Abstract
Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic gram-negative pathogen that can cause various infections, particularly in patients with compromised host defenses. P. aeruginosa forms biofilms and produces virulence factors through quorum sensing (QS) network, resulting in resistance to antibiotics. RhlI/RhlR, one of key QS systems in P. aeruginosa, is considered an attractive target for inhibiting biofilm formation and attenuating virulence factors. Several recent studies examined small molecules targeting the RhlI/RhlR system and their in vitro and in vivo biological activities. In this review, RhlR-targeted modulators, including agonists and antagonists, are discussed with particular focus on structure-activity relationship studies and outlook for next-generation anti-biofilm agents.
1. Introduction
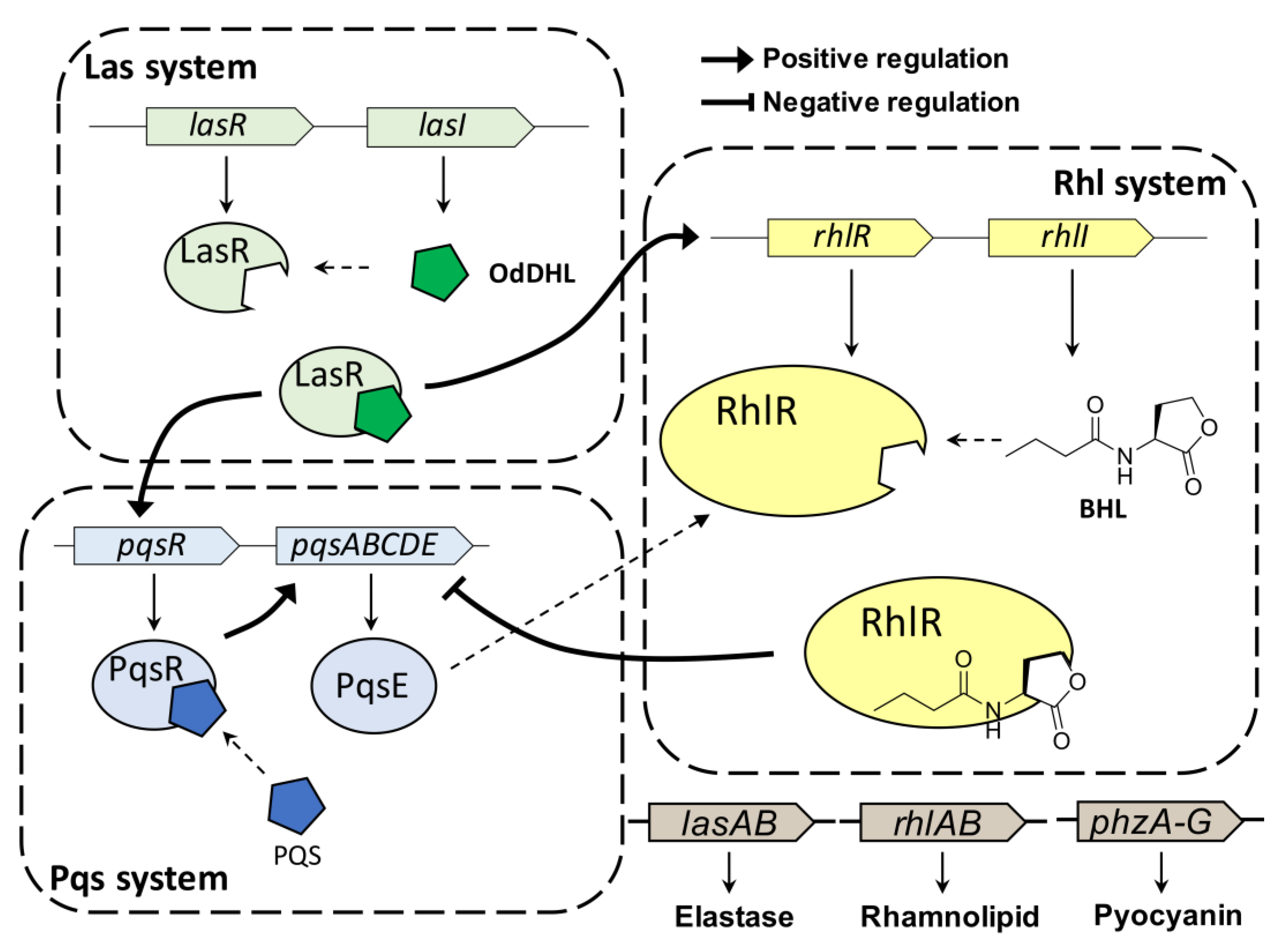
Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic human pathogen that can cause various infections, particularly in patients with compromised host defenses [1]. P. aeruginosa is one of the so-called “ESKAPE” panel pathogens (i.e., Enterococcus facium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) [2]. P. aeruginosa can form biofilms and produce virulence factors through quorum sensing (QS), resulting in resistance to antibiotics and to the host immune response [3]. QS is a cell–cell communication process that allows bacteria to share information on bacterial population density and behave as a community to respond to changes in their environment [4]. This intercellular communication process is controlled by interactions between autoinducers and their cognate receptors. P. aeruginosa has three major cellular communication QS systems (Figure 1), (i.e., LasI/LasR, RhlI/RhlR, and PQS/PqsR), which are tightly interconnected [5]. This QS network of P. aeruginosa affects the production of virulence factors, biofilm formation, and modulation of host immune responses.
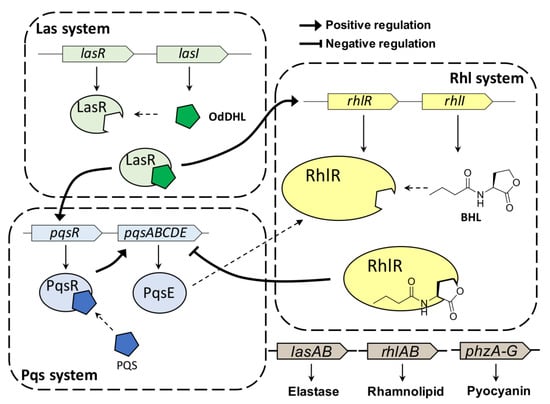
Figure 1.
QS hierarchy in P. aeruginosa. RhlR is controlled in BHL-dependent or BHL-independent manner.
P. aeruginosa uses N-acyl-L-homoserine lactones (AHLs) as QS auto-inducers, similar to other gram-negative bacteria. AHLs are typically produced by LuxI-type synthases and are recognized by the cytoplasmic LuxR-type receptor [6]. P. aeruginosa produces N-(3-oxo-dodecanoyl)-L-homoserine lactone (OdDHL) and N-butyryl-L-homoserine lactone (BHL) for the LasI/LasR and RhlI/RhlR QS systems, respectively [7]. Once the bacteria reach a certain population density threshold, AHLs bind their cognate receptor protein, thereby affecting gene expression through transcriptional activation [8]. In addition to the LasI/LasR and RhlI/RhlR QS systems, the 2-heptyl-3-hydroxy-4(1H)-quinolone (Pseudomonas quinolone signal, PQS) circuit is the third system regulated by PqsR, which relies on PQS. Recently, many studies reported the interaction between Rhl and Pqs systems [9,10,11]. RhlR negatively regulates the expression of pqsABCDE operon independently of PQS production. Additionally, PqsE, the final gene in the operon, activates RhlR. These three QS systems are controlled in a hierarchical fashion in P. aeruginosa, contributing to fighting of them [5].
However, QS has challenges of selectivity, virulence reduction, and lack of resistance against QS inhibitors to reach the treatment of people [12]. The disruption of QS signals affects indirectly or directly the disturbance between microflora QS activity and other QS-mimics dependent host-microbiota signaling [13,14]. Furthermore, P. aeruginosa promotes the development of isolates with an increased survival ability against QS inhibitor and changes their metabolism for developing resistance of QS inhibitor [15,16]. Despite the limitations of QS inhibitors, modulating the QS network between auto-inducers and their cognate receptors is still considered a promising strategy for attenuating virulence factors of P. aeruginosa [17].
The LasI/LasR system is considered a primary target and has been studied extensively because it is located at the top of the P. aeruginosa QS hierarchy [18,19,20]. Although the RhlI/RhlR system also plays an important role in the QS process of P. aeruginosa by utilizing BHL as an autoinducer, only few studies examined RhlR-targeted modulators based on the chemical structure of BHL [21,22]. Increasing research on RhlR-targeted modulators have provided the evidence that the RhlI/RhlR system play unique roles in the QS pathway of P. aeruginosa.
According to the recent report, LasR-mutants occur frequently among environmental and clinical isolates are increasing [23,24]. There are increasing evidence that such LasR-mutants have growth advantage over the wild-type for nutrient available in the infected lungs [24,25]. In addition, many clinically isolated LasR-mutants are still able to produce RhlR-dependent transcription factors [25,26]. More than half of the LasR-mutants retain LasR-independent RhlR activity [27]. Overall, it became clear that LasR-mutants are common in a variety of chronic infections and highlight the importance of RhlR role in chronic P. aeruginosa infections [28]. Furthermore, LasR becomes dispensable in P. aeruginosa when it is cultured in a low phosphate medium, suggesting RhlR is the head of the QS hierarchy under phosphate-limiting conditions [29]. Therefore, small molecule modulators targeting RhlR can be developed as novel therapeutic agents in the control of P. aeruginosa chronic infections.
This review describes structure-activity relationship (SAR) studies of RhlR-targeted agonists and antagonists and discusses RhlR-targeted drug opportunities as anti-biofilm agents. The structural relationship of RhlR-targeted modulators (agonists and antagonists) was analyzed by classifying tail, middle, and head sections, inducing detailed SAR studies compared to the previous RhlR studies [30,31]. Furthermore, the importance of developing RhlR modulators for treating patients infected with P. aeruginosa was emphasized under LasR-mutants and phosphate-limiting conditions.
2. RhlR-Targeted Modulators
2.1. RhlR-Targeted Agonists
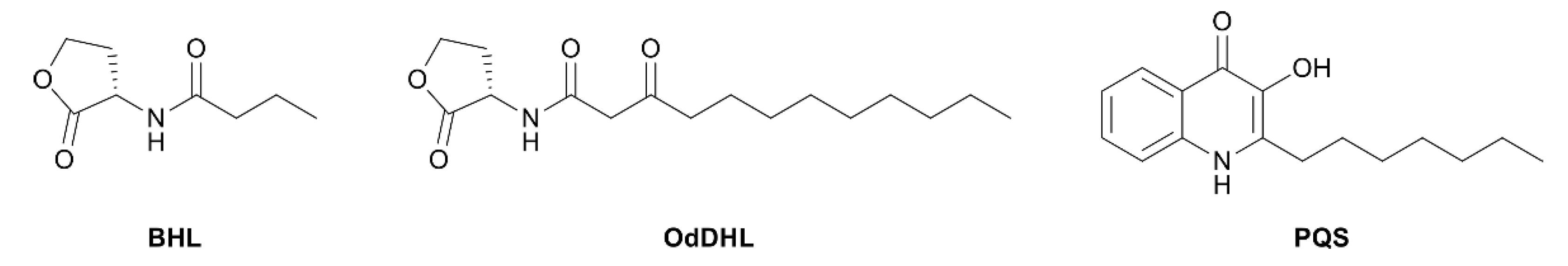
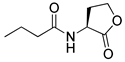
Research on RhlR-targeted modulators has mainly focused on RhlR agonists. The structural scaffold of initial RhlR agonists was based on BHL, a natural auto-inducer of RhlR (Figure 2). BHL possesses an n-butanoyl group at the tail region and a homoserine lactone moiety at the head region with an amide linkage. BHL further comprises a shorter alkyl chain than OdDHL, an auto-inducer responsive to LasR, and PQS, an auto-inducer responsive to PqsR (Figure 2).
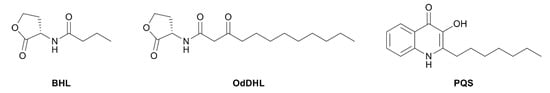
Figure 2.
Chemical structure of auto-inducers BHL, OdDHL and PQS.
Structural modification of BHL-based RhlR agonists has been implemented as follows: replacement of the homoserine lactone ring, variation of the alkyl chain, bioisosterism of the amide linkage, and absolute stereochemistry at the chiral center.
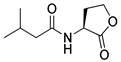
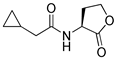
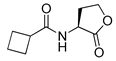
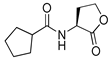
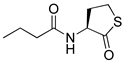
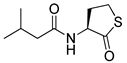
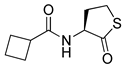
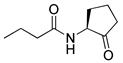
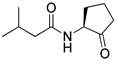
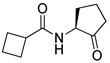
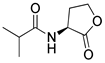
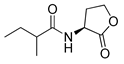
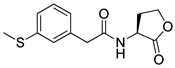
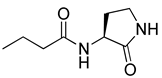
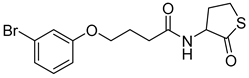
Blackwell and co-workers synthesized various BHL analogs and evaluated their EC50 (the effective concentration of a compound that gives half-maximal response) values using the RhlR reporter systems of E. coli and P. aeruginosa [32]. A dose-response curve of the most active RhlR agonists was analyzed to determine their EC50 values. They analyzed the effect of a branched alkyl chain or a cycloalkane ring at the tail region on RhlR activation. In addition, they evaluated the importance of the homoserine lactone ring at the head region regarding RhlR agonism. The BHL analog (1) with the isovaleryl group at the tail region showed stronger RhlR agonism with an EC50 value of 1.42 μM, compared to BHL (EC50 = 8.08 μM) in the P. aeruginosa reporter system (Table 1). Compound 2 with a cyclopropylacetyl group also showed strong RhlR agonism with an EC50 value of 2.76 μM in the E. coli reporter system. Introduction of cycloalkane ring such as cyclobutane (3, EC50 = 1.41 μM) or cyclopentane (4, EC50 = 1.22 μM) instead of the lactone ring enhanced RhlR agonistic properties compared to BHL in E. coli reporter system. In addition, replacement of the homoserine lactone ring with the homocysteine thiolactone ring (5, EC50 = 3.82 μM) slightly increased RhlR agonism in E. coli reporter. Furthermore, the thiolactone analogs with isovaleryl (6, EC50 = 2.58 μM) or cyclobutanyl (7, EC50 = 1.65 μM) were as potent as the corresponding the lactone analogs (1 and 3) in the P. aeruginosa RhlR reporter assay system [33], implying that the thiolactone ring can be a surrogate of the lactone ring. In particular, the thiolactone analog 6 displayed the strongest RhlR agonism with an EC50 value of 0.46 μM in the E. coli RhlR reporter assay system. When the lactone ring of BHL was replaced by cyclopentanone (8–10), RhlR activities were markedly decreased, compared to the corresponding lactone or thiolactone analogs in E. coli and P. aeruginosa reporter systems [20]. In addition, the reduction of the ketone to alcohol precluded the RhlR agonism, suggesting that the carbonyl group in the ring at the head region is essential for RhlR agonism between two different reporters [21]. Ring expansion from cyclopentanone (8) to cyclohexanone maintained RhlR agonism activity in both systems [21].

Table 1.
RhlR-targeted agonists based on BHL.
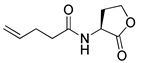
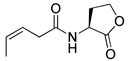
In case of homoserine lactone analogs, the extension of butyl chain to pentenyl chains at the tail region (11 and 12) slightly enhanced RhlR agonism in E. coli. In addition, the methyl branching in the propionyl (13) or butyryl (14) at the tail region showed increased RhlR agonistic activity compared to BHL in E. coli reporter system.
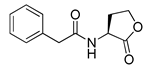
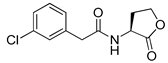
Blackwell et al. conducted comprehensive structure-activity relationship studies of BHL-based RhlR agonists by focusing on the tail region while retaining the homoserine lactone ring in the head region [34]. They introduced the substituted phenylacetyl, the substituted phenylpropionyl group at the tail region, and evaluated RhlR agonism by E. coli as summarized in Table 2.

Table 2.
RhlR-targeted agonists with variation of tail region.
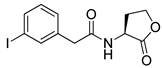
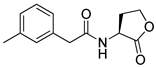
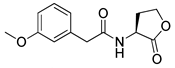
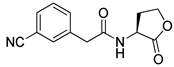
Phenylacetyl analogs (16–21) substituted with electron-withdrawing substituents (-Cl, -I, and -CN) or electron-donating groups (-CH3, -OCH3, and -SCH3) at the meta-position displayed stronger RhlR agonism than compound 15 with no substituent. The electronic effect of the substituent at the m-position had little influence on RhlR activation. In contrast, the position of the substituent significantly affected RhlR activity, making the meta-substituents more potent than para- or ortho-substituents in this series. Among m-substituted phenylacetyl analogs, compound 20 with a -CN group at the m-position was most potent, with an EC50 value of 1.7 μM in the E. coli reporter system. However, this compound showed only approximately 70% of the maximum RhlR activity, compared to BHL. In the case of phenylpropionyl analogs, three compounds (22–24) displayed EC50 values comparable to that of BHL. However, phenylpropionyl analogs, in general, were less potent than the corresponding phenylacetyl analogs, indicating that carbon chain length in the tail region is critical for maintaining and maximizing RhlR agonism. In addition, the phenylpropionyl analogs activated LasR, PqsR, and RhlR, leading to a decrease in RhlR selectivity. Interestingly, phenylacetyl analogs substituted with the bulky group at the para-position turned out to be RhlR antagonists. (See Section 2.2).
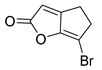
Luk and co-workers reported a non-BHL RhlR agonist. Bicyclic brominated furan compound 25, the so-called 6-bromo-4,5-dihydro-2H-cyclopenta[b]furan-2-one (5-BBF), displayed moderate RhlR agonistic activity in the PA01 system (Table 2) [35]. 5-BBF is the only compound comprising a scaffold that is not related to the homoserine lactone ring, as found in BHL analogs. However, 5-BBF was much less potent than BHL-based RhlR agonists, with an EC50 value of approximately 50 μM. Furthermore, this compound was not effective in inhibiting biofilm formation in P. aeruginosa and E. coli. And 5-BBF showed mild cytotoxic effects on human cells as ~76% of cells survived after 1 h of treatment.
2.2. RhlR-Targeted Antagonists
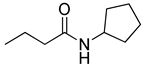
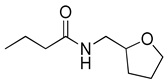
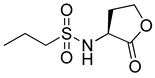
RhlR-targeted antagonists have also been developed based on BHL structure. Replacement of the lactone ring with a cyclopentane (26) or a tetrahydrofurfuryl ring (27) makes the parent molecule an antagonist, as summarized in Table 3. Compounds 26 and 27 showed 45% and 57% inhibition at 1 mM concentration in the presence of 10 μM BHL in the E. coli RhlR reporter assay, respectively [32]. In addition, compound 28 with a γ-lactam ring also showed weak antagonistic activity (35% inhibition). These results suggested that ring variation in the head region influences the properties of agonist or antagonist. With regard to the amide bond variation in the middle region, compound 29 with the sulfonamide linkage was a moderate RhlR antagonist with 55% inhibition. However, the compound with the ester linkage was neither an RhlR agonist nor an RhlR antagonist, implying that the hydrogen-bonding donor N-H is necessary for binding to RhlR in the BHL series [32]. The next modification in antagonists was implemented in the tail region.

Table 3.
RhlR-targeted antagonists.
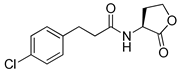
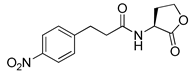
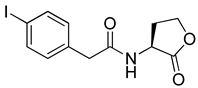
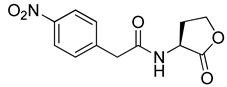
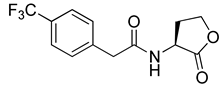
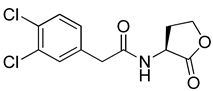
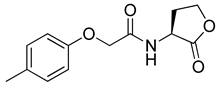
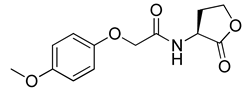
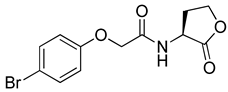
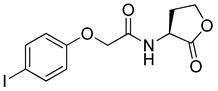
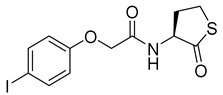
Blackwell and co-workers synthesized and evaluated various phenylacetyl analogs that are bulkier than RhlR agonists with respect to molecular size (Table 4) [34]. Compounds substituted with bulky functional groups such as -I (30), -NO2 (31), -CH3 (32), and -CF3 (33) at the para-position showed strong RhlR antagonism in the E. coli RhlR reporter system, with IC50 (the inhibitory concentration of a compound where the response is reduced by half for dose-response curves) values ranging from 8 to 24 μM. In particular, dichloro-substituted phenylacetyl analog (34) exhibited the strongest RhlR antagonism with an IC50 value of 3.4 μM in the E. coli reporter system. para-Substituted phenoxyacetyl analogs (35–39) displayed strong RhlR antagonism in the E. coli bioassay. In particular, para-iodo substituted phenoxyacetyl compound 38 showed high RhlR selectivity over LasR and PqsR in E. coli. The antagonist effect of compound 38 was observed in the P. aeruginosa reporter system with an IC50 value of 23.9 μM. However, the instability of the lactone ring in culture media precluded compound 38 from further examination [33]. Based on comprehensive SAR studies, they designed and synthesized the thiolactone analog (40) as RhlR antagonist (Table 4). Although replacement of the homoserine lactone with the homocysteine thiolactone ring decreased RhlR antagonist activities slightly, compound 40 was a strong RhlR antagonist, with an IC50 values of 19.6 μM and 31.4 μM in the E. coli and P. aeruginosa reporter systems, respectively. The thiolactone ring is generally more unstable than the lactone ring because the C-S bond strength is weaker than the C-O bond. However, stability studies showed that the thiolactone compound 40 was more stable than the corresponding lactone compound 38.

Table 4.
Phenylacetyl or phenoxyacetyl analogs as RhlR antagonists.
The EC50 or IC50 values between P. aeruginosa and E. coli reporter did not often match accurately [32,34]. P. aeruginosa has a thicker, less permeable outer membrane, which promotes efflux pathways for small molecules to be exported both in and out of the cell more easily [36,37]. The MexAB-OprM efflux pump in P. aeruginosa has been shown to play a role in the transfer of many small molecules including native and non-native AHLs [38]. Therefore, it is estimated that the substrate specificity of the MexAB-OprM efflux pump and cell membrane diffusion rate could have a significant impact on the EC50 or IC50 values in P. aeruginosa [39,40]. However, P. aeruginosa would be the most useful reporter strain for evaluating the activity of BHL analogs, as this strain is RhlR’s native background [38].
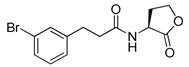
Bassler and co-workers also reported that a meta-bromo aryl homocysteine thiolactone analog (41, mBTL) was a partial agonist/partial antagonist of both RhlR and LasR in the E. coli assay system [22] (Table 5). They used E. coli BL21 carrying plasmid pET23b containing rhlR and plasmid pEVS141 containing the rhlA promoter-driving expression of gfp to measure RhlR transcriptional level more directly. To determine whether analogs act as an antagonist or agonist, BHL and the analog were reacted with reporter strain in the antagonism test, whereas only analog was reacted in the agonism test. Replacement of Br with Cl in the phenyl ring retained the mixed agonism/antagonism effect and inhibition of pyocyanin production without affecting P. aeruginosa PA14 growth [22], suggesting that RhlR is well tolerated with structural modifications in the tail region. With regard to the absolute configuration of the homocysteine thiolactone ring, the (S)-enantiomer, a natural amino acid type, was more potent than the corresponding (R)-enantiomer [22]. Treatment with mBTL results in a decrease in the average height of biofilm by 64%, delaying time to clogging of microfluidic chambers. Moreover, P. aeruginosa rapidly killed 77% of C. elegans after 24 h, but when 50 μM of mBTL was treated on C. elegans, the killing rate decreased to 23%. mBTL also reduced the killing of human lung cells by P. aeruginosa and was not toxic at 100 μM.

Table 5.
Non-BHL RhlR antagonists.
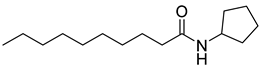
Kato and co-workers synthesized and evaluated the effects of acyl cyclopentylamides in P. aeruginosa PAO1 [41]. They distinguished the antagonism activities of LasR and RhlR with different specific reporter strains. RhlR antagonism activity was evaluated with P. aeruginosa PAO1 introduced rhlA-lacZ transcriptional fusion gene by plasmid pβ01, whereas PAO1 with plasmid pβ02 carrying lasB-lacZ transcriptional fusion gene was used for the LasR antagonism. The β-galactosidase assay revealed that N-decanoyl cyclopentylamide (42) is a weak RhlR antagonist, with an IC50 value of 90 μM for rhlA-lacZ expression in P. aeruginosa PAO1 (Table 5). However, this compound also displayed LasR-inhibitory activity with an IC50 value of 80 μM for lasB-lacZ expression due to presence of a long alkyl chain group in the tail region. 250 μM of compound 42 reduced the production of elastase, rhamnolipid, and pyocyanin to 23%, 13%, and 36%, respectively [41]. And in presence of compound 42, P. aeruginosa biofilm was not formed even after 1 week of cultivation.
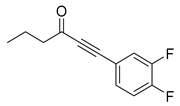
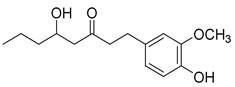
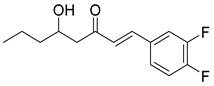
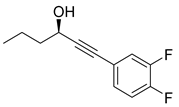
Recently, Byun and co-workers screened RhlR antagonism of gingerol analogs with various alkyl chain lengths from 4-gingerol to 10-gingerol [42]. Compound 44 (4-gingerol) with the n-butyl chain in the tail region showed 31% RhlR inhibition at 100 μM in the presence of 10 μM BHL in the E. coli QS reporter strain assay (Table 6). Based on the chemical structure of 4-gingerol, they synthesized a variety of 4-gingerol analogs and evaluated RhlR antagonism. The compound structures tested in this study were not related to that of BHL. In particular, the substituted phenyl ring was utilized in the head region, instead of the homoserine lactone ring. Furthermore, the amide linkage was replaced by a simple carbonyl group. Among the diverse substituents in the phenyl ring of the head region, compound 45 with difluoro substituents at the 3- and 4-position was the most potent, leading to the replacement of 3-OCH3 and 4-OH substituents in 4-gingerol. Compound 45 exhibited 69% RhlR inhibition at a concentration of 100 μM. Structural optimization of compound 45 resulted in the discovery of compound 43 (Table 5), which was the most potent RhlR antagonist with 86% inhibition at 100 μM, with an IC50 value of 26 μM in the E. coli RhlR reporter system. The reduction of the ketone group in compound 43 to alcohol resulted in a slight decrease in RhlR antagonism. Although the absolute configuration had little effect on RhlR inhibition, the (R)-enantiomer (46) was more potent than the corresponding (S)-enantiomer. Molecular docking studies of compound 43 with the RhlR homology model suggested that the strong π-π stacking interaction of the 3,4-difluorophenyl ring with Tyr 71 residue, which is one of the key amino acids that interact with BHL-based RhlR modulators. Molecular docking studies of the RhlR homology model with BHL analogs using Glide software by Ravi et al. also proposed that the native auto-inducer interacts strongly with the two amino acids (Thr 57 and Tyr 71) in the active site of RhlR [43]. Moreover, compound 43 displayed strong inhibition of biofilm formation in static and dynamic settings and the reduction of virulence factor production (elastase, rhamnolipid, and pyocyanin) in P. aeruginosa. In addition, compound 43 did not cause toxicity to human lung epithelial cells and alleviated the infectivity of P. aeruginosa in Tenebrio molitor larvae [44].

Table 6.
Gingerol-based RhlR antagonists.
3. Discussion and Conclusions
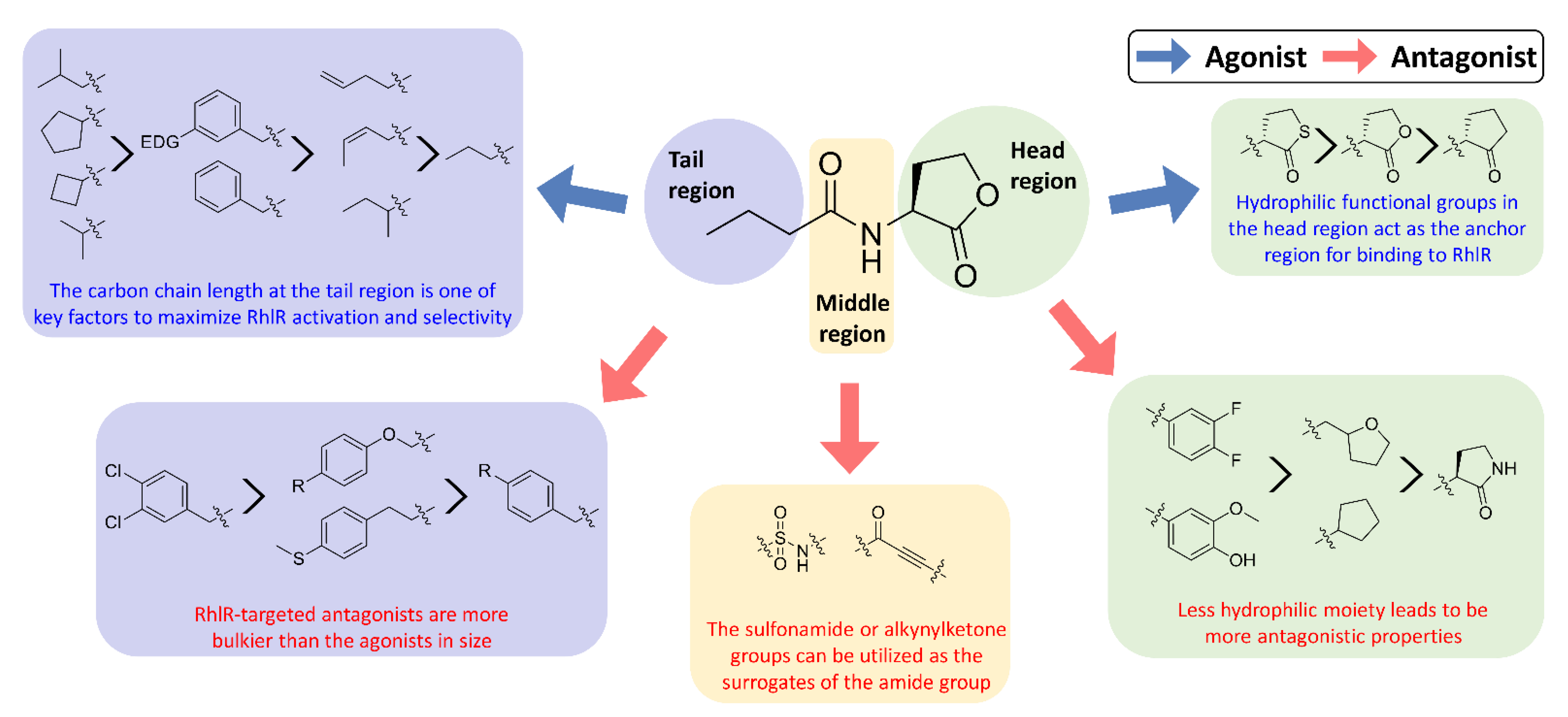
Recent SAR studies have shown the structural characteristics of RhlR-targeted agonists and antagonists. In general, receptor antagonists are more bulky in molecular size and have additional binding subpockets, compared to the corresponding agonists when they compete against the same active site of the target protein. As summarized in Figure 3, RhlR-targeted antagonists are slightly bulkier than the agonists. Homoserine lactone, homocysteine thiolactone, and cyclopentanone in the head region are commonly found in both RhlR-targeted agonists and antagonists, suggesting that a hydrophilic functional group in the head region acts as the anchor region for binding to RhlR. Replacement of the homoserine lactone with cyclopentane, tetrahydrofuran, and γ-lactam ring makes the parent molecule less hydrophilic, which leads to more antagonistic properties. In addition, introduction of the substituted phenyl ring in the head region renders the parent molecule an RhlR antagonist. In the middle region, structural modification is relatively limited compared to the head and tail regions. The sulfonamide or alkynylketone groups can be utilized as surrogates of the amide group for RhlR antagonists. In the tail region, the branched alkyls (e.g., isobutyl and isopropyl) and the cycloalkyl rings (e.g., cyclobutane and cyclopentane) were more favorable for RhlR agonism, compared with the n-propyl group in BHL. In the case of RhlR-targeted antagonists, the more bulky moieties including 2,4-dichlorophenylmethyl, p-substituted phenoxymethyl and p-substituted phenylmethyl are preferred in the tail region. However, there have been few reports on RhlR-targeted modulators to establish comprehensive SAR studies. Most QS inhibitors of P. aeruginosa target LasR because it is located at the top of the P. aeruginosa QS network hierarchy. From a viewpoint of drug discovery and development of RhlR-targeted modulators, X-ray crystal structures of RhlR in the presence or absence of a ligand should be determined and utilized. The lack of a RhlR 3D structure is a major obstacle to the discovery and development of novel potent and selective RhlR-targeted modulators through structure-based drug design.
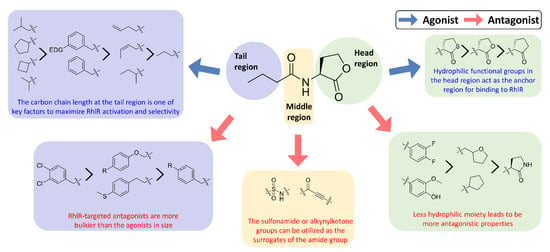
Figure 3.
SAR summary of RhlR-targeted agonists and antagonists.
P. aeruginosa is a leading cause of airway infections in patients with cystic fibrosis (CF). In isolates from CF patients with chronic P. aeruginosa infections, LasR mutations are commonly observed [24,45,46]. In these CF isolates, RhlR plays a key role in encoding virulence factors in a LasR-independent manner [28]. Dandekar et al. studied E90, a CF isolate which contains a single-base-pair deletion in lasR and uses RhlI/RhlR to mediate QS. RhlR produces QS-regulated virulence factors in E90 isolates, and it was the critical determinant of cytotoxicity in a 3-D lung epithelium cell model [28]. In general, the BHL/RhlR system activates the expression of genes encoding virulence factors including pyocyanin, rhamnolipid, and elastase [28,47]. However, Bassler and co-workers found that RhlR also responded in the absence of BHL and was responsible for BHL-independent transcription activities related to biofilm formation and virulence factor production [48]. The P. aeruginosa ΔrhlI mutant was virulent in animal infection models while the ΔrhlR mutant was avirulent, suggesting that BHL-independent regulation by RhlR may be more important for pathogenicity in P. aeruginosa infection [48]. The importance of RhlR was also supported by Ferrandon et al. who found that rhlI mutants were more virulent than rhlR mutants both in fly and in nematode intestinal infection models [49]. Furthermore, other studies show that in addition to atypical strains, the QS system can be flexible under certain environmental conditions, particularly for phosphate limitation [50,51]. When P. aeruginosa establishes infections, the phosphate level of patients undergoing chemotherapy or surgery is 0.03 mM, which is extremely low compared to healthy people (1.25 mM) [52]. Under phosphate-limiting conditions, the production of virulence factors in P. aeruginosa was increased [53,54]. Moreover, Soto-Aceves et al. discovered that LasR is indispensable to activate QS response, which suggested that RhlR is at the top of the QS hierarchy [29]. This phenomenon is supported by the fact that the activity of elastase, a LasR-specific virulence factor, is dependent on the Rhl system under phosphate-limiting conditions.
Overall, RhlR is an important QS transcription factor and may be a potential target for the treatment of P. aeruginosa infections, particularly in CF patients. Therefore, small molecule modulators targeting RhlR may be developed as novel antimicrobial agents for the control of P. aeruginosa infections. RhlR X-ray crystal structure, structural optimization of current RhlR-targeted agonists/antagonists, comprehensive in vivo efficacy studies, and synergistic effects with antibiotics will help develop and optimize the next generation of RhlR-targeted modulators. These efforts will be of use to promote preclinical and clinical studies, which may produce a proof-of-concept of targeting RhlR as a new therapeutic strategy to control P. aeruginosa infections.
Author Contributions
All authors contributed to writing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (2019R1A6A1A03031807).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Delden, C.; Iglewski, B.H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 1998, 4, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef]
- Wilder, C.N.; Diggle, S.P.; Schuster, M. Cooperation and cheating in Pseudomonas aeruginosa: The roles of the las, rhl and pqs quorum-sensing systems. ISME J. 2011, 5, 1332–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilli, A.; Bassler, B.L. Bacterial small-molecule signaling pathways. Science 2006, 311, 1113–1116. [Google Scholar] [CrossRef] [Green Version]
- Pesci, E.C.; Pearson, J.P.; Seed, P.C.; Iglewski, B.H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1997, 179, 3127–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Reyes, S.; Soberon-Chavez, G.; Cocotl-Yanez, M. The third quorum-sensing system of Pseudomonas aeruginosa: Pseudomonas quinolone signal and the enigmatic PqsE protein. J. Med. Microbiol. 2020, 69, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.R.; Paczkowski, J.E.; Jeffrey, P.D.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Inhibitor mimetic mutations in the Pseudomonas aeruginosa PqsE enzyme reveal a protein-protein interaction with the quorum-sensing receptor RhlR that is vital for virulence factor production. ACS Chem. Biol. 2021, 16, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Simanek, K.A.; Taylor, I.R.; Richael, E.K.; Lasek-Nesselquist, E.; Bassler, B.L.; Paczkowski, J.E. The PqsE-RhlR interaction regulates RhlR DNA binding to control virulence factor production in Pseudomonas aeruginosa. Microbiol. Spectr. 2022, 10, e02108–e02121. [Google Scholar] [CrossRef] [PubMed]
- Krzyzek, P. Challenges and limitations of anti-quorum sensing therapies. Front. Microbiol. 2019, 10, 2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.A.; Oliveira, R.A.; Djukovic, A.; Ubeda, C.; Xavier, K.B. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell. Rep. 2015, 10, 1861–1871. [Google Scholar] [CrossRef]
- Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical conversations in the gut microbiota. Gut Microbes 2016, 2, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Garcia-Contreras, R.; Pu, M.; Sheng, L.; Garcia, L.R.; Tomas, M.; Wood, T.K. Quorum quenching quandary: Resistance to antivirulence compounds. ISME J. 2012, 6, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Contreras, R.; Martinez-Vazquez, M.; Velazquez Guadarrama, N.; Villegas Paneda, A.G.; Hashimoto, T.; Maeda, T.; Quezada, H.; Wood, T.K. Resistance to the quorum-quenching compounds brominated furanone C-30 and 5-fluorouracil in Pseudomonas aeruginosa clinical isolates. Pathog. Dis. 2013, 68, 8–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, T.B.; Givskov, M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.T.; Noto, J.G.; Nichols-O’Neill, L.; Perez, L.J. Potent Irreversible Inhibitors of LasR Quorum Sensing in Pseudomonas aeruginosa. ACS Med. Chem. Lett. 2015, 6, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Geske, G.D.; Mattmann, M.E.; Blackwell, H.E. Evaluation of a focused library of N-aryl L-homoserine lactones reveals a new set of potent quorum sensing modulators. Bioorg. Med. Chem. Lett. 2008, 18, 5978–5981. [Google Scholar] [CrossRef] [Green Version]
- McInnis, C.E.; Blackwell, H.E. Thiolactone modulators of quorum sensing revealed through library design and screening. Biorg. Med. Chem. 2011, 19, 4820–4828. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.M.; Bu, Y.; Suga, H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 2003, 10, 81–89. [Google Scholar] [CrossRef] [Green Version]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrol, S.; Olliver, A.; Pier, G.B.; Andremont, A.; Ruimy, R. Transcription of quorum-sensing system genes in clinical and environmental isolates of Pseudomonas aeruginosa. J. Bacteriol. 2003, 185, 7222–7230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Argenio, D.A.; Wu, M.; Hoffman, L.R.; Kulasekara, H.D.; Deziel, E.; Smith, E.E.; Nguyen, H.; Ernst, R.K.; Larson Freeman, T.J.; Spencer, D.H.; et al. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol. Microbiol. 2007, 64, 512–533. [Google Scholar] [CrossRef] [Green Version]
- Feltner, J.B.; Wolter, D.J.; Pope, C.E.; Groleau, M.C.; Smalley, N.E.; Greenberg, E.P.; Mayer-Hamblett, N.; Burns, J.; Deziel, E.; Hoffman, L.R.; et al. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio 2016, 7, e01513-16. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Deziel, E.; Groleau, M.C.; Schaefer, A.L.; Greenberg, E.P. Social cheating in a Pseudomonas aeruginosa quorum-sensing variant. Proc. Natl. Acad. Sci. USA 2019, 116, 7021–7026. [Google Scholar] [CrossRef] [Green Version]
- Groleau, M.C.; Taillefer, H.; Vincent, A.T.; Constant, P.; Deziel, E. Pseudomonas aeruginosa isolates defective in function of the LasR quorum sensing regulator are frequent in diverse environmental niches. Environ. Microbiol. 2021. early view. [Google Scholar] [CrossRef]
- Cruz, R.L.; Asfahl, K.L.; Van den Bossche, S.; Coenye, T.; Crabbe, A.; Dandekar, A.A. RhlR-Regulated acyl-homoserine lactone quorum sensing in a cystic fibrosis isolate of Pseudomonas aeruginosa. mBio 2020, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Soto-Aceves, M.P.; Cocotl-Yanez, M.; Servin-Gonzalez, L.; Soberon-Chavez, G. The rhl quorum-sensing system is at the top of the regulatory hierarchy under phosphate-limiting conditions in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2021, 203, 203. [Google Scholar] [CrossRef]
- Mattmann, M.E.; Blackwell, H.E. Small molecules that modulate quorum sensing and control virulence in Pseudomonas aeruginosa. J. Org. Chem. 2010, 75, 6737–6746. [Google Scholar] [CrossRef] [Green Version]
- Duplantier, M.; Lohou, E.; Sonnet, P. Quorum sensing inhibitors to quench P. aeruginosa pathogenicity. Pharmaceuticals 2021, 14, 1262. [Google Scholar] [CrossRef] [PubMed]
- Boursier, M.E.; Moore, J.D.; Heitman, K.M.; Shepardson-Fungairino, S.P.; Combs, J.B.; Koenig, L.C.; Shin, D.; Brown, E.C.; Nagarajan, R.; Blackwell, H.E. Structure-function analyses of the N-butanoyl l-homoserine lactone quorum-sensing signal define features critical to activity in RhlR. ACS Chem. Biol. 2018, 13, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Boursier, M.E.; Combs, J.B.; Blackwell, H.E. N-Acyl l-homocysteine thiolactones are potent and stable synthetic modulators of the RhlR quorum sensing receptor in Pseudomonas aeruginosa. ACS Chem. Biol. 2019, 14, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Eibergen, N.R.; Moore, J.D.; Mattmann, M.E.; Blackwell, H.E. Potent and selective modulation of the RhlR quorum sensing receptor by using non-native ligands: An emerging target for virulence control in Pseudomonas aeruginosa. ChemBioChem 2015, 16, 2348–2356. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Abdel-Razek, O.A.; Cheng, F.; Bandyopadhyay, D.; Shetye, G.S.; Wang, G.; Luk, Y.Y. Bicyclic brominated furanones: A new class of quorum sensing modulators that inhibit bacterial biofilm formation. Biorg. Med. Chem. 2014, 22, 1313–1317. [Google Scholar] [CrossRef]
- Li, X.-Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria: An update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Kumar, A.; Schweizer, H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005, 57, 1486–1513. [Google Scholar] [CrossRef]
- Moore, J.D.; Gerdt, J.P.; Eibergen, N.R.; Blackwell, H.E. Active efflux influences the potency of quorum sensing inhibitors in Pseudomonas aeruginosa. ChemBioChem 2014, 15, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.P.; Van Delden, C.; Iglewski, B.H. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 1999, 181, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Minagawa, S.; Inami, H.; Kato, T.; Sawada, S.; Yasuki, T.; Miyairi, S.; Horikawa, M.; Okuda, J.; Gotoh, N. RND type efflux pump system mexab-oprm of pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol. 2012, 12, 70. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Ikeda, T.; Takiguchi, N.; Kuroda, A.; Ohtake, H.; Kato, J. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol. 2007, 73, 3183–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.; Ham, S.Y.; Kwon, H.; Kim, H.S.; Moon, S.; Lee, J.H.; Lim, T.; Son, S.H.; Park, H.D.; Byun, Y. Discovery and characterization of pure RhlR antagonists against Pseudomonas aeruginosa infections. J. Med. Chem. 2020, 63, 8388–8407. [Google Scholar] [CrossRef]
- Annapoorani, A.; Umamageswaran, V.; Parameswari, R.; Pandian, S.K.; Ravi, A.V. Computational discovery of putative quorum sensing inhibitors against LasR and RhlR receptor proteins of Pseudomonas aeruginosa. J. Comput. Aided Mol. Des. 2012, 26, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.Y.; Kim, H.S.; Jo, M.J.; Lee, J.H.; Byun, Y.; Ko, G.J.; Park, H.D. Combined treatment of 6-gingerol analog and tobramycin for inhibiting Pseudomonas aeruginosa infections. Microbiol. Spectr. 2021, 9, e0019221. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Buckley, D.G.; Wu, Z.; Saenphimmachak, C.; Hoffman, L.R.; D’Argenio, D.A.; Miller, S.I.; Ramsey, B.W.; Speert, D.P.; Moskowitz, S.M.; et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2006, 103, 8487–8492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, L.R.; Kulasekara, H.D.; Emerson, J.; Houston, L.S.; Burns, J.L.; Ramsey, B.W.; Miller, S.I. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J. Cyst. Fibros. 2009, 8, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Brint, J.M.; Ohman, D.E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 1995, 177, 7155–7163. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Moustafa, D.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathog. 2017, 13, e1006504. [Google Scholar] [CrossRef]
- Haller, S.; Franchet, A.; Hakkim, A.; Chen, J.; Drenkard, E.; Yu, S.; Schirmeier, S.; Li, Z.; Martins, N.; Ausubel, F.M.; et al. Quorum-sensing regulator RhlR but not its autoinducer RhlI enables Pseudomonas to evade opsonization. EMBO Rep. 2018, 19, e1006504. [Google Scholar] [CrossRef]
- Jensen, V.; Lons, D.; Zaoui, C.; Bredenbruch, F.; Meissner, A.; Dieterich, G.; Munch, R.; Haussler, S. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J. Bacteriol. 2006, 188, 8601–8606. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Ahator, S.D.; Zhang, L.-H. Molecular mechanisms of phosphate stress activation of Pseudomonas aeruginosa quorum sensing systems. mSphere 2020, 5, e00119-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.; Zaborina, O.; Holbrook, C.; Zaborin, A.; Alverdy, J. Depletion of intestinal phosphate after operative injury activates the virulence of P. aeruginosa causing lethal gut-derived sepsis. Surgery 2008, 144, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, L.E.; Price-Whelan, A.; Petersen, A.; Whiteley, M.; Newman, D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006, 61, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.A.; Newman, D.K. Both toxic and beneficial effects of pyocyanin contribute to the lifecycle of Pseudomonas aeruginosa. Mol. Microbiol. 2018, 110, 995–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).