Ethyl Acetate Fraction of Punica granatum and Its Galloyl-HHDP-Glucose Compound, Alone or in Combination with Fluconazole, Have Antifungal and Antivirulence Properties against Candida spp.
Abstract
:1. Introduction
2. Results
2.1. In Silico Analysis of the Biological Activities of G-HHDP-G and Its Hepatotoxic Action
2.2. Antifungal Activity
2.3. In Vitro Interaction between PgEA/FCZ and G-HHDP-G/FCZ
2.4. Antibiofilm Effect
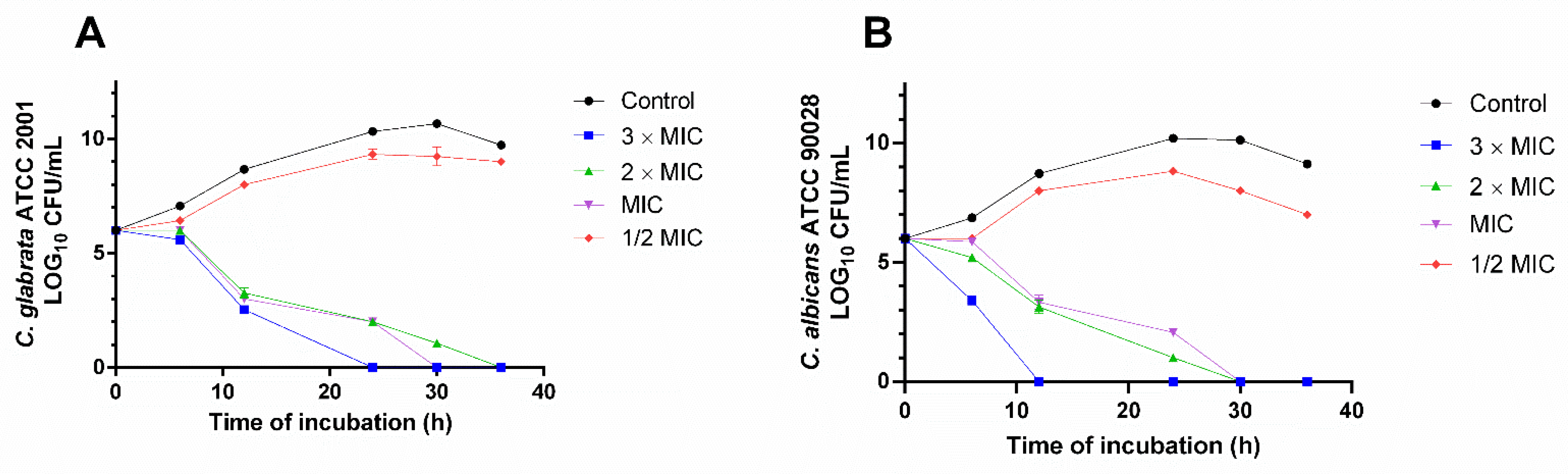
2.5. Time Kill-Curve Assay
2.6. Phospholipase Assay
3. Discussion
4. Materials and Methods
4.1. Preparation of PgEA and Isolation/Identification of G-HHDP-G
4.2. In Silico Analysis
4.2.1. Prediction of the Biological Activities of G-HHDP-G In Silico
4.2.2. In Silico Analysis of G-HHDP-G Hepatic Toxicity
4.3. In Vitro Analysis
4.3.1. Candida Strains and Growth Conditions
4.3.2. MIC Determination
4.3.3. In Vitro Interaction Assays between PgEA + FCZ and G-HHDP-G + FCZ
4.3.4. Effect of PgEA and G-HHDP-G on Candida Biofilms
4.3.5. Time Kill-Curve Assay
4.3.6. PgEA, G-HHDP-G, and FCZ Interference in Phospholipase Production
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Althaus, V.A.; Regginato, A.; Bossetti, V.; Schmidt, J.C. Espécies Se Candida spp. Em Isolados Clínicos e Suscetibilidade a Antifúngicos de Uso Hospitalar. Saúde e Pesqui. 2015, 8, 7. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Khan, I.; Shukla, S.; Kumar, P.; Rather, I.A.; Park, Y.H.; Huh, Y.S.; Han, Y.K. Invasive Fungal Infections and Their Epidemiology: Measures in the Clinical Scenario. Biotechnol. Bioprocess Eng. 2019, 24, 436–444. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Fakhim, H.; Vaezi, A.; Javidnia, J.; Nasri, E.; Mahdi, D.; Diba, K.; Badali, H. Candida Africana Vulvovaginitis: Prevalence and Geographical Distribution. J. Mycol. Med. 2020, 30, 100966. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Noverr, M.C. The Emerging World of the Fungal Microbiome. Trends Microbiol. 2013, 21, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Cerdeira, C.; Gregorio, M.C.; Molares-Vila, A.; López-Barcenas, A.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Sánchez-Blanco, E.; Arenas-Guzmán, R.; Hernandez-Castro, R. Biofilms and Vulvovaginal Candidiasis. Colloids Surf. B Biointerfaces 2019, 174, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.S.; Braga, K.R.G.S.; Vieira, C.A.; Souza, W.W.R.; Chávez-Pavoni, J.H.; Araújo, C.d.; Goulart, L.S. Genotypic Analysis of Secreted Aspartyl Proteinases in Vaginal Candida Albicans Isolates. J. Bras. Patol. e Med. Lab. 2018, 54, 28–33. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; de Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catania, M.R. Antifungal and Anti-Biofilm Activity of the First Cryptic Antimicrobial Peptide from an Archaeal Protein against Candida spp. Clinical Isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida Albicans Targets That Potentially Synergize with Fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef]
- Dantas-Medeiros, R.; Zanatta, A.C.; de Souza, L.B.F.C.; Fernandes, J.M.; Amorim-Carmo, B.; Torres-Rêgo, M.; Fernandes-Pedrosa, M.d.F.; Vilegas, W.; Araújo, T.A.d.S.; Michel, S.; et al. Antifungal and Antibiofilm Activities of B-Type Oligomeric Procyanidins From Commiphora Leptophloeos Used Alone or in Combination with Fluconazole against Candida spp. Front. Microbiol. 2021, 12, 613155. [Google Scholar] [CrossRef] [PubMed]
- Fuentefria, A.M.; Pippi, B.; Dalla Lana, D.F.; Donato, K.K.; de Andrade, S.F. Antifungals Discovery: An Insight into New Strategies to Combat Antifungal Resistance. Lett. Appl. Microbiol. 2018, 66, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The Global Problem of Antifungal Resistance: Prevalence, Mechanisms, and Management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Antifungal Resistance: Current Trends and Future Strategies to Combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal Activity of Natural Compounds vs. Candida Spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics 2022, 11, 73. [Google Scholar] [CrossRef]
- Degaspari, C.H.; Dutra, A.P.C. Propriedades fitoterápicas da romã (Punica granatum L.). Visão Acadêmica 2011, 12, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Lavaee, F.; Motaghi, D.; Jassbi, A.R.; Jafarian, H.; Ghasemi, F.; Badiee, P. Antifungal Effect of the Bark and Root Extracts of Punica Granatum on Oral Candida Isolates. Curr. Med. Mycol. 2018, 4, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.J.M.C.R.; Gonçalves, J.S.; Dourado, Á.W.A.; De Sousa, E.M.; Brito, N.M.; Silva, L.K.; Batista, M.C.A.; De Sá, J.C.; Monteiro, C.R.A.V.; Fernandes, E.S.; et al. Punica granatum L. Leaf Extract Attenuates Lung Inflammation in Mice with Acute Lung Injury. J. Immunol. Res. 2018, 2018, 6879183. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, A.J.M.C.R.; Mendes, A.R.S.; Neves, M.D.F.d.J.; Prado, C.M.; Bittencourt-Mernak, M.I.; Santana, F.P.R.; Lago, J.H.G.; de Sá, J.C.; da Rocha, C.Q.; de Sousa, E.M.; et al. Galloyl-Hexahydroxydiphenoyl (HHDP)-Glucose Isolated from Punica granatum L. Leaves Protects against Lipopolysaccharide (LPS)-Induced Acute Lung Injury in BALB/c Mice. Front. Immunol. 2019, 10, 1978. [Google Scholar] [CrossRef] [Green Version]
- Lansky, E.P.; Newman, R.A. Punica Granatum (Pomegranate) and Its Potential for Prevention and Treatment of Inflammation and Cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Esmat, A. Hepatoprotective and Antioxidant Effect of Ellagitannins and Galloyl Esters Isolated from Melaleuca Styphelioides on Carbon Tetrachloride-Induced Hepatotoxicity in HepG2 Cells. Pharm. Biol. 2016, 54, 1727–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, K.T. In Silico Prediction of Toxicity and Its Applications for Chemicals at Work. Toxicol. Environ. Health Sci. 2020, 12, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bag, A.; Chattopadhyay, R.R. Synergistic Antibiofilm Efficacy of a Gallotannin 1,2,6-Tri-O-Galloyl-β-D-Glucopyranose from Terminalia Chebula Fruit in Combination with Gentamicin and Trimethoprim against Multidrug Resistant Uropathogenic Escherichia Coli Biofilms. PLoS ONE 2017, 12, e0178712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, G.C.d.O.; Vasconcelos, C.C.; Lopes, A.J.O.; Cartágenes, M.d.S.d.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Prem Kumar, K.; Samlin, S.; Siva, B.; Sudharshan, R.; Vignesswary, A.; Divya, K. Punica Granatum as a Salutiferous Superfruit in the Treatment of Oral Candidiasis—An in-Vitro Study. J. Oral Maxillofac. Pathol. 2020, 24, 188. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols Profile of Pomegranate Leaves and Their Role in Green Synthesis of Silver Nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Brighenti, V.; Iseppi, R.; Pinzi, L.; Mincuzzi, A.; Ippolito, A.; Messi, P.; Sanzani, S.M.; Rastelli, G.; Pellati, F. Antifungal Activity and DNA Topoisomerase Inhibition of Hydrolysable Tannins from Punica granatum L. Int. J. Mol. Sci. 2021, 22, 4175. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.C.; de Ávila, R.I.; Zara, A.L.S.A.; Santos, A.S.; Ataídes, F.; Freitas, V.A.Q.; Costa, C.R.; Valadares, M.C.; Silva, M.d.R.R. Punicalagin Triggers Ergosterol Biosynthesis Disruption and Cell Cycle Arrest in Cryptococcus Gattii and Candida Albicans: Action Mechanisms of Punicalagin against Yeasts. Braz. J. Microbiol. 2020, 51, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of Punicalagin as the Bioactive Compound behind the Antimicrobial Activity of Pomegranate (Punica granatum L.) Peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, T.; Wang, D.; Yang, Y.; Sun, W.; Liu, J.; Sun, S. Synergistic Antifungal Effect of Fluconazole Combined with Licofelone against Resistant Candida Albicans. Front. Microbiol. 2017, 8, 2101. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Endo, E.H.; Garcia Cortez, D.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Potent Antifungal Activity of Extracts and Pure Compound Isolated from Pomegranate Peels and Synergism with Fluconazole against Candida Albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; Ishikiriama, B.L.C.; Ribeiro Lopes, M.M.; de Castro, R.D.; Garcia, C.R.; Porto, V.C.; Santos, C.F.; Neppelenbroek, K.H.; Lara, V.S. Antifungal Activity of Punicalagin–Nystatin Combinations against Candida Albicans. Oral Dis. 2020, 26, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; de Moura, M.C.; Gomes, F.S.; da Silva Trentin, D.; Silva de Oliveira, A.P.; de Mello, G.S.V.; da Rocha Pitta, M.G.; de Melo Rego, M.J.B.; Coelho, L.C.B.B.; Macedo, A.J.; et al. PgTeL, the Lectin Found in Punica Granatum Juice, Is an Antifungal Agent against Candida Albicans and Candida Krusei. Int. J. Biol. Macromol. 2018, 108, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida Albicans-Biology, Molecular Characterization, Pathogenicity, and Advances in Diagnosis and Control—An Update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida Glabrata, Candida Parapsilosis and Candida Tropicalis: Biology, Epidemiology, Pathogenicity and Antifungal Resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, P.P.; Rossoni, R.D.; de Souza, C.M.; Scorzoni, L.; Fenley, J.D.C.; Junqueira, J.C. Candida Biofilms: An Update on Developmental Mechanisms and Therapeutic Challenges. Mycopathologia 2020, 185, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Bakkiyaraj, D.; Nandhini, J.R.; Malathy, B.; Pandian, S.K. The Anti-Biofilm Potential of Pomegranate (Punica granatum L.) Extract against Human Bacterial and Fungal Pathogens. Biofouling 2013, 29, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.L.M.; Saldanha, L.L.; da Silva, R.A.; Pinke, K.H.; da Costa, E.F.; Porto, V.C.; Dokkedal, A.L.; Lara, V.S. Antimicrobial Activity of Denture Adhesive Associated with Equisetum Giganteumand Punica Granatum-Enriched Fractions against Candida Albicans Biofilms on Acrylic Resin Surfaces. Biofouling 2018, 34, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villis, P.C.M.; de Macedo, A.T.; Furtado, H.L.A.; Fontenelle, P.H.C.; Gonçalves, I.S.; Mendes, T.L.; Motta, B.L.A.; Marinho, P.L.L.; Pinheiro, A.J.M.C.R.; Lima-Neto, L.G.; et al. A Study of the Disruptive Effect of the Acetate Fraction of Punica Granatum Extract on Cryptococcus Biofilms. Front. Microbiol. 2021, 11, 568258. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida Albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida Albicans Biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zago, P.M.W.; Dos Santos Castelo Branco, S.J.; De Albuquerque Bogéa Fecury, L.; Carvalho, L.T.; Rocha, C.Q.; Madeira, P.L.B.; De Sousa, E.M.; De Siqueira, F.S.F.; Paschoal, M.A.B.; Diniz, R.S.; et al. Anti-Biofilm Action of Chenopodium Ambrosioides Extract, Cytotoxic Potential and Effects on Acrylic Denture Surface. Front. Microbiol. 2019, 10, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriphannam, C.; Nuanmuang, N.; Saengsawang, K.; Amornthipayawong, D.; Kummasook, A. Anti-Fungal Susceptibility and Virulence Factors of Candida spp. Isolated from Blood Cultures. J. Mycol. Med. 2019, 29, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Nciki, S.; Oderinlo, O.O.; Gulube, Z.; Osamudiamen, P.M.; Idahosa, K.C.; Patel, M. Mezoneuron Benthamianum Inhibits Cell Adherence, Hyphae Formation, and Phospholipase Production in Candida Albicans. Arch. Microbiol. 2020, 202, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.C.F.; Pinheiro, A.J.M.C.R.; Araújo, J.G.G.; De Oliveira, R.A.G.; Silva, S.N.; Abreu, I.C.; De Sousa, E.M.; Fernandes, E.S.; Luchessi, A.D.; Silbiger, V.N.; et al. Anti-Inflammatory Effects of a Pomegranate Leaf Extract in LPS-Induced Peritonitis. Planta Med. 2016, 82, 1463–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI Standard M27; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 4th ed. Clinical and Laboratory Standards Institute: Wayne, UK, 2017.
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Gu, W.; Guo, D.; Zhang, L.; Xu, D.; Sun, S. The Synergistic Effect of Azoles and Fluoxetine against Resistant Candida Albicans Strains Is Attributed to Attenuating Fungal Virulence. Antimicrob. Agents Chemother. 2016, 60, 6179–6188. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, D.; Yu, C.; Huang, X.; Li, X.; Sun, S. Strong Synergism of Dexamethasone in Combination with Fluconazole against Resistant Candida Albicans Mediated by Inhibiting Drug Efflux and Reducing Virulence. Int. J. Antimicrob. Agents 2017, 50, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.A.; Gouveia, L.F.; Taylor, E.L.S.; Resende-Stoianoff, M.A.; Pianetti, G.A.; César, I.C.; Santos, D.A. Dynamic Interaction between Fluconazole and Amphotericin B against Cryptococcus Gattii. Antimicrob. Agents Chemother. 2012, 56, 2553–2558. [Google Scholar] [CrossRef] [Green Version]
- Price, M.F.; Wilkinson, A.N.D.; Gentry, L.O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 1982, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]





| Activities | PASS Predictions of G-HHDP-G | |
|---|---|---|
| Pa | Pi | |
| Anti-infective | 0.962 | 0.003 |
| Antioxidant | 0.895 | 0.003 |
| Hepatoprotective | 0.883 | 0.002 |
| Anti-inflammatory | 0.775 | 0.008 |
| Immunostimulant | 0.752 | 0.011 |
| Antifungal | 0.692 | 0.015 |
| Activities | PASS Predictions of FCZ | |
|---|---|---|
| Pa | Pi | |
| Antifungal | 0.726 | 0.008 |
| ATPase inhibitor of phospholipid translocation | 0.480 | 0.069 |
| Cell wall synthesis inhibitor | 0.351 | 0.002 |
| NADPH inhibitor-cytochrome-c2 reductase | 0.366 | 0.134 |
| Cytochrome | Predicted Values of Inhibitory Effect | |
|---|---|---|
| G-HHDP-G | FCZ | |
| CYP1A2 | NT (0.8) * | T (0.606) ** |
| CYP2C19 | NT (0.872) * | NT (0.775) * |
| CYP2C9 | NT (0.796) * | T (0.698) ** |
| CYP2D6 | NT (0.734) * | T (0.502) ** |
| CYP3A4 | NT (0.704) * | T (0.572) ** |
| Strains | MIC (µg/mL) | ||
|---|---|---|---|
| PgEA | G-HHDP-G | FCZ | |
| C. albicans ATCC 90028 | 125 ± 0 | >500 ± 0 | 8 ± 0 |
| C. albicans CAS | 250 ± 0 | >500 ± 0 | 8 ± 0 |
| C. glabrata ATCC 2001 | 31.25 ± 0 | 125 ± 0 | 16 ± 0 |
| C. glabrata FJF | 31.25 ± 0 | 31.25 ± 0 | 4 ± 0 |
| Strain | MIC in Combination (µg/mL) | FICI | |||||
|---|---|---|---|---|---|---|---|
| FCZ | PgEA | G-HHDP-G | FICIFCZ+PgEA | It | FICIFCZ+G-HHDP-G | It | |
| C. albicans ATCC 90028 | 8 | 3.9 | - | 0.32 | SYN | - | - |
| C. albicans CAS | 1 | 7.8 | - | 0.36 | SYN | - | - |
| C. glabrata ATCC 2001 | 4 | 15.6 | 31.2 | 0.45 | SYN | 0.47 | SYN |
| C. glabrata FJF | 0.5 | 7.8 | 7.8 | 0.49 | SYN | 0.37 | SYN |
| Treatments | Precipitation Zone | Phospholipase Activity | ||
|---|---|---|---|---|
| C. Albicans ATCC 90028 | C. Albicans CAS | C. Glabrata ATCC 2001 | ||
| Control | 0.76 | 0.67 | 0.68 | H/VH/VH |
| PgEA MIC | 0.91 | 0.70 | 0.73 | VL/H/H |
| PgEA MIC/2 | 0.93 | 0.73 | 0.75 | VL/H/H |
| G-HHDP-G MIC | --- | --- | 0.76 | H |
| G-HHDP-G MIC/2 | --- | --- | 0.84 | L |
| FCZ MIC | 0.82 | 0.71 | 0.72 | L/H/H |
| FCZ MIC/2 | 0.81 | 0.70 | 0.70 | L/H/H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendonça, A.M.S.; Monteiro, C.d.A.; Moraes-Neto, R.N.; Monteiro, A.S.; Mondego-Oliveira, R.; Nascimento, C.E.C.; da Silva, L.C.N.; Lima-Neto, L.G.; Carvalho, R.C.; de Sousa, E.M. Ethyl Acetate Fraction of Punica granatum and Its Galloyl-HHDP-Glucose Compound, Alone or in Combination with Fluconazole, Have Antifungal and Antivirulence Properties against Candida spp. Antibiotics 2022, 11, 265. https://doi.org/10.3390/antibiotics11020265
Mendonça AMS, Monteiro CdA, Moraes-Neto RN, Monteiro AS, Mondego-Oliveira R, Nascimento CEC, da Silva LCN, Lima-Neto LG, Carvalho RC, de Sousa EM. Ethyl Acetate Fraction of Punica granatum and Its Galloyl-HHDP-Glucose Compound, Alone or in Combination with Fluconazole, Have Antifungal and Antivirulence Properties against Candida spp. Antibiotics. 2022; 11(2):265. https://doi.org/10.3390/antibiotics11020265
Chicago/Turabian StyleMendonça, Aline Michelle Silva, Cristina de Andrade Monteiro, Roberval Nascimento Moraes-Neto, Andrea Souza Monteiro, Renata Mondego-Oliveira, Camila Evangelista Carnib Nascimento, Luís Cláudio Nascimento da Silva, Lidio Gonçalves Lima-Neto, Rafael Cardoso Carvalho, and Eduardo Martins de Sousa. 2022. "Ethyl Acetate Fraction of Punica granatum and Its Galloyl-HHDP-Glucose Compound, Alone or in Combination with Fluconazole, Have Antifungal and Antivirulence Properties against Candida spp." Antibiotics 11, no. 2: 265. https://doi.org/10.3390/antibiotics11020265
APA StyleMendonça, A. M. S., Monteiro, C. d. A., Moraes-Neto, R. N., Monteiro, A. S., Mondego-Oliveira, R., Nascimento, C. E. C., da Silva, L. C. N., Lima-Neto, L. G., Carvalho, R. C., & de Sousa, E. M. (2022). Ethyl Acetate Fraction of Punica granatum and Its Galloyl-HHDP-Glucose Compound, Alone or in Combination with Fluconazole, Have Antifungal and Antivirulence Properties against Candida spp. Antibiotics, 11(2), 265. https://doi.org/10.3390/antibiotics11020265










