic Indices
2.3. Activity of Small Intestine Digestive Enzyme
2.4. Antioxidant Capacity of Intestinal Mucosa
2.5. SCFA Concentration in Intestine Contents
2.6. Microbiota Community Structure of Ileum
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Sample Collection
4.3. Growth Performance Measurement
4.4. Intestinal Histomorphology Analyses
4.5. Digestive Enzyme Activity Examination
4.6. Antioxidant Indices Examination
4.7. Short-Chain Fatty Acids (SCFA) Concentration Determination
4.8. Bacterial 16S rRNA Gene Analyses
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yalcin, S.; Özkan, S.; Türkmut, L.; Siegel, P.B. Responses to Heat Stress in Commercial and Local Broiler Stocks. 1. Performance traits. Br. Poult. Sci. 2001, 42, 149–152. [Google Scholar] [CrossRef]
- Abdelqader, A.M.; Abuajamieh, M.; Hammad, H.M.; Al-Fataftah, A. Effects of Dietary Butyrate Supplementation on Intestinal Integrity of Heat-Stressed Cockerels. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1115–1121. [Google Scholar] [CrossRef]
- Song, B.; Li, H.; Wu, Y.; Zhen, W.; Wang, Z.; Xia, Z.; Guo, Y. Effect of Microencapsulated Sodium Butyrate Dietary Supplementation on Growth Performance and Intestinal Barrier Function of Broiler Chickens Infected with Necrotic Enteritis. Anim. Feed Sci. Technol. 2017, 232, 6–15. [Google Scholar] [CrossRef]
- Sapsuha, Y.; Suprijatna, E.; Kismiati, S.; Sugiharto, S. Combination of Probiotic and Phythobiotic as an Alternative for Antibiotic Growth Promoter for Broilers Chickens—A Review. Livest. Res. Rural. Dev. 2021, 33, 49. [Google Scholar]
- Ahsan, U.; Cengiz, Ö.; Raza, I.; Kuter, E.; Chacher, M.F.A.; Iqbal, Z.; Umar, S.; Çakir, S. Sodium Butyrate in Chicken Nutrition: The Dynamics of Performance, Gut Microbiota, Gut Morphology, and Immunity. Worlds Poult. Sci. J. 2016, 72, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Krüger, M.; Basiouni, S.; Ederk, I.; Rodloff, A. Susceptibility of Extendedspectrum ß-Lactamase (ESBL)-Producing Enterobacteriaaceae to Roundup. Ger. J. Microbiol. 2021, 1, 7–15. [Google Scholar] [CrossRef]
- Kaonga, N.; Hang′ombe, B.M.; Lupindu, A.M.; Hoza, A.S. Detection of CTX-M-Type Extended Spectrum Beta-Lactamase Producing Salmonella Typhimurium in Commercial Poultry Farms in Copperbelt Province, Zambia. Ger. J. Vet. Res. 2021, 2, 27–34. [Google Scholar] [CrossRef]
- Serajus, S.; Seon-Woo, K.; Haley, B.J.; Van, K.; Debabrata, B. Alternative Growth Promoters Modulate Broiler Gut Microbiome and Enhance Body Weight Gain. Front. Microbiol. 2017, 8, 2088. [Google Scholar] [CrossRef]
- Attia, Y.A.; Abd El-Hamid, A.E.; Ellakany, H.F.; Bovera, F.; Al-Harthi, M.A.; Ghazaly, S.A. Growing and Laying Performance of Japanese Quail Fed Diet Supplemented with Different Concentrations of Acetic Acid. Ital. J. Anim. Sci. 2013, 12, 222–230. [Google Scholar] [CrossRef]
- Attia, Y.A.; Ellakany, H.F.; Abd El-Hamid, A.E.; Bovera, F.; Ghazaly, S.A. Control of Salmonella Enteritidis Infection in Male Layer Chickens by Acetic Acid and/or Prebiotics, Probiotics and Antibiotics. Arch. Geflügelk 2012, 76, 239–245. [Google Scholar]
- Al-Harthi, M.A.; Attia, Y.A. Effect of Citric Acid on the Nutritive Value of Olive Cake in Broiler Diets. Eur. Poult. Sci. 2016, 80, 1–14. [Google Scholar] [CrossRef]
- Attia, Y.; Al-Harthi, M.; El-Kelawy, M. Utilisation of Essential Oils as a Natural Growth Promoter for Broiler Chickens. Ital. J. Anim. Sci. 2019, 18, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Oluwafemi, R.A.; Olawale, I.; Alagbe, J.O. Recent Trends in the Utilization of Medicinal Plants as Growth Promoters in Poultry Nutrition—A Review. Rev. Agric. Vet. Sci. 2020, 4, 5–11. [Google Scholar]
- Lan, R.; Zhao, Z.; Li, S.; An, L. Sodium Butyrate as an Effective Feed Additive to Improve Performance, Liver Function and Meat Quality in Broilers under Hot Climatic Condition. Poult. Sci. 2020, 99, 5491–5500. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Alagawany, M.; Elwan, H.A.M.; Fathi, M.A.; Farag, M.R. Effect of Sodium Butyrate on Intestinal Health of Poultry—A Review. Ann. Anim. Sci. 2020, 20, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, W.; Gao, F.; Zhou, G. Effect of Sodium Butyrate on Intestinal Inflammatory Response to Lipopolysaccharide in Broiler Chickens. Can. J. Anim. Sci. 2015, 95, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Ji, J.; Qu, H.; Wang, J.; Shu, D.M.; Wang, Y.; Liu, T.F.; Li, Y.; Luo, C.L. Effects of Sodium Butyrate on Intestinal Health and Gut Microbiota Composition during Intestinal Inflammation Progression in Broilers. Poult. Sci. 2019, 98, 4449–4456. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium Butyrate Attenuates High-Fat Diet-Induced Steatohepatitis in Mice by Improving Gut Microbiota and Gastrointestinal Barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Pedroso, A.A.; Mallo, J.J.; Puyalto, M.; Kim, W.K.; Applegate, T.J. Sodium Butyrate Improved Performance While Modulating the Cecal Microbiota and Regulating the Expression of Intestinal Immune-Related Genes of Broiler Chickens. Poult. Sci. 2017, 96, 3981–3993. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Sodium Butyrate Improved Intestinal Immune Function Associated with nf-Kappa b and p38mapk Signalling Pathways in Young Grass Carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef]
- Finnie, I.A.; Dwarakanath, A.D.; Taylor, B.A.; Rhodes, J.M. Colonic Mucin Synthesis Is Increased by Sodium Butyrate. Gut 1995, 36, 93–99. [Google Scholar] [CrossRef] [Green Version]
- González-Ortiz, G.; Dos Santos, T.T.; Vienola, K.; Vartiainen, S.; Apajalahti, J.; Bedford, M.R. Response of Broiler Chickens to Xylanase and Butyrate Supplementation. Poult. Sci. 2019, 98, 3914–3925. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Achanta, M.; Schreiber, N.B.; Bommineni, Y.R.; Dai, G.; Jiang, W.; Lamont, S.; Lillehoj, H.S.; Beker, A.; Teeter, R.G.; et al. Butyrate Enhances Disease Resistance of Chickens by Inducing Antimicrobial Host Defense Peptide Gene Expression. PLoS ONE 2011, 6, e27225. [Google Scholar] [CrossRef]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef]
- Lan, R.X.; Li, S.Q.; Zhao, Z.; An, L.L. Sodium Butyrate as an Effective Feed Additive to Improve Growth Performance and Gastrointestinal Development in Broilers. Vet. Med. Sci. 2020, 6, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Xiao, Z.; An, W.; Dong, Y.; Zhang, B. Dietary Sodium Butyrate Improves Intestinal Development and Function by Modulating the Microbial Community in Broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef]
- Zhang, W.H.; Jiang, Y.; Zhu, Q.F.; Gao, F.; Dai, S.F.; Chen, J.; Zhou, G.H. Sodium Butyrate Maintains Growth Performance by Regulating the Immune Response in Broiler Chickens. Br. Poultry Sci. 2011, 52, 292–301. [Google Scholar] [CrossRef]
- Moquet, P.C.A.; Onrust, L.; Van Immerseel, F.; Ducatelle, R.; Hendriks, W.H.; Kwakkel, R.P. Importance of Release Location on the Mode of Action of Butyrate Derivatives in the Avian Gastrointestinal Tract. Worlds Poult. Sci. J. 2016, 72, 61–80. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of Probiotic Inclusion Levels in Broiler Nutrition on Growth Performance, Nutrient Digestibility, Plasma Immunoglobulins, and Cecal Microflora Composition. Poult. Sci. 2010, 89, 58–67. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469. [Google Scholar] [CrossRef]
- El Aidy, S.; Van Den Bogert, B.; Kleerebezem, M. The Small Intestine Microbiota, Nutritional Modulation and Relevance for Health. Curr. Opin. Biotechnol. 2015, 32, 14–20. [Google Scholar] [CrossRef]
- Cotter, P.D. Small Intestine and Microbiota. Curr. Opin. Gastroenterol. 2011, 27, 99–105. [Google Scholar] [CrossRef]
- Onrust, L.; Ducatelle, R.; Van Driessche, K.; De Maesschalck, C.; Vermeulen, K.; Haesebrouck, F.; Eeckhaut, V.; Van Immerseel, F. Steering Endogenous Butyrate Production in the Intestinal Tract of Broilers as a Tool to Improve Gut Health. Front. Vet. Sci. 2015, 2, 75. [Google Scholar] [CrossRef]
- Chamba, F.; Puyalto, M.; Ortiz, A.; Torrealba, H.; Mallo, J.J.; Riboty, R. Effect of Partially Protected Sodium Butyrate on Performance, Digestive Organs, Intestinal Villi and E. coli Development in Broilers Chickens. Int. J. Poult. Sci. 2014, 13, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.D.; Bayir, H.O.; Cosby, D.E.; Cox, N.A.; Williams, S.M.; Fowler, J. Evaluation of Encapsulated Sodium Butyrate on Growth Performance, Energy Digestibility, Gut Development, and Salmonella Colonization in Broilers. Poult. Sci. 2017, 96, 3638–3644. [Google Scholar] [CrossRef]
- Lan, R.; Li, S.; Chang, Q.; An, L.; Zhao, Z. Sodium Butyrate Enhances Growth Performance and Intestinal Development in Broilers. Czech J. Anim. Sci. 2020, 65, 1–12. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, Y. Effects of Dietary Sodium Butyrate Supplementation on the Intestinal Morphological Structure, Absorptive Function and Gut Flora in Chickens. Anim. Feed Sci. Technol. 2007, 132, 240–249. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Khattak, F.; Ashraf, S.; Yousaf, M.S.; Rehman, H. Effect of Sodium Butyrate on Performance, Immune Status, Microarchitecture of Small Intestinal Mucosa and Lymphoid Organs in Broiler Chickens. Asian Australas. J. Anim. Sci. 2017, 30, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Torki, M. Study on Usage Period of Dietary Protected Butyric Acid on Performance, Carcass Characteristics, Serum Metabolite Levels and Humoral Immune Response of Broiler Chickens. J. Anim. Vet. Adv. 2009, 8, 1702–1709. [Google Scholar] [CrossRef]
- Katoh, K.; Yajima, T. Effects of Butyric Acid and Analogues on Amylase Release from Pancreatic Segments of Sheep and Goats. Pflug. Arch. Eur. J. Phy. 1989, 413, 256–260. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Lu, C.; Ahmad, H.; Zhang, H.; He, J.; Zhang, L.; Wang, T. Influence of Butyrate Loaded Clinoptilolite Dietary Supplementation on Growth Performance, Development of Intestine and Antioxidant Capacity in Broiler Chickens. PLoS ONE 2016, 11, e0154410. [Google Scholar] [CrossRef] [Green Version]
- Jazi, V.; Foroozandeh, A.D.; Toghyani, M.; Dastar, B.; Koochaksaraie, R.R. Effects of Pediococcus Acidilactici, Mannan-Oligosaccharide, Butyric Acid and Their Combination on Growth Performance and Intestinal Health in Young Broiler Chickens Challenged with Salmonella Typhimurium. Poult. Sci. 2018, 97, 2034–2043. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila Is a Promising Probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Li, J.; Xing, T.; Zhang, L.; Gao, F. Combined Effects of Xylo-Oligosaccharides and Coated Sodium Butyrate on Growth Performance, Immune Function, and Intestinal Physical Barrier Function of Broilers. Anim. Sci. J. 2021, 92, e13545. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of Dietary Polyphenol-Rich Grape Products on Intestinal Microflora and Gut Morphology in Broiler Chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Lumpkins, B.; Mathis, G.; Williams, S.M.; Fowler, J. Evaluation of Encapsulated Sodium Butyrate with Varying Releasing Times on Growth Performance and Necrotic Enteritis Mitigation in Broilers. Poult. Sci. 2019, 98, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, S.; Zhong, R.; Wan, F.; Chen, L.; Liu, L.; Yi, B.; Zhang, H. Olive Fruit Extracts Supplement Improve Antioxidant Capacity via Altering Colonic Microbiota Composition in Mice. Front. Nutr. 2021, 8, 645099. [Google Scholar] [CrossRef]
- Memon, M.A.; Dai, H.; Wang, Y.; Xu, T.; ul Aabdin, Z.; Bilal, M.S.; Chandra, R.A.; Shen, X. Efficacy of Sodium Butyrate in Alleviating Mammary Oxidative Stress Induced by Sub-Acute Ruminal Acidosis in Lactating Goats. Microb. Pathog. 2019, 137, 103781. [Google Scholar] [CrossRef]
- Liu, W.; La, A.L.T.Z.; Evans, A.; Gao, S.; Yu, Z.; Bu, D.; Ma, L. Supplementation with Sodium Butyrate Improves Growth and Antioxidant Function in Dairy Calves before Weaning. J. Anim. Sci. Biotechnol. 2021, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, W.H.; Gao, F.; Zhou, G.H. Micro-Encapsulated Sodium Butyrate Attenuates Oxidative Stress Induced by Corticosterone Exposure and Modulates Apoptosis in Intestinal Mucosa of Broiler Chickens. Anim. Prod. Sci. 2014, 55, 587–594. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Farmanbar, N.; Abdollahi, M. Oxidative Stress Status and DNA Damage in Saliva of Human Subjects with Oral Lichen Planus and Oral Squamous Cell Carcinoma. J. Oral. Pathol. Med. 2012, 41, 736–740. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Hassan, S.S. Responses of Broiler Chicken to Different Oil Levels within Constant Energy Levels from 20 to 40 Days of Age under Hot Weather Conditions. Ital. J. Anim. Sci. 2021, 20, 664–676. [Google Scholar] [CrossRef]
- Henningsson, Å.; Björck, I.; Nyman, M. Short-Chain Fatty Acid Formation at Fermentation of Indigestible Carbohydrates. Näringsforskning 2001, 45, 165–168. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Wang, J.; Ma, H.; Zhang, B.; Wang, S. The Protective Effects of 2′-Fucosyllactose against E. coli O157 Infection Are Mediated by the Regulation of Gut Microbiota and the Inhibition of Pathogen Adhesion. Nutrients 2020, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial Community Mapping in Intestinal Tract of Broiler Chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Makled, M.N.; Abouelezz, K.F.M.; Gad-Elkareem, A.E.G.; Sayed, A.M. Comparative Influence of Dietary Probiotic, Yoghurt, and Sodium Butyrate on Growth Performance, Intestinal Microbiota, Blood Hematology, and Immune Response of Meat-Type Chickens. Trop. Anim. Health Prod. 2019, 51, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, F.; Yang, Y.; Lepp, D.; Yu, H.; Ruan, Z.; Yang, C.; Yin, Y.; Hou, Y.; Leeson, S.; et al. Dietary Butyrate Glycerides Modulate Intestinal Microbiota Composition and Serum Metabolites in Broilers. Sci. Rep. 2018, 8, 4940. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Jha, R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance, and Health of Poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Rothrock, M.J.; Vieira, B.S.; Mallo, J.J.; Puyalto, M.; Hofacre, C.; Applegate, T.J. Supplementation of Protected Sodium Butyrate Alone or in Combination with Essential Oils Modulated the Cecal Microbiota of Broiler Chickens Challenged with Coccidia and Clostridium Perfringens. Front. Sustain. Food Syst. 2018, 2, 72. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y.; He, X.; et al. The Chicken Gut Metagenome and the Modulatory Effects of Plant-Derived Benzylisoquinoline Alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y. Genus Kitasatospora, Taxonomic Features and Diversity of Secondary Metabolites. J. Antibiot. 2017, 70, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, S.Y.; Kostopoulos, I.; De Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Ajuwon, K. Butyrate Modifies Intestinal Barrier Function in IPEC-J2 Cells through a Selective Upregulation of Tight Junction Proteins and Activation of the Akt Signaling Pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Belzer, C.; De Vos, W.M. Microbes Inside—From Diversity to Function: The Case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential Modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of Host Peripheral Lipid Metabolism and Histone Acetylation in Mouse Gut Organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef] [Green Version]
- Hatayama, H.; Iwashita, J.; Kuwajima, A.; Abe, T. The Short Chain Fatty Acid, Butyrate, Stimulates MUC2 Mucin Production in the Human Colon Cancer Cell Line, LS174T. Biochem. Biophys. Res. Commun. 2007, 356, 599–603. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; Van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The Regulation of Intestinal Mucin MUC2 Expression by Short-Chain Fatty Acids: Implications for Epithelial Protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [Green Version]






| Item | CON | ANT | CSB |
|---|---|---|---|
| BWG, g | |||
| D 1–21 | 552 ± 29.4 b | 573 ± 13.1 ab | 589 ± 42.8 a |
| D 22–42 | 1349 ± 111 b | 1468 ± 88.1 a | 1428 ± 119 ab |
| D 1–42 | 1902 ± 131 b | 2041 ± 84.5 a | 2017 ± 145 ab |
| FI, g | |||
| D 1–21 | 713 ± 19.5 | 700 ± 19.4 | 719 ± 40.2 |
| D 22–42 | 2177 ± 125 | 2214 ± 105 | 2215 ± 186 |
| D 1–42 | 2890 ± 142 | 2914 ± 103 | 2933 ± 215 |
| F/G | |||
| D 1–21 | 1.29 ± 0.0501 a | 1.22 ± 0.0256 b | 1.22 ± 0.0646 b |
| D 22–42 | 1.62 ± 0.0861 a | 1.51 ± 0.0503 b | 1.55 ± 0.0644 ab |
| D 1–42 | 1.52 ± 0.0615 a | 1.43 ± 0.0383 b | 1.46 ± 0.0436 b |
| Item (% Unless Noted) | 1 to 21 Days Old | 22 to 42 Days of Old |
|---|---|---|
| Ingredients | ||
| Corn (7.9%, crude protein) | 55.00 | 58.80 |
| Soybean meal (43.6%, crude protein) | 36.30 | 32.27 |
| Soybean oil | 4.15 | 5.00 |
| Dicalcium phosphate | 1.80 | 1.62 |
| Sodium chloride | 0.30 | 0.30 |
| Limestone | 0.90 | 0.67 |
| Choline chloride (50%) | 0.10 | 0.10 |
| L-Lysine·HCl (99%) | 0.21 | 0.10 |
| DL-Methionine (98%) | 0.24 | 0.14 |
| Premix 1 (1%) | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Calculated Nutrient levels | ||
| Metabolizable Energy (Mcal/kg) | 2.97 | 3.06 |
| Crude Protein | 21.1 | 19.6 |
| Available Phosphorus | 0.46 | 0.39 |
| Calcium | 1.05 | 0.82 |
| Lysine | 1.32 | 1.10 |
| Methionine | 0.58 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Bai, H.; Deng, F.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics. Antibiotics 2022, 11, 132. https://doi.org/10.3390/antibiotics11020132
Zhao H, Bai H, Deng F, Zhong R, Liu L, Chen L, Zhang H. Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics. Antibiotics. 2022; 11(2):132. https://doi.org/10.3390/antibiotics11020132
Chicago/Turabian StyleZhao, Huaibao, Hai Bai, Fuli Deng, Ruqing Zhong, Lei Liu, Liang Chen, and Hongfu Zhang. 2022. "Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics" Antibiotics 11, no. 2: 132. https://doi.org/10.3390/antibiotics11020132
APA StyleZhao, H., Bai, H., Deng, F., Zhong, R., Liu, L., Chen, L., & Zhang, H. (2022). Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics. Antibiotics, 11(2), 132. https://doi.org/10.3390/antibiotics11020132








