Abstract
The Quorum-sensing system in Pseudomonas aeruginosa is responsible for the pathogenicity and the production of virulence factors and biofilm formation. Dihydropyrrolones were previously found to act as inhibitors of QS-dependent bacterial phenotypes. In this study, a range of dihydropyrrolone (DHP) analogues was synthesized via the lactone-lactam conversion of lactone intermediates followed by the formation of novel acetylene analogues of dihydropyrrolones from brominated dihydropyrrolones via Sonogashira coupling reactions in moderate to high yields. Upon biological testing, the most potent compounds, 39–40 and 44, showed higher bacterial quorum-sensing inhibitory (QSI) activity against P. aeruginosa reporter strain at 62.5 µM. Structure–activity relationship studies revealed that di-alkynyl substituent at the exocyclic position of DHPs possessed higher QSI activities than those of mono-alkynyl DHPs. Moreover, a hexyl-substituent at C3 of DHPs was beneficial to QSI activity while a phenyl substituent at C4 of DHPs was detrimental to QSI activity of analogues.
1. Introduction
Pseudomonas aeruginosa regulates its pathogenicity through an intercellular density-dependent communication system mediated by the binding of signaling molecules to quorum-sensing (QS) receptors such as LasR, which, upon its activation, modulates the expression of multiple genes responsible for the production of various virulence factors (e.g., pyocyanin, rhamnolipids, and pyoverdine), biofilm formation, the swarming motility, and antibiotic resistance. While conventional antibiotics exert increased selective pressure on bacteria, resulting in the development of antibiotic resistance [1,2,3], inhibition of quorum sensing interrupts the cell-to-cell coordination without exerting a selective pressure on bacteria. This may reduce the chance of bacterial resistance. Therefore, antagonists of LasR are considered an effective alternative strategy with a novel mode of actions for the treatment of infections while preventing bacterial resistance [1].
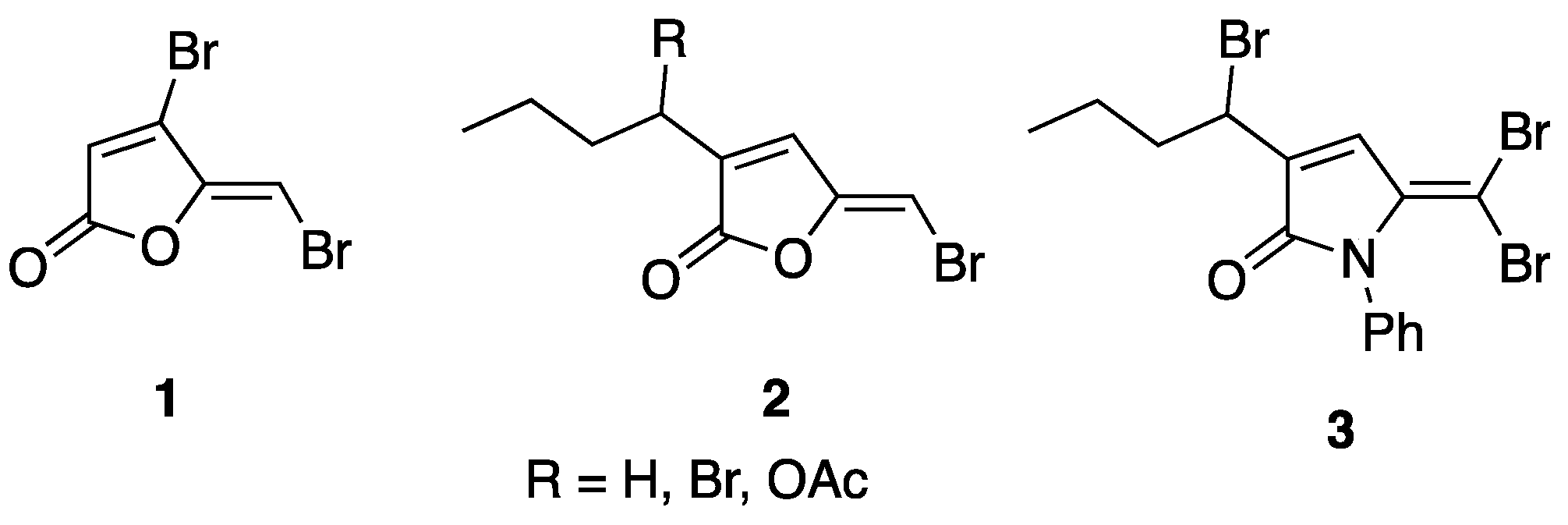
Fimbrolides, or halogenated furanones such as 5-dibrominated furanones 1 and 2 (Figure 1), were first synthesized in 1997 by Manny et al. [4] and are known to possess antimicrobial and quorum-sensing inhibitory (QSI) activities [5,6,7]. They were previously found to act as QS inhibitors of AHL that mediate QS phenotypes [5,6]. We previously reported the design, synthesis, and evaluation of fimbrolide–nitric oxide donor hybrids as antimicrobial agents [7]. However, their therapeutic use is limited by their instability, ease of hydrolysis, and toxicity [8]. Nevertheless, fimbrolides or 5-dibrominated furnaones can be utilized as a starting template for chemical manipulation into 1,5-dihydropyrrol-2-ones (DHPs) such as compound 3. The dihydropyrrole-2-one moiety is present in several classes of biologically important natural and synthetic molecules such as rolipram, pulchellalactam [9], and Jatropham [10]. The 1,5-dihydropyrrol-2-one is an isosteric five-membered ring that is structurally related to furanones but contains a cyclic amide bond in substituting the hydrolytically and enzymatically labile cyclic ester in the natural and synthetic furanones. The lactam ring is hydrolytically more stable, which reduces the susceptibility of the ring-opening reaction by either enzymatically or chemical lactonolysis. [11,12] Several DHPs were found to possess antimicrobial activity, QS, and biofilm inhibitory activities [13] such as those reported by our group [14,15,16], including DHPs with a thioether [17] or a seleno-urea [18] moiety. In addition, the biological activities of DHP-related compounds were also studied by other research groups [19,20,21,22], including rubrolide derivatives, which showed antibacterial [23], anticancer [24], and herbicidal [25] activities. Moreover, a recent paper by Ma et al. also reported the synthesis and anti-virulence activities of several furanones and aryl-substituted pyrrolidone derivatives as quorum sensing inhibitors [26].
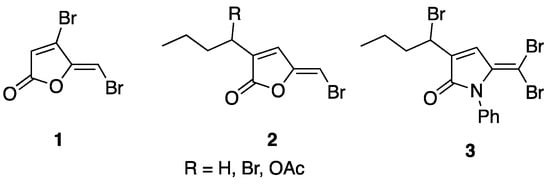
Figure 1.
Furanones and DHP examples.
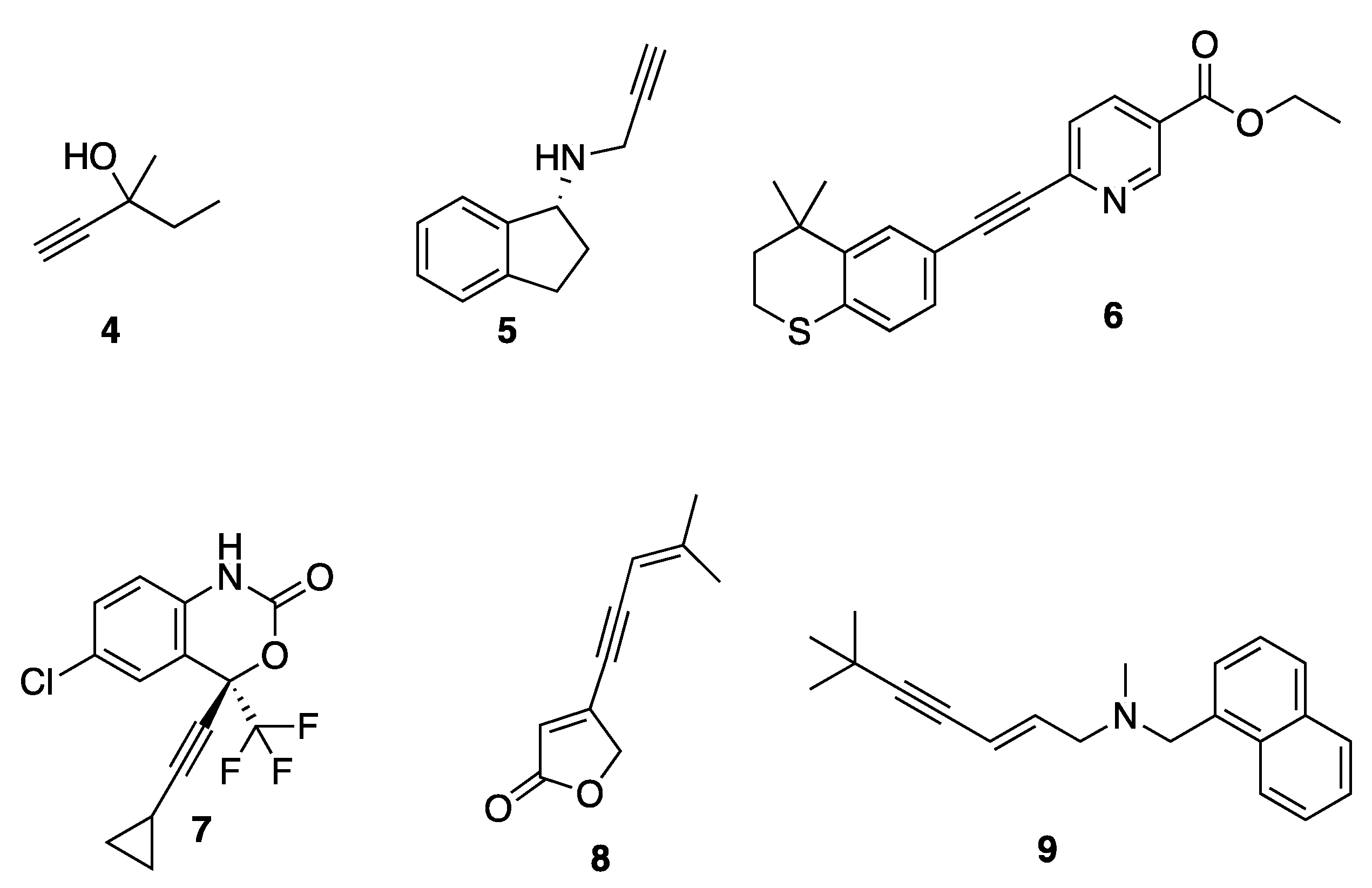
The acetylene functionality is frequently found in many synthetic products as well as natural products that can be isolated from a wide range of plant species, fungal and bacterial cultures, and marine sponges and corals [27,28,29]. Acetylene groups are commonly used in medicinal chemistry for their electronic effects, equivalent to aromatic rings and providing structural rigidity (Figure 2) [30]. They are present in some of the sedative-hypnotic drugs such as meparfynol 4, ethinamate, and the antiparkinsonian rasagiline 5 [30]. They are also present in the synthetic retinoid tazarotene 6, which is used for the treatment of acne, psoriasis, and photo-aging, as well as the antiviral compound efavirenz 7, the orally active contraceptives ethynyl-estradiol, norethindrone, and the implantable contraceptive etonogestrel [30]. Acetylene is also found in the natural pesticide falcarinol, the nervous system toxin oenanthotoxin, and the furanone-containing natural product cleviolide 8. [31].
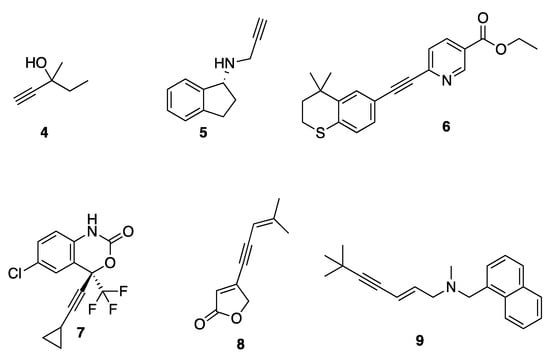
Figure 2.
Examples of acetylene in pharmaceuticals.
The installation of acetylene moieties into molecules can be achieved via the Sonogashira cross-coupling reaction between an organohalide and a terminal alkyne. The Sonogashira reaction was first reported in 1975 by Kenkichi Sonogashira and his co-workers. Since then, it has been one of the most important and commonly used cross-coupling reactions for forming carbon-carbon bonds. It is sometimes considered as important as the Suzuki–Miyaura reaction for the synthesis of compounds that are useful for medicinal chemistry research [32]. The Sonogashira cross-coupling reaction was featured in the synthesis of acetylenic derivatives of furanones [31,33] and quinolines with potential antimicrobial activity [34], as well as 2-aminoimidazole with antibiofilm activity [35]. It was also employed in the total synthesis of enediyne-containing antibiotics such as calicheamicin and dynemicin [36]. Many pharmaceutically important molecules were synthesized via the Sonogashira reaction, including terbinafine 9 (lamisil), which is used to treat fungal infections, and the aforementioned tazarotene 6.
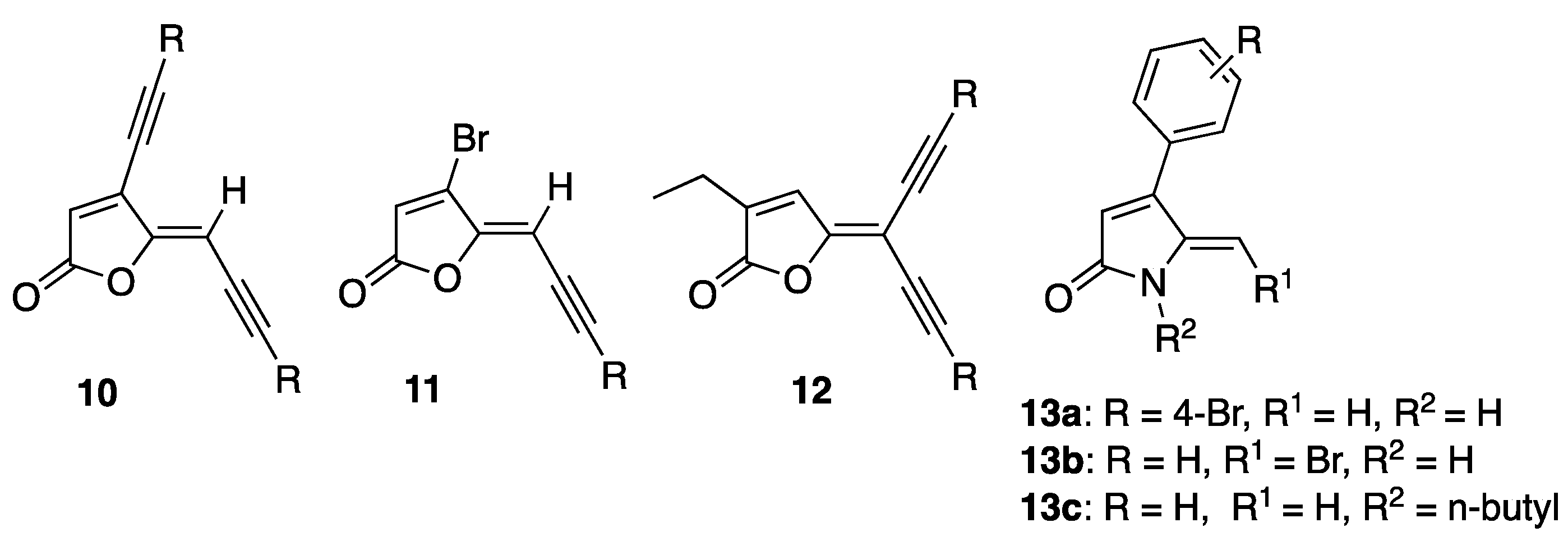
Recently, our group reported the synthesis of acetylene-containing furanones 10–12, which possessed low to moderate quorum-sensing inhibitory (QSI) activity, from brominated furanones 1 via Sonogashira cross-coupling reaction (Figure 3) [37]. In addition, we developed a method to convert brominated furanones into DHPs using a ring-opening/ring-closing lactamization reaction with amine nucleophiles [38]. We also reported the structural activity relationships (SAR) of our DHPs-based AHL mimics [15]. Dihydropyrrolones (DHPs) 13a–c that were synthesized from their corresponding furanones using the ring-opening/ring-closing lactamisation reaction demonstrated good QSI and antibiofilm activities against P. aeruginosa [15] and E. coli [16] with minimal effect on bacterial growth [15,16]. Furthermore, our work on the synthesis of a series of thioether containing DHP analogues as PqsR antagonist and novel seleno- and thio-urea containing dihydropyrrol-2-one (DHP) analogues as LasR antagonists [18] was also reported. Prompted by these previous findings [14,15,18,37] and the possibility of exploiting the brominated exocyclic alkene at the 5-position of DHP, herein we aimed to introduce acetylene group(s) at the 5-position of the DHP and investigate their effect on the biological activity.
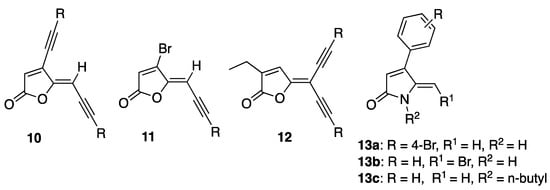
Figure 3.
Acetylene-containing furanones based on our recent work.
2. Results and Discussion
2.1. Synthesis
In this study, we investigated the introduction of acetylene substituents via the Sonogashira cross-coupling reaction of brominated DHPs to give acetylene-substituted DHPs. The reaction typically involves the use of a palladium catalyst and copper as a co-catalyst to form a carbon–carbon bond between a terminal alkyne, which serves as the coupling partner, and an aryl or vinyl halide [32,39].
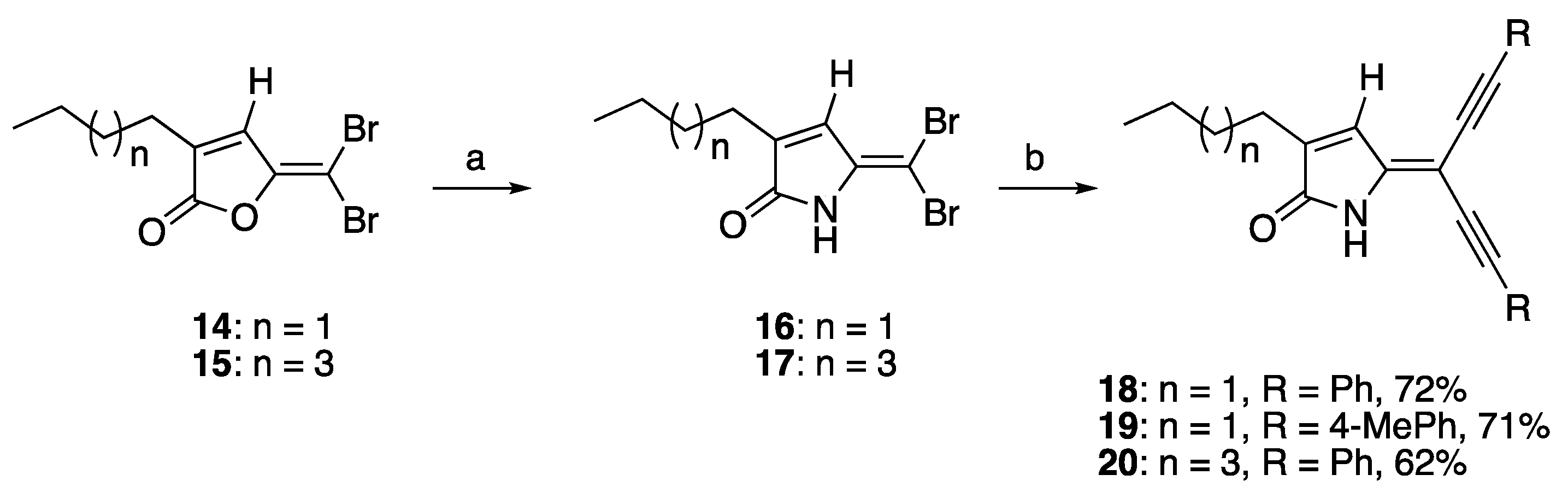
The synthesis of acetylene substituted DHPs was initially attempted by the lactamisation of the previously reported Sonogashira furanone products [37] with aqueous ammonia and/or alkylamine (e.g., propylamine) at room temperature and monitored by TLC. However, this synthetic strategy was proven to be unsuccessful. This could be attributed to the reduced reactivity of the acetylene-containing furanones towards lactamisation. Therefore, an alternative synthetic pathway for the synthesis of compounds 18–20 was sought. This alternative pathway involved the lactamisation of the brominated furanones 14–15 prior to the Sonogashira reaction. The lactamisation reaction of brominated furanones 14 and 15 using ammonia gas successfully afforded brominated DHPs 16 and 17. The Sonogashira reaction was then carried out between the brominated DHPs 16–17 and phenylacetylene or 4-methylphenylacetylene (2.5 eq.) with the presence of CuI (0.1 eq.), bistriphenylphosphine)palladium(II) dichloride (PdCl2(PPh3)2 (0.1 eq.), and triethylamine (TEA, 1 eq.) in degassed THF under reflux conditions to give the di-alkynylated NH DHPs 18–20 in 62–72% yields as reported in our recently published conference paper (Scheme 1) [40]. During the optimization of rection process, it was found that heating was crucial for the success of the reaction and could minimize the formation of the acetylene homo-adduct. Moreover, it was found that the use of triethylamine as the solvent and the base could also result in the formation of the Sonogashira products, although the use of THF as the solvent in this reaction afforded the Sonogashira products in the highest yield [40].
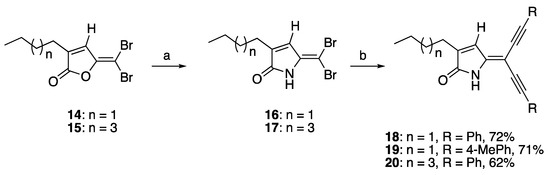
Scheme 1.
Lactamisation of furanone and Sonogashira coupling reaction of compounds 18–20. Reaction conditions: (a) ammonia gas, DCM, r.t.; (b) PdCl2(PPh3)2 CuI, TEA, terminal alkyne, THF, reflux [40].
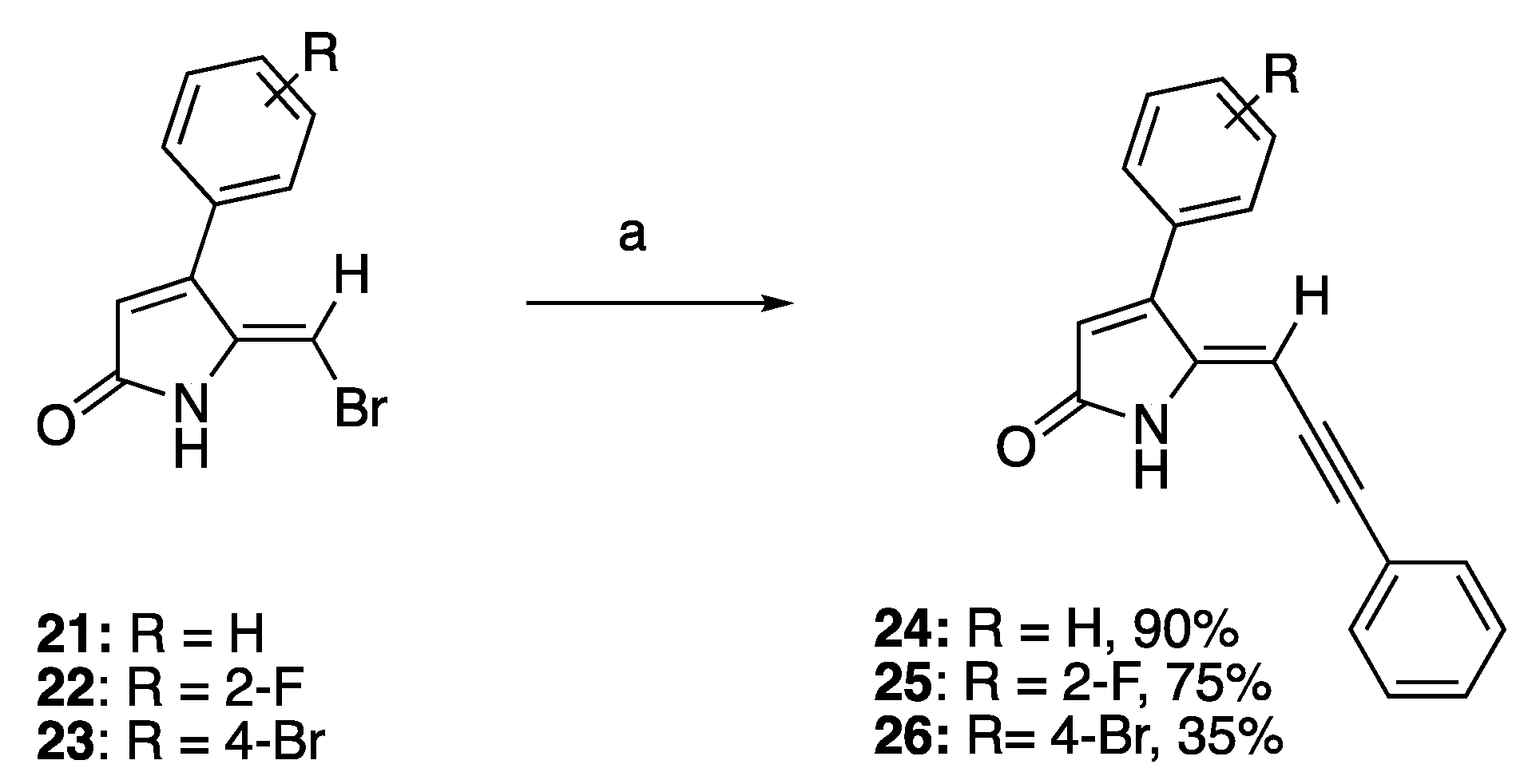
Recently, we reported the QS inhibitory activities of 5-methylene-4-phenyl-1,5-dihydro-2H-pyrrol-2-ones such as compounds 21–23, which can be synthesized via the lactone-lactam conversion method [15,41]. The synthesis began with the acid-catalyzed condensation of phenylacetones with glyoxylic acid, producing 5-hydroxyfuranones. In the key lactone-lactam conversion step, furanones were treated with thionyl chloride followed by aqueous ammonia to provide the intermediate 5-hydroxylactams, which were subsequently subjected to radical bromination with N-bromosuccinimide (NBS) to give 5-bromo-methylene-5-hydroxylactams, followed by dehydration to obtain the target compounds 21–23.
To investigate the effect of introduction of a bulky mono-substituted acetylene group to the exocyclic vinylic alkene of compounds 21–23 while keeping the phenyl moiety on C4 on the QS activity of the compounds, 5-(bromomethylene) DHPs 21–23 were subjected to the Sonogashira coupling reaction with phenylacetylene, CuI, and palladium catalyst to generate compounds 24–26 in 35–90% yield (Scheme 2). While compounds 24 and 25 were synthesized in good yields of 90% and 75%, respectively, the low yield of compound 26 could be due to its poor solubility in organic solvent and high adsorption on silica gel, leading to considerable loss of the compound upon purification.
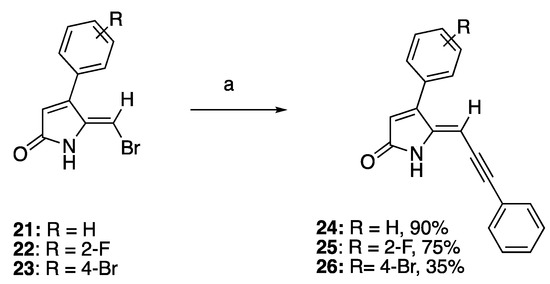
Scheme 2.
Sonogashira synthesis of 4-phenyl DHP derivatives. Reaction conditions: (a) PdCl2(PPh3)2, CuI, TEA, argon, alkyne, THF, and reflux.
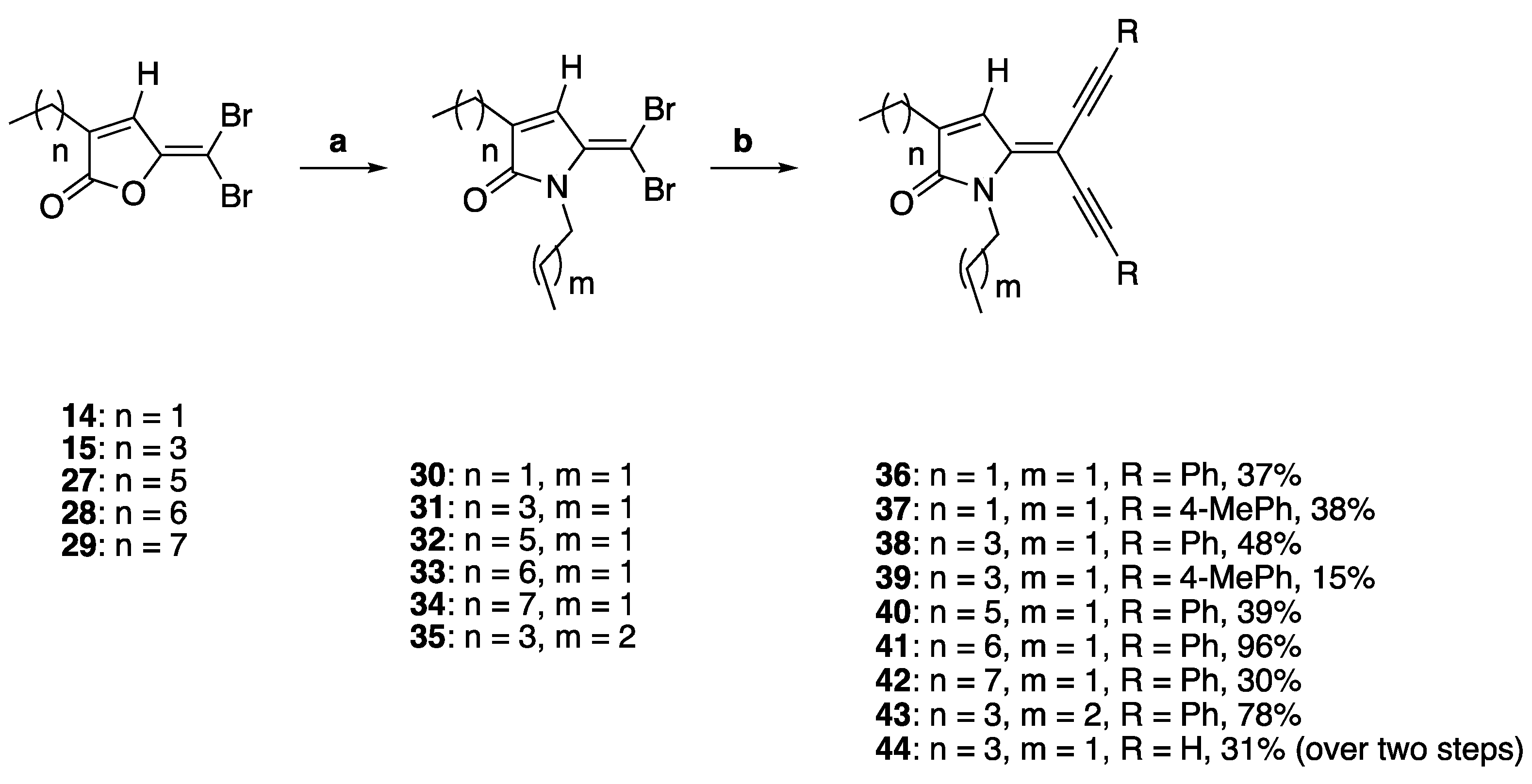
To study the effect of introducing alkyl groups at the ring nitrogen, the synthesis of compounds 36–44 was investigated (Scheme 3). The precursor furanones 14–15 and 27–29 were synthesized following our previously reported procedure using sulfuric acid-catalyzed cyclization of brominated 2-alkyl-levulinic acids [4,7,42].
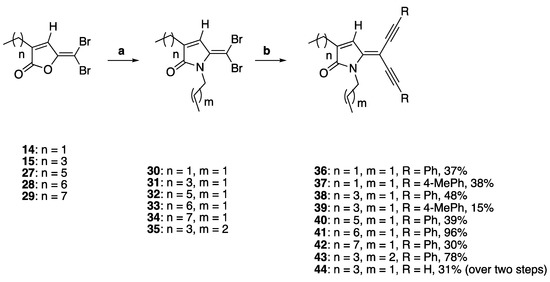
Scheme 3.
DHP analogues containing diacetylene groups and substituents at the ring nitrogen. Reaction conditions: (a) propylamine or butylamine, DCM, r.t., and 3 h; (b) PdCl2(PPh3)2, CuI, TEA, argon, alkyne, THF, and reflux.
Following the synthesis of the precursor furanones 14–15 and 27–29, the N-propyl analogues 30–34 were obtained in 30–96% yields by lactamization using excess equivalent of propylamine in dichloromethane, while the synthesis of 35 was synthesized using the same synthetic procedure for compounds 30–34 but with butylamine instead of propylamine [43]. The 5-dibromo DHPs 30 and 31 were reacted with phenylacetylene to give compounds 36 and 38 in 37% and 48% yield. Alternatively, the 5-dibromo DHPs 30 and 31 were also reacted with p-tolylacetylene to give alkyne compounds 37 and 39 in moderate to low yields of 37% and 15%, respectively. The longer chain 5-dibromo DHPs 40–43 were also synthesized from their corresponding 5-dibromo DHPs 30–35 in the analogous reactions in 30–96% yields. Finally, compound 44 with a terminal acetylene group was synthesized over two steps, by firstly reacting compound 31 with trimethylsilyl-protected acetylene followed by the deprotection of the trimethylsilyl protecting group to yield the terminal acetylene group.
The structures of the Sonogashira products 18–20, 24–26, and 36–44 were confirmed by spectroscopic analysis including 1H NMR, 13C NMR, and mass spectrometry (see Supplementary Materials File S1). In the acetylene products, the C4 hydrogen resonance of 18–20 and 36–44 was observed to shift downfield in the Sonogashira coupling products at 7.13–7.26 ppm compared to 7.02 ppm in the DHP intermediates. The additional phenyl ring proton resonances for compounds 18–20 and 36–44 were observed at 7.20–7.60 ppm in the aromatic region of the 1H NMR spectra. Meanwhile, the newly formed acetylene carbons were confirmed by 13C NMR and were observed at 83.0–98.0 ppm. The number of aliphatic carbons was also confirmed by 13C NMR for compounds 18–20 and 36–44 as these carbons resonated in the aliphatic region between 22.0 and 40.0 ppm. The CH2 adjacent to the ring nitrogen was observed at 30.0–40.0 ppm in the 13C NMR spectrum. The exocyclic singlet protons (C=CHBr) adjacent to the newly formed acetylene phenyl of the Sonogashira products 24–26 were observed upfield at 5.47–5.59 ppm compared to 6.16–6.35 ppm in the DHP intermediates 21–23.
2.2. X-ray Analysis of Sonogashira DHP Compounds
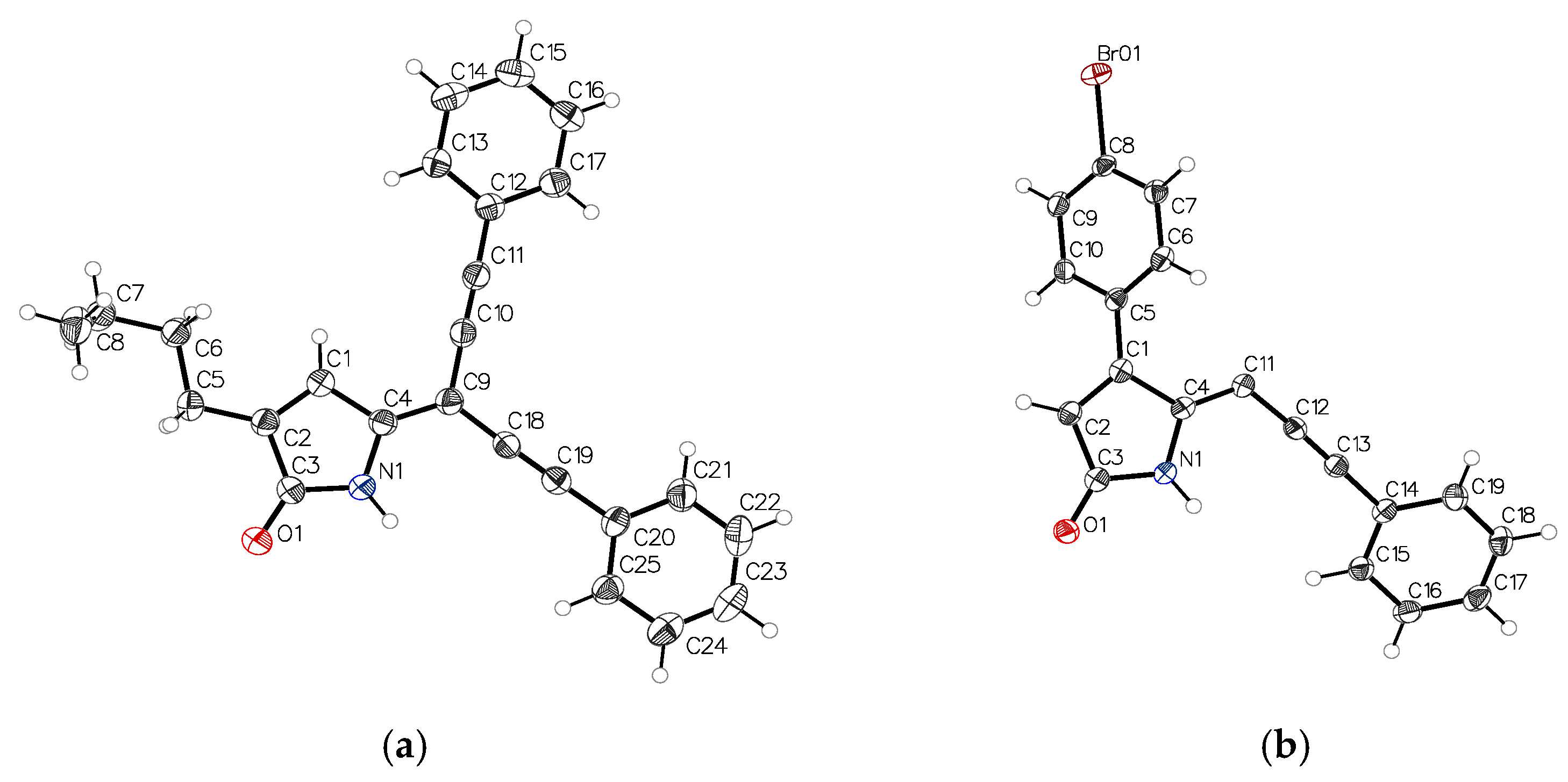
To confirm the structures of the acetylene compounds 18 (C3-butyl, C4-H) and 26 (C4-(p-Br-phenyl), these compounds were crystallized from acetonitrile to give yellow needles and yellow plate-shaped crystals, respectively, that were suitable for single crystal X-ray diffraction. The ORTEP views of molecules 18 and 26 along with the labelling of atoms are presented in Figure 4a,b.
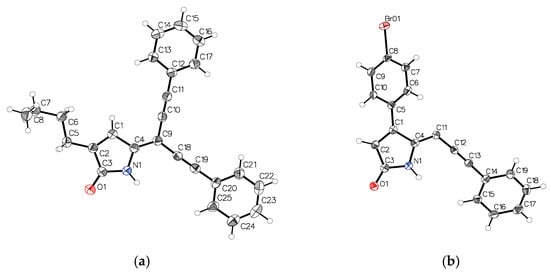
Figure 4.
ORTEP representation of compounds 18 (a) and 26 (b).
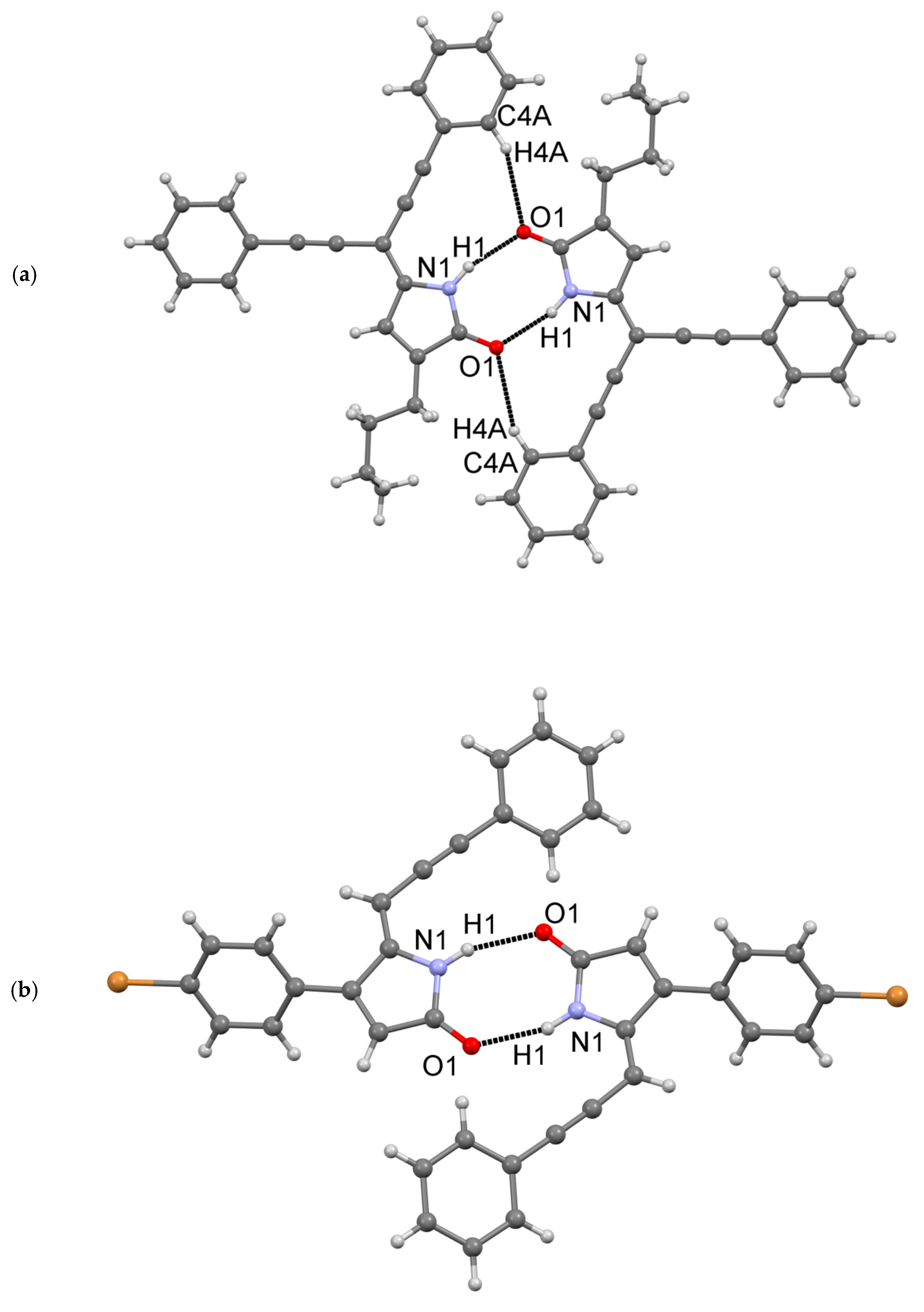
Compound 18 with a butyl group at C3 is essentially planar, whereas molecule 26 with a p-bromophenyl group at C4 is not planar. In 26, the phenyl ring C5-C10 is rotated about the C1-C5 bond by −54.3°, presumably to avoid the short H…H contact of the hydrogens from carbon atoms C6 and C11. Both 18 (Figure 5a) and 26 (Figure 5b) self-associate in a very similar fashion via N…O hydrogen bonds. An additional C-H…O interaction (“hydrogen bond”) was also observed in 18.
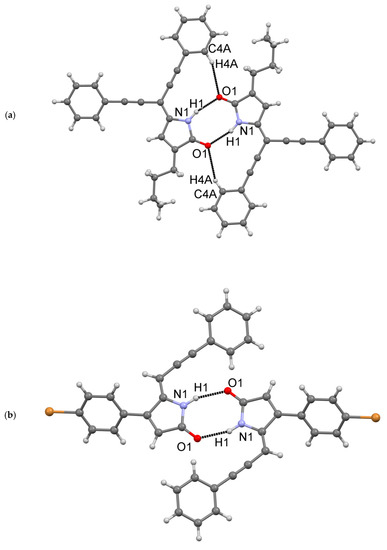
Figure 5.
Dimeric association via H-bonding interactions in structures of compounds 18 (a) and (b) 26.
2.3. QS Inhibition
QS inhibition assay was performed on the synthesized acetylene DHPs to evaluate their efficacy and to study their structure–activity relationship (SAR). These compounds were tested against P. aeruginosa MH602, a reporter strain that measures the level of green fluorescent protein (GFP), following the method reported by Hentzer et al. [5] An inhibitor is expected to reduce the expression and the production of GFP. In this study, the liquid cultures of the P. aeruginosa reporter strain MH602 were incubated in various concentrations (250, 125, and 62.5 µM) of the synthesized compounds and the fluorescence of GFP at λ = 535 nm was recorded. A fimbrolide, furanone 30 (1), was used as a positive control to validate the assay protocol. The optical density (OD) at 600 nm was also measured to determine the potential effect of the tested compounds on bacterial growth to ensure that their QSI activity was not due to bactericidal effects.
The results for the tested compounds are presented in Table 1. The percentage QS inhibition of the compounds was calculated as the percentage difference of GFP intensity between the sample and the control at the same time point when the fluorescence reached its maximum value in the control. The acetylene compounds reduced QS by 43–80% at 250 µM, 28–66% at 125 µM, and 24–56% at 62.5 µM. Moreover, most of the alkyne derivatives did not have a substantial effect on the viability of bacterial cells.

Table 1.
Percentage QS inhibition of the tested compounds against P. aeruginosa reporter strain MH602.
Among the di-alkynyl DHPs 18–20 with a C3 alkyl group but no substituent at N1-position, molecules 18 and 20 bearing a terminal phenyl ring possessed higher QS inhibition at 62.5 µM (QSI = 47.8% and 39.3%, respectively) than compound 19 bearing a p-methylphenyl group (QSI = 24.1%). Interestingly, while the installation of the phenylacetylene groups generally increased the QSI activities of the analogues, the introduction of a methyl-substituent at the terminal phenyl rings decreased the QSI activity significantly. DHP 19 with a methyl-substituent at the terminal phenyl rings had a significantly lower QSI activity of 24.1% when compared to its parent compounds 18 (QSI = 47.8%) with unsubstituted terminal phenyl rings and 16 (QSI = 44.7%) without the phenylacetylene moiety at 62.5 µM.
On the other hand, the N-unsubstituted 4-aryl DHPs 24 (C4-phenyl), 25 (C4-2-fluorophenyl), and 26 (C4-4-bromophenyl) with a single acetylene unit at the exocyclic position showed lower QS inhibition compared to their parent molecules 21–23 without the acetylene substituents [15]. The observed reduction in QS activity could be attributed to the bulky phenyl and acetylene groups, which hinder the molecules from binding to the bacterial receptor protein. This observation is consistent with lower activities of the mono- and di-substituted acetylene-derived furanones reported by Biswas et al. [37].
Among the di-alkynyl DHPs bearing aliphatic chains at both C3 and N1, compound 44 with unsubstituted terminal acetylene moieties possessed the highest QSI activities at all tested concentrations. The introduction of a terminal phenyl ring was detrimental to QSI activity as the corresponding compound 38 with a terminal phenyl ring at the acetylene moieties showed a lower QSI of 40.6% compared to compound 44 with a QSI of 55.8% at 62.5 µM. Interestingly, the effect of the introduction of an alkyl group at N1-position of the DHP on the QSI activity of the analogues depends on the substituent at the terminal phenyl rings of the acetylene moieties. For compounds with a phenylacetylene moiety, the introduction of a methyl-substituent at N1 position decreased the QSI activity slightly as N-methyl-substituted compound 38 possessed a lower QSI of 40.6% compared to its corresponding unsubstituted compound 18 (QSI = 47.8%) at 62.5 µM. In contrast, the introduction of a methyl-substituent at N1 position of DHP bearing a p-methylphenylacetylene moiety enhanced the QSI activity of the analogue significantly as N-methyl-substituted compound 39 possessed a higher QSI of 54.1% compared to its corresponding unsubstituted compound 19 (QSI = 24.1%) at 62.5 µM. In addition, lengthening the N-methyl-substituent to N-ethyl-substituent was found to be detrimental to QSI activity as N-ethyl-substituted compound 43 exhibited a lower QSI of 24.3% compared to the corresponding N-methyl-substituted compound 38, which exhibited a QSI of 40.6% at 62.5 µM. Moreover, compounds with different aliphatic chain length at C3 were also synthesized to investigate the optimum chain length for the highest QSI activity. It was found that a hexyl-substituent at C3 was optimum for QSI activity, in which compound 40 with a hexyl-substituent at C3 possessed the highest QSI of 52.9% at 62.5 µM among this series of compounds. Compounds with a longer or shorter alkyl chain length generally showed lower QSI activities.
3. Experimental
3.1. Quorum-Sensing Inhibition Assay for PAMH602
The P. aeruginosa MH602 PlasB::gfp (ASV) reporter strain was used. An overnight culture was prepared in Luria–Bertani (LB10) media supplemented with gentamycin (40 μM). This bacterial culture solution was diluted (1 in 100) with LB10 supplemented with gentamycin (15 μM). Stock solutions of the synthesized compounds were prepared at 20 mM in DMSO. Compounds were pipetted into each well with final concentrations of 250, 125, and 62.5 μM (in triplicate) with a final volume of 200 μL with the prepared bacterial culture. The negative control was prepared containing 200 μL of the bacterial culture without the tested compounds. The plates were incubated at 37 °C for 15 h. The plates were measured for GFP expression (fluorescence: excitation 485 nm, emission 535 nm) using a microplate reader (Wallac Victor, Perkin-Elmer, Minneapolis-Saint Paul, MN, USA), and the cell growth was also assessed by recording the OD at 600 nm.
3.2. Methodology for X-ray Crystallography
A suitable single crystal of 18 (CCDC 2131315) and 26 (CCDC 2131273) obtained from the recrystallisation of the corresponding compound from acetonitrile was selected under a polarizing microscope (Leica M165Z) mounted on a MicroMount (MiTeGen, Ithaca, NY, USA) consisting of a thin polymer tip with a wicking aperture. The X-ray diffraction measurements were carried out on a Bruker D8 Quest Single Crystal diffractometer with Photon II detector at 150 K by using IμS 3.0 Microfocus Source with Mo-Kα radiation (λ = 0.710723 Å). The single crystal, mounted on the goniometer using cryo loops for intensity measurements, was coated with paraffin oil and then quickly transferred to the cold stream using an Oxford Cryo stream 800 attachment. Symmetry-related absorption corrections using the program SADABS were applied and the data were corrected for Lorentz and polarization effects using Bruker APEX3 software [44]. The structure was solved by ShelxT (intrinsic phasing) [45] and the full-matrix least-square refinement was carried out using Shelxl [46] in Olex2 [47]. The non-hydrogen atoms were refined anisotropically. The molecular graphic was generated using program Olex2 [47] (Supplementary Materials Tables S3 and S4).
Crystal data: compound 18 C25H21NO (Table S1), M.W. = 351.4490, Monoclinic. Cell dimensions: a = 8.8068 (7) Å, b = 20.8376 (15) Å, c =10.6832 (6), and beta = 104.884 (2) Å; compound 26 C19H12BrNO (Table S2), M.W. = 350.2150, Monoclinic, Cell dimensions: a = 11.944 (4) Å, b = 9.578 (3) Å, c =13.798 (4), and beta = 105.385 (6) Å. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre with publication numbers CCDC 2131315 and CCDC 2131273. A copy of the data can be obtained free of charge from CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK, or by e-mail: deposit@ccdc.cam.ac.uk.
3.3. Synthesis Procedures
3.3.1. General Information
Commercially available reagents were purchased from standard suppliers such as Sigma Aldrich, Alfa Aesar, Combi-Blocks, and Oakwood Chemicals. The synthetic procedures have been reported for all compounds as general methods and appropriate references have been given for known compounds. Melting points were measured using an OptiMelt melting point apparatus and are reported without correction. High-resolution mass spectra were recorded by the Bioanalytical Mass Spectrometry Facility, UNSW, on an Orbitrap LTQ XL ion trap mass spectrometer using a nanospray (nano-electrospray) ionization source under positive ESI mode. The 1H and 13C NMR spectra were determined in the designated solvent on a Bruker DPX 300 spectrometer or a Bruker Avance 400 spectrometer. Chemical shifts (δ) are quoted in parts per million (ppm) internally referenced relative to the solvent nuclei. Multiplicities in 1H NMR are assigned as follows: brs, broad singlet; s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; sext, sextet; m, multiplet; or as a combination (e.g., dd, dt, td, etc.). The coupling constant (J) in hertz, integration, and proton count was also reported.
3.3.2. Synthesis of Brominated DHPs’ Intermediates 30–35
The 5-Br DHPs were synthesized from furanones 14–15 and 27–29. Furanones (1 mmol) were dissolved in DCM (5 mL) and the solution was cooled in an ice bath before the addition of propylamine (4 eq., 4 mmol), and then the reaction mixture was left to stir for 2 h. The solvent was evaporated and the oil residue was dehydrated with TFA (1 eq.) to give intermediates 30 to 34, which were purified by flash chromatography with 50% dichloromethane in hexane to give intermediate DHPs 30 to 34. Synthetic procedures were reported of other intermediates 16, 17, and 35 previously [7,43].
3.3.3. Sonogashira Coupling Reaction Procedure
A mixture of acetylene (2.5 eq.) and TEA (1 eq.) in THF was purged with argon or nitrogen for 20 min before the addition of the 5-bromo DHPs (1 mmol), CuI (0.1 eq.), and PdCl2(PPh3)2 (0.1 eq.), and the mixture was heated at 60 °C for 18 h. The THF in the reaction mixture was evaporated, redissolved in DCM, washed with 2 M HCl (5 mL × 2), and the organic layer was dried over sodium sulphate. The crude mixtures were purified by flash chromatography using gradient solvent mixture of dichloromethane and hexane (10 to 50% gradient).
3.4. Compounds’ Full Characterizations
5-(Dibromomethylene)-3-ethyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (30)
Yellow semi-solid (30%); 1H NMR (CDCl3, 400 MHz): δ 0.91 (t, J = 8.0 Hz, 3H, CH3), 1.20 (t, J = 8.0 Hz, 3H, CH3), 1.61–1.70 (m, 2H, CH2), 2.34–2.38 (m, 2H, CH2), 3.93–3.97 (m, 2H, CH2), 7.02 (s, 1H, C4-H); 13C NMR (CDCl3, 100 MHz): δ 10.8 (CH3), 11.7 (CH3), 18.9 (CH2), 23.4 (CH2), 42.3 (CH2), 73.5 (C), 131.3 (CH), 140.2 (C), 140.7 (C), 171.9 (C=O); IR (ATR): νmax 753, 878, 1044, 1451, 1688, 2925, 2967 cm−1; UV-VIS (MeOH): λmax 285 nm (ε 13,859 cm−1M−1); HRMS (C10H1379Br2NO) calcd m/z 343.9256 [M + Na]+, obsd m/z 343.9258 [M + Na]+ and HRMS (C10H1381Br2NO) calcd m/z 347.9215 [M + Na]+, obsd m/z 349.9215 [M + Na]+.
3-Butyl-5-(Dibromomethylene)-1-propyl-1,5-dihydro-2H-pyrrol-2-one (31)
Yellow semi-solid (61%); 1H NMR (CDCl3, 400 MHz): δ 0.94–0.98 (m, 6H, CH3 × 2), 1.28–1.44 (m, 2H, CH2), 1.54–1.70 (m, 6H, CH2 × 3), 2.32–2.36 (m, 2H, CH2), 3.93–3.95 (m, 2H, CH2) 7.02 (s, 1H, C4-H); 13C NMR (CDCl3, 100 MHz): δ 10.8 (CH3), 13.8 (CH3), 22.4 (CH2), 23.4 (CH2), 25.2 (CH2), 29.6 (CH2), 42.3 (CH2), 73.1(C), 131.8 (CH), 138.8 (C), 140.7 (C), 172.1 (C); IR (ATR): νmax 694, 851, 1188, 1345, 1508, 1709, 3171 cm-−1; UV-VIS (MeOH): λmax 285 nm (ε 13,920 cm−1M−1); HRMS (C12H1779Br2NO) calcd m/z 349.9750 [M + H]+, obsd m/z 349.9751 [M + H]+ and HRMS (C12H1781Br2NO) calcd m/z 353.9709 [M + H]+, obsd m/z 353.9709 [M + H]+.
5-(Dibromomethylene)-3-hexyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (32)
Yellow semi-solid (58%); 1H NMR (CDCl3, 400 MHz): δ 0.91 (t, J = 8.0 Hz, 6H, CH3 × 2), 1.30–1.40 (m, 6H, CH2 × 3), 1.54–1.68 (m, 4H, CH2 × 2), 2.31–2.35 (m, 2H, CH2), 3.93–3.97 (m, 2H, CH2), 7.02 (s, C4-H); 13C NMR (CDCl3, 100 MHz): δ 10.9 (CH3), 14.1 (CH3), 22.5 (CH2), 23.4 (CH2), 25.5 (CH2), 27.5 (CH2), 28.9 (CH2), 31.5 (CH2), 42.3 (CH2), 70.5 (C), 131.8 (CH), 138.9 (C), 140.7 (C), 172.1 (C=O); IR (ATR): νmax 753, 816, 1172, 1440, 1594, 1694, 2923 cm−1; UV-VIS (MeOH): λmax 285 nm (ε 53,601 cm−1M−1); HRMS (C14H21Br2NO) calcd m/z 378.0063 [M + H]+, obsd m/z 378.0065 [M + H]+ and HRMS (C14H2181Br2NO) calcd m/z 382.0022 [M + H]+, obsd m/z 382.0023 [M + H]+.
5-(Dibromomethylene)-3-heptyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (33)
Yellow semi-solid (87%); 1H NMR (CDCl3, 400 MHz): δ 0.90–0.93 (m, 6H, CH3), 1.28–1.36 (m, 10H, CH2), 1.61–1.66 (m, 2H, CH2) 2.31–2.36 (m, 2H, CH2), 3.95 (t, J = 8.0 Hz, CH2), 7.02 (s, 1H, CH); 13C NMR (CDCl3, 100 MHz): δ 10.9 (CH3), 14.1 (CH3), 22.6 (CH2), 23.4 (CH2), 25.5 (CH2), 27.5 (CH2), 28.9 (CH2), 29.3 (CH2), 31.7 (CH2), 42.3 (CH2), 73.5 (C); UV-VIS (MeOH): λmax 285 nm (ε 56,642 cm−1M−1); HRMS (C15H23Br2NO) calcd m/z 392.0219 [M + H]+, obsd m/z 392.0219 [M + H]+ and HRMS (C15H2381Br2NO) calcd m/z 396.0178 [M + H]+, obsd m/z 396.0175 [M + H]+.
5-(Dibromomethylene)-3-octyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (34)
Yellow semi-solid (68%); 1H NMR (CDCl3, 400 MHz): δ 0.90–0.97 (m, 6H, CH3), 1.90–1.93 (m, 12H, CH2), 1.57–2.35 (m, CH2), 2.33 (t, J = 8.0 Hz, 2H, CH2), 3.95 (t, J = 8.0 Hz, 2H, CH2), 7.03 (s, 1H, CH); 13C NMR (CDCl3, 100 MHz): δ 10.9 (CH3), 14.1 (CH3), 22.7 (CH2), 23.4 (CH2), 25.5 (CH2), 27.5 (CH2), 29.2 (CH2), 29.3 (CH2), 31.8 (CH2), 42.4 (CH2), 73.9 (C), 131.9 (CH), 138.8 (C), 140.7 (C), 172.2 (C=O); IR (ATR): νmax 751, 824, 1132, 1197, 1459, 1687, 2922 cm−1; UV-VIS (MeOH): λmax 285 nm (ε 15,452 cm−1M−1); HRMS (C16H2579Br2NO) calcd m/z 406.0376 [M + H]+, obsd m/z 406.0380 [M + H]+ and HRMS (C16H2581Br2NO) calcd m/z 410.0335 [M + H]+, obsd m/z 410.0338 [M + H]+.
DHP Acetylene Analogues
3-Butyl-5-(1,5-diphenylpenta-1,4-diyn-3-ylidene)-1,5-dihydro-2H-pyrrol-2-one (18)
Yellow solid (72%); m.p. 215 °C; 1H NMR (CDCl3, 400 MHz): δ 0.93 (t, J = 8.0 Hz, 3H, CH3), 1.38–1.48 (m, 2H, CH3), 1.58–1.61 (m, 2H, CH2), 2.40–2.42 (q, J = 8.0 Hz, 2H, CH2), 7.11 (s, 1H, C4-H), 7.26–7.38 (m, 6H, ArH), 7.53–7.57 (m, 4H, ArH), 8.06 (s, NH); 13C NMR (CDCl3, 100 MHz): δ 13.9 (CH3), 22.5 (CH2), 25.4 (CH2), 29.9 (CH2), 65.9 (=C-Br2), 83.8 (C), 86.9 (C), 93.5 (C), 97.5 (C), 122.1 (C), 122.5 (C), 128.0 (C), 128.4 (CH), 128.5 (CH), 128.8 (C), 129.2 (C), 131.6 (CH), 131.7 (CH), 141.1 (C), 148.6 (C), 170.6 (C=O); IR (ATR): νmax 752, 848, 1124, 1486, 1685, 3155 cm−1; UV-VIS (MeOH): λmax 390 nm (ε 12,962 cm−1M−1) 295 (10,741); HRMS (C25H21NO) calcd m/z 352.1696 [M + H]+, obsd m/z 352.1695 [M + H]+.
3-butyl-5-(1,5-di-p-tolylpenta-1,4-diyn-3-ylidene)-1,5-dihydro-2H-pyrrol-2-one (19)
Yellow solid (71%); m.p. 148 °C; 1H NMR (CDCl3, 400 MHz): δ 0.97 (t, J = 8.0 Hz, 3H, CH3), 1.41–1.46 (m, 2H, CH2), 1.59–1.65 (m, 4H, CH2), 2.42–2.46 (m, 2H, CH2), 7.13 (s, 1H, C4-H), 7.19–7.21 (m, 4H, ArH), 7.28–7.46 (m, 4H, ArH), 7.87 (s, NH); 13C NMR (CDCl3, 100 MHz): δ 13.8 (CH3), 21.6 (CH2), 22.5 (CH2), 25.4 (CH2), 30.0 (CH2), 83.5 (=C-Br2), 87.4 (C), 87.4 (C), 93.7 (C), 97.8 (C), 119.0 (C), 128.0 (C), 129.2 (CH), 131.5 (CH), 139.1 (C), 139.5 (C), 141.8 (C), 148.0 (C), 170.5 (C=O); IR (ATR): νmax 753, 812, 1090, 1508, 1685, 2959 cm−1; UV-VIS (MeOH): λmax 390 nm (ε 5560 cm−1M−1) 300 (5370); HRMS (C27H25NO) calcd m/z 380.2009 [M + H]+, obsd m/z 380.2009 [M + H]+.
5-(1,5-Diphenylpenta-1,4-diyn-3-ylidene)-3-hexyl-1,5-dihydro-2H-pyrrol-2-one (20)
Yellow solid (62%); m.p. 156 °C; 1H NMR (CDCl3, 400 MHz): δ 0.92 (t, J = 8.0 Hz, 3H, CH3), 1.28–1.40 (m, 6H, CH2), 1.42–1.66 (m, 2H, CH2), 2.42–2.46 (m, 2H, CH2), 7.14 (s, 1H, C4-H), 7.28–7.41 (m, 6H, ArH), 7.56–7.60 (m, 4H, ArH), 8.11 (s, NH); 13C NMR (CDCl3, 100 MHz): δ 14.1 (CH3), 22.5 (CH2), 25.7 (CH2), 27.9 (CH2), 29.1 (CH2), 31.6 (CH2), 83.8 (C), 86.9 (C), 93.5 (C), 97.5 (C), 122.1 (C), 128.0 (C), 128.5 (CH), 128.9 (C), 129.2 (C), 131.6 (CH), 141.1 (C), 148.6 (C), 170.6 (C=O); IR (ATR): νmax 752, 845, 1096, 1370, 1488, 1684, 2918 cm–1; UV-VIS (MeOH): λmax 390 nm (ε 5655 cm−1M−1), 295 (4934); HRMS (C27H25NO) calcd m/z 380.2009 [M + H]+, obsd m/z 380.2010 [M + H]+.
(Z)-4-Phenyl-5-(3-phenylprop-2-yn-1-ylidene)-1,5-dihydro-2H-pyrrol-2-one (24)
Brown semi-solid (90%); 1H NMR (CDCl3, 400 MHz): δ 5.59 (s, 1H, CH), 6.25 (s, 1H, C3-H), 7.37–7.47 (m, 3H, ArH), 7.48–7.56 (m, 7H, ArH), 8.30 (s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 84.9 (C), 92.9 (C), 101.4 (C), 121.6 (ArC), 122.5 (CH), 128.5 (ArCH × 2), 125.6 (ArCH), 128.9 (ArCH × 2), 129.1 (ArCH), 129.7 (ArCH), 131.3 (ArC), 131.7 (ArCH), 145.9 (C), 150.5 (C), 169.5 (C=O); IR (ATR): νmax 691, 758, 1682, 3140 cm—1; UV-VIS (MeOH): λmax 363 nm (ε 29,137 cm−1M−1); HRMS (C19H13NO) calcd m/z 294.0889 [M+Na]+, obsd m/z 294.0887 [M+Na]+.
(Z)-4-(2-Fluorophenyl)-5-(3-phenylprop-2-yn-1-ylidene)-1,5-dihydro-2H-pyrrol-2-one (25)
Red semi-solid (75%); 1H NMR (CDCl3, 400 MHz): δ 5.47 (s, 1H, CH), 6.35 (s, 1H, C3-H), 7.21–7.28 (m, 2H, ArH), 7.38–7.39 (m, 7H, ArH), 7.45–7.53 (m, 4H, ArH), 8.53 (s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 84.8 (C), 92.8 (C), 101.4 (C), 116.3 (ArCH), 118.9 (ArC), 122.4 (CH), 124.2 (ArC), 124.3 (ArCH), 128.0 (ArC), 128.6 (ArCH × 2), 129.1 (ArCH), 130.8 (ArCH), 131.4 (ArCH), 131.8 (ArCH × 2), 143.7 (C), 158.9 (C), 169.5 (C=O); IR (ATR): νmax 745, 831, 1688, 3139 cm–1; UV-VIS (MeOH): λmax 364 nm (ε 36,707 cm−1M−1); HRMS (C19H12F1NO) calcd m/z 312.0795 [M+Na]+, obsd m/z 312.0795 [M+Na]+.
(Z)-4-(4-Bromophenyl)-5-(3-phenylprop-2-yn-1-ylidene)-1,5-dihydro-2H-pyrrol-2-one (26)
Yellow semi-solid (35%); 1H NMR (CDCl3, 400 MHz): δ 5.54 (s, 1H, CH), 6.25 (s, 1H, C3-H), 7.31–7.40 (m, 5H, ArH), 7.51–7.54 (m, 2H, ArH), 7.61–7.65 (m, 2H, ArH), 8.09 (s, 1H, NH); 13C NMR (CDCl3, 100 MHz): δ 84.7 (C), 92.9 (C), 101.7 (C), 121.8 (CH), 122.3 (ArC), 124.3 (ArC), 128.6 (ArCH × 2), 129.2 (ArCH), 130.0 (ArCH × 2), 130.0 (ArC), 131.6 (ArCH), 132.2 (ArCH), 145.5 (C), 149.2 (C), 169.3 (C=O); IR (ATR): νmax 750, 817, 1682, 3138 cm–1; UV-VIS (MeOH): λmax 367 nm (ε 34,700 cm−1M−1); HRMS (C19H1279Br1NO) calcd m/z 371.9995 [M+Na]+, obsd m/z 371.9995 [M+Na]+ and HRMS (C19H1281Br1NO) calcd m/z 373.9974 [M+Na]+, obsd m/z 373.9974 [M+Na]+.
5-(1,5-Diphenylpenta-1,4-diyn-3-ylidene)-3-ethyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (36)
Yellow semi-solid (37%); 1H NMR (CDCl3, 400 MHz): δ 0.93 (t, J = 8.0 Hz, 3H, CH3), 1.23–1.29 (m, 4H, CH3), 1.78–1.83 (m, 2H, CH2), 2.44–2.50 (m, 2H, CH2), 4.12–4.16 (m, 2H, CH2), 7.22 (s, 1H, C4-H), 7.38–7.40 (m, 6H, ArH), 7.52–7.57 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 11.2 (CH3), 11.9 (CH3), 19.0 (CH2), 23.4 (CH2), 42.2 (CH2), 85.4 (C), 86.2 (C), 92.3 (C), 96.2 (C), 122.6 (C), 128.5 (CH), 128.6 (C), 129.0 (C), 131.4 (C), 131.6 (C), 139.7 (CH), 139.7 (C), 148.4 (C), 170.9 (C=O), IR (ATR): νmax 752, 1040, 1154, 1440, 1569, 1689, 2963 cm–1; UV-VIS (MeOH): λmax 395 nm (ε 36,349 cm−1M−1), 295 (31,086); HRMS (C26H23NO) calcd m/z 366.1852 [M + H]+, obsd m/z 366.1854 [M + H]+.
5-(1,5-Di-p-tolylpenta-1,4-diyn-3-ylidene)-3-ethyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (37)
Yellow semi-solid (38%); 1H NMR (CDCl3, 400 MHz): δ 0.92 (t, J = 8.0 Hz, 3H, CH3), 1.22–1.26 (m, 3H, CH3), 1.76–1.82 (m, 2H, CH2), 2.40–2.43 (bs, 6H, CH3), 2.44–2.47 (m, 2H, CH2), 4.11–4.15 (m, 2H, CH2), 7.18–7.22 (m, 5H, C4-H & ArH), 7.42–7.46 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 11.2 (CH3), 12.0 (CH3), 19.0 (CH2), 21.6 (CH2), 23.4 (CH2), 42.2 (CH2), 85.0 (C), 86.7 (C), 87.0 (C), 92.4 (C), 96.5 (C), 119.6 (C), 129.0 (CH), 129.2 (CH), 131.4 (CH), 138.9 (C), 139.4 (C), 147.9 (C), 170.9 (C=O); IR (ATR): νmax 813, 853, 1197, 1442, 1565, 1694, 2924 cm–1; UV-VIS (MeOH): λmax 395 nm (ε 6265 cm−1M−1), 393 (6186); HRMS (C28H27NO) calcd m/z 394.2165 [M + H]+, obsd m/z 394.2165 [M + H]+.
3-Butyl-5-(1,5-diphenylpenta-1,4-diyn-3-ylidene)-1-propyl-1,5-dihydro-2H-pyrrol-2-one (38)
Yellow semi-solid (48%); 1H NMR (CDCl3, 400 MHz): δ 0.91–0.99 (m, 6H, CH3), 1.40–1.46 (m, 2H, CH2), 1.52–1.66 (m, 2H, CH2), 1.78–1.83 (m, 2H, CH2), 2.42–2.46 (m, 2H, CH2), 4.12–4.16 (m, 2H, CH2), 7.22 (s, 1H, C4-H), 7.38–7.40 (m, 6H, ArH), 7.52–7.57 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 11.2 (CH3), 13.9 (CH3), 22.5 (CH2), 23.5 (CH2), 25.4 (CH2), 29.9 (CH2), 42.2 (CH2), 85.5 (C), 86.2 (C), 86.5 (C), 92.2 (C), 96.2 (C), 122.6 (C), 122.8 (C), 128.4 (CH), 128.5 (CH), 128.7 (C), 129.0 (C), 129.7 (C), 131.4 (CH), 131.5 (CH), 138.3 (C), 148.4 (C), 171.1 (C=O); IR (ATR): νmax 752, 1045, 1322, 1487, 1569, 1689, 2956 cm–1; UV-VIS (MeOH): λmax 390 nm (ε 30,999 cm−1M−1), 300 (26,121); HRMS (C28H27NO) calcd m/z 394.2165 [M + H]+, obsd m/z 394.2164 [M + H]+.
3-Butyl-5-(1,5-di-p-tolylpenta-1,4-diyn-3-ylidene)-1-propyl-1,5-dihydro-2H-pyrrol-2-one (39)
Yellow semi-solid (15%); 1H NMR (CDCl3, 400 MHz): δ 0.95–0.99 (m, 6H, CH3), 1.40–1.45 (m, 2H, CH2), 1.60–1.66 (m, 2H, CH2), 1.76–1.82 (m, 2H, CH2), 2.40 (s, 6H, CH3), 2.41–2.47 (m, 2H, CH2), 4.11–4.16 (m, 2H, CH2), 7.18–24 (m, 5H, ArH & C4-H), 7.33–7.62 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 11.2 (CH3), 13.9 (CH3), 21.5 (CH2), 21.6 (CH2), 22.5 (CH2), 23.4 (CH2), 25.4 (CH2), 30.0 (CH2), 42.2 (CH2), 85.0 (C), 85.7 (C), 86.9 (C), 92.4 (C), 96.5 (C), 119.6 (C), 119.8 (C), 129.2 (CH), 129.2 (CH), 129.5 (C), 129.6 (C), 129.7 (C), 131.3 (CH), 131.5 (CH), 141.4 (C), 147.9 (C), 171.1 (C=O); IR (ATR): νmax 812, 1019, 1170, 1440, 1507, 1689, 2956 cm–1; UV-VIS (MeOH): λmax 400 nm (ε 25,717 cm−1M−1), 300 (25,464); HRMS (C30H31NO) calcd m/z 422.2478 [M + H]+, obsd m/z 422.2475 [M + H]+.
5-(1,5-Diphenylpenta-1,4-diyn-3-ylidene)-3-hexyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (40)
Yellow semi-solid (39%); 1H NMR (CDCl3, 400 MHz): δ 0.91–0.98 (m, 6H, CH3), 1.32–1.42 (m, 6H, CH2), 1.60–1.65 (m, 2H, CH2), 1.78–1.83 (m, 2H, CH2), 2.41–2.45 (m, 2H, CH2), 4.12–4.16 (m, 2H, CH2), 7.22 (s, 1H, C4-H), 7.38–7.40 (m, 6H, ArH), 7.52–7.58 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 11.2 (CH3), 14.1 (CH3), 22.6 (CH2), 23.5 (CH2), 25.7 (CH2), 27.8 (CH2), 29.1 (CH2), 31.6 (CH2), 42.3 (CH2), 85.5 (C), 86.2 (C), 86.5 (C), 92.2 (C), 96.2 (C), 122.6 (C), 122.8 (C), 128.4 (CH), 128.5 (CH), 128.7 (C), 129.0 (C), 129.6 (C), 131.4 (CH), 131.6 (CH), 138.4 (C), 148.4 (C), 171.1 (C=O); IR (ATR): νmax 752, 1096, 1154, 1441, 1569, 1690, 2925 cm–; UV-VIS (MeOH): λmax 390 nm (ε 34,662 cm−1M−1), 300 (29,285); HRMS (C30H31NO) calcd m/z 422.2478 [M + H]+, obsd m/z 422.2475 [M + H]+.
5-(1,5-Diphenylpenta-1,4-diyn-3-ylidene)-3-heptyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (41)
Yellow semi-solid (96%); 1H NMR (CDCl3, 400 MHz): δ 0.89–0.94 (m, 6H, CH3), 1.30–1.34 (m, 8H, CH2), 1.36–1.42 (m, 2H, CH2), 1.59–1.83 (m, 2H, CH2), 2.41–2.45 (m, 2H, CH2), 4.12–4.16 (m, 2H, CH2), 7.22 (s, 1H, C4-H), 7.38–7.40 (m, 6H, ArH), 7.52–7.57 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 11.2 (CH3), 14.1 (CH3), 22.7 (CH2), 23.4 (CH2), 25.7 (CH2), 27.9 (CH2), 29.0 (CH2), 29.4 (CH2), 31.7 (CH2), 42.2 (CH2), 85.5 (C), 86.2 (C), 86.5 (C), 92.2 (C), 96.2 (C), 122.6 (C), 122.8 (C), 128.4 (CH), 128.5 (CH), 128.7 (C), 129.0 (C), 129.6 (C), 131.4 (CH), 131.6 (CH), 138.4 (C), 148.4 (C), 171.1 (C=O); IR (ATR): νmax 752, 1096, 1153, 1322, 1441, 1570, 1693, 2924 cm–1; UV-VIS (MeOH): λmax 390 nm (ε 26,340 cm−1M−1), 295 (24,641); HRMS (C31H33NO) calcd m/z 436.2635 [M + H]+, obsd m/z 436.2633 [M + H]+.
5-(1,5-Diphenylpenta-1,4-diyn-3-ylidene)-3-octyl-1-propyl-1,5-dihydro-2H-pyrrol-2-one (42)
Yellow semi-solid (30%); 1H NMR (CDCl3, 400 MHz): δ 0.92–0.94 (m, 6H, CH3), 1.30–1.40 (m, 10H, CH2), 1.60–1.65 (m, 2H, CH2), 1.78–1.83 (m, 2H, CH2), 2.41–2.45 (m, 2H, CH2), 4.12–4.16 (m, 2H, CH2), 7.22 (s, 1H, C4-H), 7.38–7.40 (m, 6H, ArH), 7.52–7.57 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 11.2 (CH3), 14.1 (CH3), 22.7 (CH2), 23.5 (CH2), 25.7 (CH2), 27.9 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2), 31.8 (CH2), 42.2 (CH2), 85.5 (C), 86.2 (C), 86.5 (C), 92.2 (C), 96.2 (C), 122.6 (C), 122.8 (C), 128.4 (CH), 128.5 (CH), 128.7 (C), 129.0 (C), 129.6 (C), 131.4 (CH), 131.6 (CH), 138.4 (C), 148.4 (C), 171.1 (C=O); IR (ATR): νmax 752, 1096, 1322, 1440, 1570, 1692, 2923 cm–1; UV-VIS (MeOH): λmax 395 nm (ε 47,392 cm−1M−1), 300 (38,669); HRMS (C32H35NO) calcd m/z 450.2791 [M + H]+, obsd m/z 450.2788 [M + H]+.
1,3-Dibutyl-5-(1,5-diphenylpenta-1,4-diyn-3-ylidene)-1,5-dihydro-2H-pyrrol-2-one (43)
Yellow semi-solid (78%); 1H NMR (CDCl3, 400 MHz): δ 0.90–0.99 (m, 6H, CH3), 1.28–1.48 (m, 4H, CH2), 1.56–1.66 (m, 2H, CH2), 1.71–1.79 (m, 2H, CH2), 2.40–2.46 (m, 2H, CH2), 4.15–4.19 (m, 2H, CH2), 7.22 (m, 1H, C4-H), 7.36–7.40 (m, 6H, ArH), 7.51–7.58 (m, 4H, ArH); 13C NMR (CDCl3, 100 MHz): δ 13.8 (CH3), 13.9 (CH3), 20.0 (CH2), 22.5 (CH2), 22.4 (CH2), 29.9 (CH2), 32.4 (CH2), 40.6 (CH2), 85.5 (C), 86.2 (C), 86.5 (C), 92.2 (C), 96.2 (C), 122.6 (C), 122.8 (C), 128.4 (CH), 128.5 (CH), 128.7 (C), 129.0 (C), 129.7 (C), 131.4 (CH), 131.6 (CH), 138.3 (C), 148.4 (C), 171.1 (C=O); IR (ATR): νmax 751, 801, 1096, 1154, 1437, 1487, 1570, 1689, 2925 cm−1; UV-VIS (MeOH): λmax 385 nm (ε 26,773 cm−1M−1), 300 (25,306); HRMS (C29H29NO) calcd m/z 408.2322 [M + H]+, obsd m/z 408.2323 [M + H]+.
3-Butyl-5-(penta-1,4-diyn-3-ylidene)-1-propyl-1,5-dihydro-2H-pyrrol-2-one (44)
Yellow semi-solid (31%); 1H NMR (CDCl3, 400 MHz): δ 0.86–0.95 (m, 6H, CH3), 1.35–1.40 (m, 2H, CH2), 1.52–1.68 (m, 4H, CH2), 2.35–2.39 (m, 2H, CH2), 3.21 (s, 1H), 3.41 (s, 1H), 3.93–3.97 (m, 2H, CH2), 7.07 (s, 1H, C4-H); 13C NMR (CDCl3, 100 MHz): δ 10.6 (CH3), 13.8 (CH3), 22.4 (CH2), 23.3 (CH2), 25.2 (CH2), 29.7 (CH2), 41.9 (CH2), 79.0 (C), 79.9 (C), 80.4 (C), 83.2 (C), 84.4 (C), 129.6 (CH), 139.2 (C), 151.2 (C), 170.9 (C=O); IR (ATR): νmax 696, 855, 1018, 1169, 1364, 1377, 1457, 1694, 2930, 2959 cm−1; UV-VIS (MeOH): λmax 305 nm (ε 2558 cm−1M−1); HRMS (C16H19NO) calcd m/z 242.1539 [M + H]+, obsd m/z 242.1540 [M + H]+.
4. Conclusions
Fifteen novel alkyne analogues of DHPs with various substitution patterns and aliphatic chain lengths were successfully synthesized via lactamisation and Sonogashira coupling reactions in moderate to high yields. The Sonogashira reaction was carried out with DHPs and alkynes in the presence of CuI, palladium catalyst PdCl2(PPh3)2, and TEA. Biological testing demonstrated that several compounds showed moderate activity against the P. aeruginosa MH602 reporter strain with little bactericidal effect. The present study represents the first application of the Sonogashira reaction to DHP scaffolds for the synthesis of novel bacterial QS inhibitors.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics11020151/s1: Table S1: X-ray Experimental details for compound 18, Table S2: X-ray Experimental details for compound 26, Table S3: Selected geometric parameters, Table S4: Selected hydrogen-bond parameters, File S1: NMR spectra for 1H NMR and 13C NMR.
Author Contributions
Conceptualization, B.A., G.I. and N.K.; methodology, B.A., T.T.Y. and S.S.; resources, N.K. and D.S.B.; X-ray data and analysis, B.A. and M.B.; writing—original draft preparation, B.A. and T.T.Y.; writing—review and editing, B.A. and T.T.Y.; supervision, N.K. and D.S.B.; funding acquisition, B.A., N.K. and D.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Linkage Project grant (LP150100752) and a Discovery Project grant (DP180100845) from the Australian Research Council.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
We thank the NMR and Bioanalytical Mass Spectrometry Facility (BMSF) facilities at UNSW. The authors would like to acknowledge the Saudi Ministry of Education and King Khalid University for an Endowment Fund and financial support given to B. Almohaywi.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Welsh, M.A.; Blackwell, H.E. Chemical probes of quorum sensing: From compound development to biological discovery. FEMS Microbiol. Rev. 2016, 40, 774–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, C.; Greenberg, E.P. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Manny, A.J.; Kjelleberg, S.; Kumar, N.; de Nys, R.; Read, R.W.; Steinberg, P. Reinvestigation of the sulfuric acid-catalysed cyclisation of brominated 2-alkyllevulinic acids to 3-alkyl-5-methylene-2 (5H)-furanones. Tetrahedron 1997, 53, 15813–15826. [Google Scholar] [CrossRef]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Kutty, S.K.; Barraud, N.; Pham, A.; Iskander, G.; Rice, S.A.; Black, D.S.; Kumar, N. Design, synthesis, and evaluation of fimbrolide–nitric oxide donor hybrids as antimicrobial agents. J. Med. Chem. 2013, 56, 9517–9529. [Google Scholar] [CrossRef]
- Wagner, S.; Sommer, R.; Hinsberger, S.; Lu, C.; Hartmann, R.W.; Empting, M.; Titz, A. Novel strategies for the treatment of Pseudomonas aeruginosa infections. J. Med. Chem. 2016, 59, 5929–5969. [Google Scholar] [CrossRef]
- Li, W.-R.; Lin, S.T.; Hsu, N.-M.; Chern, M.-S. Efficient total synthesis of pulchellalactam, a CD45 protein tyrosine phosphatase inhibitor. J. Org. Chem. 2002, 67, 4702–4706. [Google Scholar] [CrossRef]
- Mase, N.; Nishi, T.; Takamori, Y.; Yoda, H.; Takabe, K. First synthesis of (R)-(−)-5-hydroxy-3-methyl-3-pyrrolin-2-one (jatropham) by lipase-catalyzed kinetic resolution. Tetrahedron Asymmetry 1999, 10, 4469–4471. [Google Scholar] [CrossRef]
- Yates, E.A.; Philipp, B.; Buckley, C.; Atkinson, S.; Chhabra, S.R.; Sockett, R.E.; Goldner, M.; Dessaux, Y.; Cámara, M.; Smith, H. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 2002, 70, 5635–5646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byers, J.T.; Lucas, C.; Salmond, G.P.; Welch, M. Nonenzymatic turnover of an Erwinia carotovora quorum-sensing signaling molecule. J. Bacteriol. 2002, 184, 1163–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, T.; Gahan, C.G.; O’Sullivan, T.P. Structure–activity relationships of furanones, dihydropyrrolones and thiophenones as potential quorum sensing inhibitors. Future Med. Chem. 2020, 12, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Almohaywi, B.; Taunk, A.; Wenholz, D.S.; Nizalapur, S.; Biswas, N.N.; Ho, K.K.; Rice, S.A.; Iskander, G.; Black, D.S.; Griffith, R. Design and synthesis of lactams derived from mucochloric and mucobromic acids as Pseudomonas aeruginosa quorum sensing inhibitors. Molecules 2018, 23, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almohaywi, B.; Yu, T.T.; Iskander, G.; Chan, D.S.; Ho, K.K.; Rice, S.; Black, D.S.; Griffith, R.; Kumar, N. Dihydropyrrolones as bacterial quorum sensing inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.-K.; Gardner, C.R.; Sekhar, K.V.C.; Biswas, N.N.; Nizalapur, S.; Rice, S.A.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis, quorum sensing inhibition and docking studies of 1, 5-dihydropyrrol-2-ones. Bioorg. Med. Chem. 2015, 23, 7366–7377. [Google Scholar] [CrossRef]
- Sabir, S.; Suresh, D.; Subramoni, S.; Das, T.; Bhadbhade, M.; Black, D.S.; Rice, S.A.; Kumar, N. Thioether-linked dihydropyrrol-2-one analogues as PqsR antagonists against antibiotic resistant Pseudomonas aeruginosa. Bioorg. Med. Chem. 2021, 31, 115967. [Google Scholar] [CrossRef]
- Sabir, S.; Yu, T.T.; Kuppusamy, R.; Almohaywi, B.; Iskander, G.; Das, T.; Willcox, M.D.; Black, D.S.; Kumar, N. Novel Seleno-and Thio-urea-Containing Dihydropyrrol-2-one Analogues as Antibacterial Agents. Antibiotics 2021, 10, 321. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Khan, S.A.; Mujeeb, M.; Bhandari, A. Design, synthesis, molecular properties and antimicrobial activities of some novel 2 (3H) pyrrolone derivatives. J. Saudi Chem. Soc. 2015, 19, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Husain, A.; Alam, M.M.; Shaharyar, M.; Lal, S. Antimicrobial activities of some synthetic butenolides and their pyrrolone derivatives. J. Enzym. Inhib. Med. Chem. 2010, 25, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Abdelbaset, M.S.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Ramadan, M.; Abdelrahman, M.H. Pyridazinones and pyrrolones as promising Scaffolds in Medicinal Chemistry. J. Adv. Biomed. Pharm. Sci. 2019, 2, 19–28. [Google Scholar] [CrossRef]
- Koz’minykh, V.; Igidov, N.; Zykova, S.; Kolla, V.; Shuklina, N.; Odegova, T. Synthesis and pharmacological activity of 3-hydroxy-1, 5-diaryl-4-pivaloyl-2, 5-dihydro-2-pyrrolones. Pharm. Chem. J. 2002, 36, 188–191. [Google Scholar] [CrossRef]
- Miranda, A.C.; Barbosa, L.C.; Masood, M.A.; Varejao, J.O.; Sordi, M.; Benfatti, C.S.A.; Pimenta, A.L. Inhibitory Effect on Biofilm Formation of Pathogenic Bacteria Induced by Rubrolide Lactam Analogues. ACS Omega 2018, 3, 18475–18480. [Google Scholar] [CrossRef]
- Pereira, U.; Moreira, T.; Barbosa, L.; Maltha, C.; Bomfim, I.; Maranhão, S.; Moraes, M.; Pessoa, C.; Barros-Nepomuceno, F. Rubrolide analogues and their derived lactams as potential anticancer agents. MedChemComm 2016, 7, 345–352. [Google Scholar] [CrossRef]
- Pereira, U.A.; Barbosa, L.C.; Demuner, A.J.; Silva, A.A.; Bertazzini, M.; Forlani, G. Rubrolides as model for the development of new lactones and their aza analogs as potential photosynthesis inhibitors. Chem. Biodivers. 2015, 12, 987–1006. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, P.; Qin, Y.; Zhang, N.; Teng, Y.; Venter, H.; Ma, S. Design and synthesis of aryl-substituted pyrrolidone derivatives as quorum sensing inhibitors. Bioorg. Chem. 2020, 105, 104376. [Google Scholar] [CrossRef]
- Maretina, I.A.; Trofimov, B.A. Enediyne antibiotics and their models: New potential of acetylene chemistry. Russ. Chem. Rev. 2006, 75, 825–845. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef] [Green Version]
- Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in organic synthesis: Recent progress and new uses. Molecules 2018, 23, 2442. [Google Scholar] [CrossRef] [Green Version]
- Wermuth, C.G. The Practice of Medicinal Chemistry; Academic Press: Cambridge, MA, USA, 2015; pp. 319–357. [Google Scholar]
- Boukouvalas, J.; Côté, S.; Ndzi, B. Facile access to 4-(1-alkynyl)-2 (5H)-furanones by Sonogashira coupling of terminal acetylenes with β-tetronic acid bromide: Efficient synthesis of cleviolide. Tetrahedron Lett. 2007, 48, 105–107. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Bellina, F.; Falchi, E.; Rossi, R. Regioselective synthesis of cytotoxic 4-(1-alkynyl)-substituted 2-(5H)-furanones. Tetrahedron 2003, 59, 9091–9100. [Google Scholar] [CrossRef]
- Bonacorso, H.G.; Rodrigues, M.B.; Feitosa, S.C.; Coelho, H.S.; Alves, S.H.; Keller, J.T.; Rosa, W.C.; Ketzer, A.; Frizzo, C.P.; Martins, M.A. Synthesis and antimicrobial screening of 2-alkyl (aryl)-7-chloro-6-fluoro-4-(trifluoromethyl)-quinolines and their phenylacetylene derivatives, promoted by Sonogashira cross-coupling reaction. J. Fluor. Chem. 2018, 205, 49–57. [Google Scholar] [CrossRef]
- Richards, J.J.; Melander, C. Synthesis of a 2-aminoimidazole library for antibiofilm screening utilizing the Sonogashira reaction. J. Org. Chem. 2008, 73, 5191–5193. [Google Scholar] [CrossRef]
- Wang, D.; Gao, S. Sonogashira coupling in natural product synthesis. Org. Chem. Front. 2014, 1, 556–566. [Google Scholar] [CrossRef]
- Biswas, N.N.; Iskander, G.M.; Mielczarek, M.; Yu, T.T.; Black, D.S.; Kumar, N. Alkyne-substituted fimbrolide analogues as novel bacterial quorum-sensing inhibitors. Aust. J. Chem. 2018, 71, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Goh, W.K.; Iskander, G.; Black, D.S.; Kumar, N. An efficient lactamization of fimbrolides to novel 1, 5-dihydropyrrol-2-ones. Tetrahedron Lett. 2007, 48, 2287–2290. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Almohaywi, B.; Iskander, G.; Black, D.; Kumar, N. Antimicrobial Discovery, Development, Stewardship and Susceptibility Testing. In Proceedings of the 1st International Electronic Conference on Antibiotics—The Equal Power of Antibiotics and Antimicrobial Resistance, Online, 8–17 May 2021; Volume 68. [Google Scholar]
- Almohaywi, B.; Iskander, G.; Yu, T.T.; Bhadbhade, M.; Black, D.S.; Kumar, N. Copper-mediated Chan-Evans-Lam N-arylation of 5-methylene-4-aryl-1, 5-dihydro-2H-pyrrol-2-one derivatives. Tetrahedron Lett. 2018, 59, 811–814. [Google Scholar] [CrossRef]
- Biswas, N.N.; Kutty, S.K.; Iskander, G.M.; Mielczarek, M.; Bhadbhade, M.M.; Gardner, C.R.; Black, D.S.; Kumar, N. Synthesis of brominated novel N-heterocycles: New scaffolds for antimicrobial discovery. Tetrahedron 2016, 72, 539–546. [Google Scholar] [CrossRef]
- Kutty, S.K. Novel Nitric Oxide Donors as Antimicrobial Agents. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2012. [Google Scholar]
- Bruker. APEX3, SAINT-Plus and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).