Perspective, Opportunities and Challenges in Using Fennel (Foeniculum vulgare) in Poultry Health and Production as an Eco-Friendly Alternative to Antibiotics: A Review
Abstract
:1. Introduction
2. Uses of Fennel Seeds as an Alternative to Antibiotics
2.1. Growth Performance in Poultry
2.2. Egg Production and Quality Traits
2.3. Antimicrobial and Immune Stimulating Effects
2.4. Antioxidant Activity
2.5. Hematology and Biochemistry
3. Challenges and Way Forward
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, R.U.; Naz, S. The applications of probiotics in poultry production. World’s Poult. Sci. J. 2013, 69, 621–632. [Google Scholar] [CrossRef]
- Khan, R.U.; Rahman, Z.U.; Javed, I.; Muhammad, F. Supplementation of vitamins, probioitics and proteins on oxidative stress, enzymes and hormones in post-moulted male broiler breeder. Arch. Tierz. 2013, 61, 607–616. [Google Scholar]
- Khan, R.; Rahman, Z.U.; Javed, I.; Muhammad, F. Serum antioxidants and trace minerals as influenced by vitamins, probiotics and proteins in broiler breeders. J. Appl. Anim. Res. 2014, 42, 249–255. [Google Scholar] [CrossRef]
- Alam, S.; Masood, S.; Zaneb, H.; Rabbani, I.; Khan, R.U.; Shah, M.; Ashraf, S.; Alhidary, I.A. Effect of Bacillus ce-reus and phytase on the expression of musculoskeletal strength and gut health in Japanese quail (Coturnix japonica). Poult. Sci. J. 2020, 57, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Zaneb, H.; Masood, S.; Khan, R.U.; Mobashar, M.; Khan, I.; Din, S.; Khan, M.S.; Rehman, H.R.; Tinelli, H.A. Single or combined applications of zinc and multi-strains probiotic on intestinal histomorphology of broilers under cy-clic heat stress. Probiotics Antimicrob. Proteins 2020, 12, 473–480. [Google Scholar] [CrossRef]
- Shah, M.; Zaneb, H.; Masood, S.; Khan, R.U.; Din, S.; Shakirullah, K.I.; Tariq, A.; Rehman, H. Ameliora-tive effect of zinc and multistrain probiotic on muscle and bone characteristics in broiler reared under cyclic heat stress. Pak. J. Zool. 2019, 51, 1041–1046. [Google Scholar] [CrossRef]
- Shah, M.; Zaneb, H.; Masood, S.; Khan, R.U.; Ashraf, S.; Sikandar, A.; Faseeh, H.U.R.; Rehman, H. Effect of die-tary supplementation of zinc and multi-microbe probiotic on growth traits and alteration of intestinal architecture in broiler. Probiotics Antimicrob. Proteins 2019, 11, 931–937. [Google Scholar] [CrossRef]
- Khan, R.U.; Rahman, Z.U.; Javed, I.; Muhammad, F. Effect of vitamins, protein level and probiotics on immune response of molted male broiler breeders. J. Anim. Physiol. Anim. Nutr. 2014, 98, 620–627. [Google Scholar] [CrossRef]
- Chand, N.; Faheem, H.; Khan, R.U.; Qureshi, M.S.; Alhidary, I.A.; Abudabos, A.M. Anticoccidial effect of mannanoli-gosacharide against experimentally induced coccidiosis in broiler. Environ. Sci. Pollut. Res. 2016, 23, 23:14414–14421. [Google Scholar] [CrossRef]
- Chand, N.; Shamsullah, R.; Khan, R.U.; Mobashar, M.; Naz, S.; Rowghani, I.; Khan, M.A. Mannanoligosac-charide (MOS) in broiler ration during the starter phase: 1. growth performance and intestinal histomorpholgy. Pak. J. Zool. 2019, 51, 173–176. [Google Scholar]
- Tufail, M.; Chand, N.; Rafiullah, A.S.; Khan, R.U.; Mobashar, M.; Naz, S. Mannanoligosaccharide (MOS) in broiler diet during the finisher phase: 2. growth traits and intestinal histomorphology. Pak. J. Zool. 2019, 51, 597–602. [Google Scholar] [CrossRef]
- Haq, I.U.; Hafeez, A.; Khan, R.U. Protective effect of Nigella sativa and Saccharomyces cerevisiae on zootechnical characteristics, fecal Escherichia coli and hematopoietic potential in broiler infected with experimental Colibacillosis. Livest. Sci. 2020, 239, 104119. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; Alagawany, M.; Arif, M.; Emam, M.; Saeed, M.; Arain, M.A.; Siyal, F.A.; Patra, A.; Elnesr, S.S.; Khan, R.U. The uses of microbial phytase as a feed additive in poultry nutrition—A review. Ann. Anim. Sci. 2018, 18, 639–658. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.; Ali, R.; Khan, R.U.; Khan, S.; Chand, N.; Tariq, A. Nutritional Evaluation of Two Sorghum Varieties in Broiler Fortified with Phytase. Pak. J. Zool. 2019, 51. [Google Scholar] [CrossRef]
- Jabbar, A.; Tahir, M.; Khan, R.U.; Ahmad, N. Interactive effect of exogenous protease enzyme and dietary crude protein levels on growth and digestibility indices in broiler chickens during the starter phase. Trop. Anim. Heal. Prod. 2020, 53, 1–5. [Google Scholar] [CrossRef]
- Jabbar, A.; Tahir, M.; Alhidary, I.A.; Abdelrahman, M.A.; Albadani, H.; Khan, R.U.; Selvaggi, M.; Laudadio, V.; Tufarelli, V. Impact of Microbial Protease Enzyme and Dietary Crude Protein Levels on Growth and Nutrients Digestibility in Broilers over 15–28 Days. Animals 2021, 11, 2499. [Google Scholar] [CrossRef]
- Hafeez, A.; Iqbal, S.; Sikandar, A.; Din, S.; Khan, I.; Ashraf, S.; Khan, R.; Tufarelli, V.; Laudadio, V. Feeding of Phytobiotics and Exogenous Protease in Broilers: Comparative Effect on Nutrient Digestibility, Bone Strength and Gut Morphology. Agriculture 2021, 11, 228. [Google Scholar] [CrossRef]
- Nazeer, N.; Uribe-Diaz, S.; Rodriguez-Lecompte, J.C.; Ahmed, M. Antimicrobial peptides as an alternative to relieve antimicrobial growth promoters in poultry. Br. Poult. Sci. 2021, 62, 672–685. [Google Scholar] [CrossRef]
- Silveira, R.F.; Roque-Borda, C.A.; Vicente, E.F. Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: An overview. Anim. Nutr. 2021, 7, 896–904. [Google Scholar] [CrossRef]
- Li, M.; Lin, H.; Jing, Y.; Wang, J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020, 99, 3643–3654. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Ahn, J.M.; Cho, J.H.; Kim, J.Y.; Kang, D.K.; Kim, S.W.; Kim, H.B.; Kim, I.H. Bacteriophage cocktail supplementation improves growth performance, gut microbiome and production traits in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Hussein, M.A.; Rehan, I.F.; Rehan, A.F.; Eleiwa, N.Z.; Abdel-Rahman, M.A.M.; Fahmy, S.G.; Ahmed, A.S.; Youssef, M.; Diab, H.M.; Batiha, G.E.; et al. Egg Yolk IgY: A Novel Trend of Feed Additives to Limit Drugs and to Improve Poultry Meat Quality. Front. Vet. Sci. 2020, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Chelliappan, B.; Michael, A.; Vega, C.G.; Zhong, F.; Zhang, X.; Morgan, P.M. Applications of IgY in Veterinary Medicine. In IgY-Technology: Production and Application of Egg Yolk Antibodies; Springer: New York, NY, USA, 2021; pp. 205–235. [Google Scholar]
- Hayat, T.A.; Sultan, R.U.; Khan, S.; Khan, Z.H.; Ullah, R.; Aziz, T. Impact of organic acid on some liver and kidney function tests in Japanese quails, Coturnix coturnix japonica. Pak. J. Zool. 2014, 46, 1179–1182. [Google Scholar]
- Sultan, A.; Ullah, T.; Khan, S.; Khan, R.U. Effect of organic acid supplementation on the performance and ileal microflora of broiler during finishing period. Pak. J. Zool. 2015, 47, 635–639. [Google Scholar]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broiler exposed to Salmonella typhimurium challenge during the starter phase. J. Appl. Anim. Res. 2017, 45, 538–542. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.U.; Naz, S.; Javadani, M.; Nikousefat, Z.; Selvaggi, M.; Tufarelli, V.; Laudadio, V. The use of turmeric (Curcuma longa) in poultry diets. World’s Poult. Sci. J. 2012, 68, 97–103. [Google Scholar] [CrossRef]
- Khan, R.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Qureshi, M.; Laudadio, V. Potential applications of ginger (Zingiber officinale) in poultry diets. World’s Poult. Sci. J. 2012, 68, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Laudadio, V. Thymus vulgaris: Alternative to antibiotics in poultry feed. World’s Poult. Sci. J. 2012, 68, 401–408. [Google Scholar] [CrossRef]
- Khan, R.U.; Nikosefat, Z.; Tufarelli, V.; Naz, S.; Javdani, M.; Laudadio, V. Garlic (Allium sativa) supplementation in poultry diet: Effect on production and physiology. World’s Poult. Sci. J. 2012, 68, 417–424. [Google Scholar] [CrossRef]
- Khan, R.; Rahman, Z.-U.; Javed, I.; Muhammad, F. Effect of vitamins, probiotics and protein on semen traits in post-molt male broiler breeders. Anim. Reprod. Sci. 2012, 135, 85–90. [Google Scholar] [CrossRef]
- Alzawqari, M.H.; Al-Baddany, A.A.; Al-Baadani, H.H.; Alhidary, I.A.; Khan, R.U.; Aqil, G.M.; Abdurab, A. Effect of feeding dried sweet orange (Citrus sinensis) peel and lemon grass (Cymbopogon citratus) leaves on growth performance, carcass traits, serum metabolites and antioxidant status in broiler during the finisher phase. Environ. Sci. Pollut. Res. 2016, 23, 17077–17082. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Shah, S.A.A.; Khan, R.U.; Ullah, Q.; Naz, S. Effect of diet supplemented with phytogenics and protease enzyme on performance, serum biochemistry and muscle histomorphology in broilers. J. Appl. Anim. Res. 2020, 48, 326–330. [Google Scholar] [CrossRef]
- Hafeez, A.; Sohail, M.; Ahmad, A.; Shah, M.; Din, S.; Khan, I.; Shuiab, M.; Nasrullah; Shahzada, W.; Iqbal, M.; et al. Selected herbal plants showing enhanced growth performance, ileal digestibility, bone strength and blood metabolites in broilers. J. Appl. Anim. Res. 2020, 48, 448–453. [Google Scholar] [CrossRef]
- Hafeez, A.; Ullah, Z.; Khan, R.U.; Ullah, Q.; Naz, S. Effect of diet supplemented with essential coconut oil on per-formance and intestinal injury in broiler exposed to avian coccidiosis. Trop. Anim. Health Prod. 2020, 52, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Hafeez, A.; Ullah, Q.; Naz, S.; Khan, R.U. Protective effect of Aloe vera on growth performance, leu-cocyte count and intestinal injury in broiler chicken infected with coccidiosis. J. Appl. Anim. Res. 2020, 48, 252–256. [Google Scholar] [CrossRef]
- Chand, N.; Ali, P.; Alhidary, I.; Abdelrahman, M.; Albadani, H.; Khan, M.; Seidavi, A.; Laudadio, V.; Tufarelli, V.; Khan, R. Protective Effect of Grape (Vitis vinifera) Seed Powder and Zinc-Glycine Complex on Growth Traits and Gut Health of Broilers Following Eimeria tenella Challenge. Antibiotics 2021, 10, 186. [Google Scholar] [CrossRef]
- Israr, M.; Chand, N.; Khan, R.U.; Alhidary, I.A.; Abdelrhman, M.M.; Al-Baddani, H.H.; Laudadio, V.; Tufarelli, V. Dietary grape (Vitis vinifera) seeds powder and organic Zn-gly chelate complex for mitigating heat stress in broiler chickens: Growth parameters, malanodialdehyde, paraoxonase-1 and antibody titre. Agriculture 2021, 11, 1087. [Google Scholar] [CrossRef]
- Khan, R.U.; Khan, A.; Naz, S.; Ullah, Q.; Laudadio, V.; Tufarelli, V.; Ragni, M. Potential Applications of Moringa oleifera in Poultry Health and Production as Alternative to Antibiotics: A Review. Antibiotics 2021, 10, 1540. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Alobre, M.M.; Abdelrahman, M.M.; Al-Baadani, H.H.; Swelum, A.A.; Khan, R.U.; Alhidary, I.A. The Effects of Different Levels of Sunflower Hulls on Reproductive Performance of Yearly Ewes Fed with Pelleted Complete Diets. Agriculture 2021, 11, 959. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Al-Baadani, H.H.; Al-Badwi, M.A.; Abdelrahman, M.M.; Alhidary, I.A.; Khan, R.U. Effects of Sunflower Hulls on Productive Performance, Digestibility Indices and Rumen Morphology of Growing Awassi Lambs Fed with Total Mixed Rations. Vet. Sci. 2021, 8, 174. [Google Scholar] [CrossRef]
- Khan, A.; Tahir, M.; Alhidary, I.; Abdelrahman, M.; Swelum, A.A.; Khan, R.U. Role of dietary Moringa oleifera leaf extract on productive parameters, humoral immunity and lipid peroxidation in broiler chicks. Anim. Biotechnol. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Tahir, M.; Naz, S.; Khan, N.A.; Khan, R.U. In vtro efficacy and ameliorating effect of Moringa oleifera on growth, carcass, stress and digestibility of nutrients in Eschertchia coli-infected broilers. J. Appl. Anim. Res. 2022. [Google Scholar]
- Alhidary, I.A.; Abdelrahman, M.M.; Alyemni, A.H.; Khan, R.; Al-Saiady, M.Y.; Amran, R.A.; Alshamiry, F.A. Effect of alfalfa hay on growth performance, carcass characteristics, and meat quality of growing lambs with ad libitum access to total mixed rations. Rev. Bras. de Zootec. 2016, 45, 302–308. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Alhidary, I.; Alyemni, A.H.; Khan, R.U.; Bello, A.R.S.; Al-Saiady, M.Y.; Amran, R.A. Effect of alfalfa hay on rumen fermentation patterns and serum biochemical profile of growing Naemi lambs with ad libitum access to total mixed rations. Pak. J. Zool. 2017, 49, 1519–1522. [Google Scholar] [CrossRef]
- Alhidary, I.; Rehman, Z.; Khan, R.; Tahir, M. Anti-aflatoxin activities of milk thistle (Silybum marianum) in broiler. World’s Poult. Sci. J. 2017, 73, 559–566. [Google Scholar] [CrossRef]
- Rahman, S.U.; Khan, S.; Chand, N.; Sadique, U.; Khan, R.U. In vivo effects of Allium cepa L. on the selected gut microflora and intestinal histomorphology in broiler. Acta Histochem. 2017, 119, 446–450. [Google Scholar] [CrossRef]
- Chand, N.; Naz, S.; Irfan, M.; Khan, R.U.; Rehman, Z.U. Effect of sea buckthorn (Hippophae rhamnoides L.) seed supplementation on egg quality and cholesterol of Rhode Island Red × Fayoumi laying hens. Korean J. Food Sci. Anim. Resour. 2018, 38, 468–475. [Google Scholar]
- Aćimović, M.; Zeremski, T.; Kiprovski, B.; Brdar-Jokanović, M.; Popović, V.; Koren, A.; Sikora, V. Nepeta ca-taria—Cultivation, Chemical Composition and Biological Activity. J. Agron. Technol. Eng. Manag. 2021, 4, 620–634. [Google Scholar]
- Popović, S.; Puvača, N.; Peulić, T.; Ikonić, P.; Spasevski, N.; Kostadinović, L.J.; Đuragić, O. The use-fulness of dietary es-sential oils mixture supplementation on quality aspect of poultry meat. J. Agron. Technol. Eng. Manag. 2019, 2, 335–343. [Google Scholar]
- Nastić, N.; Gavrić, A.; Vladić, J.; Vidović, S.; Aćimović, M.; Puvača, N.; Brkić, I. pruce (Picea abies (L.). H. Karst): Different Approaches for Extraction of Valuable Chemical Compounds. J. Agron. Technol. Eng. Manag. 2020, 3, 437–447. [Google Scholar]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill, A review of its botany, phytochemistry, pharma-cology, contemporary application and toxicology. Int. Bio. Med. Res. 2014, 2014, 842674. [Google Scholar]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel seeds from Pakistan. Flav. Frag. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Q.; Wang, X. Efficacy of herbal medicine (cinnamon/fennel/ginger) for primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. J. Int. Med. Res. 2020, 48, 300060520936179. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Qi, M.; Li, T.; Shao, Q.; Fu, R. Headspace solvent microextraction-gas chromatography–mass spectrometry for the analysis of volatile compounds from Foeniculum vulgare Mill. J. Pharm. Biomed. Anal. 2006, 41, 791–797. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and Antimicrobial Activity of Foeniculum vulgare and Crithmum maritimum Essential Oils. Planta Medica 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Romila, R.M.A. Hacked by SOSO H. H Iraqi-Cracker. M. Sci. Ph.D. Thesis, University of Cairo Egypt, Cairo, Egypt, 2001. [Google Scholar]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant activity of chemical components from sage (Saliva officinalis L.) and Oregano (Thymus vulgaris L.) measured by the oil stability index methods. J. Agri. Food Chem. 2002, 50, 1845–1851. [Google Scholar] [CrossRef]
- Gende, L.B.; Maggi, M.D.; Fritz, R.; Eguaras, M.J.; Bailac, P.N.; Ponzi, M.I. Antimicrobial Activity of Pimpinella anisum and Foeniculum vulgare Essential Oils Against Paenibacillus larvae. J. Essent. Oil Res. 2009, 21, 91–93. [Google Scholar] [CrossRef]
- Mehra, N.; Tamta, G.; Nand, V. A review on nutritional value, phytochemical and pharmacological attributes of Foeniculum vulgare Mill. J. Pharmacogn. Phytochem. 2021, 10, 1255–1263. [Google Scholar] [CrossRef]
- Al-Sagan, A.A.; Khalil, S.; Hussein, E.O.S.; Attia, Y.A. Effects of Fennel Seed Powder Supplementation on Growth Performance, Carcass Characteristics, Meat Quality, and Economic Efficiency of Broilers under Thermoneutral and Chronic Heat Stress Conditions. Animals 2020, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Gharehsheikhlou, H.R.; Chamani, M.; Seidavi, A.R.; Sadeghi, A.A.; Mohiti-Asli, M. Effect of fennel and savory essential oils on performance, carcass characteristics and blood parameters of broilers. J. Livest. Sci. 2018, 9, 23–31. [Google Scholar]
- Ragab, M.S.; Namra, M.M.M.; Aly, M.M.M.; Fathi, M.A. Impact of inclusion fennel seeds and thyme dried leaves in broiler diets on some productive and physiological performance during summer season. Egypt Poult. Sci. 2013, 33, 197–219. [Google Scholar]
- Vakili, R. Effect of Plant Extract of Fennel and Thyme with and without Flax on Egg Performance and Quality in Laying Hens. Iran. J. Anim. Sci. Res. 2011, 3, 243–249. [Google Scholar]
- Saki, A.A.; Kalantar, M.; Rahmatnejad, E.; Mirzaaghatabar, F. Health characteristics and performance of broiler chicks in response to trigonella foenum graenaecium and Foeniculum vulgare. Anim. Sci. J. 2014, 4, 387–391. [Google Scholar]
- Henda, A.M. Response of growing japanese quail to different level of fennel seed meal. Egypt. Poult. Sci. J. 2014, 15, 795–807. [Google Scholar]
- Ragab, M.S. Effects of using fennel seeds in growing japanese quail diets varying in their protein content with or without enzyme supplementation. Fayoum J. Agric. Res. Dev. 2007, 21, 113–136. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Selim, S.; Hussein, E. Effect of supplementing layer hen diet with phytogenic feed additives on per-formance, egg quality, egg lipid peroxidation and blood biochemical constituents. Anim. Nutr. 2018, 4, 394–400. [Google Scholar] [CrossRef]
- Zahira, A.; Abdullah, W.; Sand, R.; Majal, K. Effect of dietary supplementation of coriander and fennel seed powder and their mixture on productional and physiological performance of broiler. Al-qadis. Vet. Med. Sci. J. 2017, 17, 135–149. [Google Scholar]
- Gharaghani, H.; Shariatmadari, F.; Torshizi, M.A. Effect of Fennel (Foeniculum vulgare Mill.) Used as a Feed Additive on The Egg Quality of Laying Hens Under Heat Stress Anim. Prod. Sci. J. 2015, 25, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Soltan, M.A.; Shewita, R.S.; El-Katcha, M.I. Effect of dietary Anise seed supplementation on growth performance, immune response, carcass trait and some blood parameters of broiler chickens. Int. J. Poult. Sci. 2008, 7, 1078–1088. [Google Scholar] [CrossRef] [Green Version]
- Ghiasvand, A.R.; Khatibjoo, A.; Mohammadi, Y.; Gharaei, M.A.; Shirzadi, H. Effect of fennel essential oil on performance, serum biochemistry, immunity, ileum morphology and microbial population, and meat quality of broiler chickens fed corn or wheat-based diet. Br. Poult. Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Milica, G.A.; Kostadinović, L.M.; Puvaca, N.M.; Popovic, S.J.; Urosevic, M.I. Phytochemical constituents of selected plants from Apiaceae family and their biological effects in poultry. Food Feed. Res. 2016, 43, 35–41. [Google Scholar]
- Buğdaycı, K.E.; Oğuz, F.K.; Oğuz, M.N.; Kuter, E. Effects of fennel seed supplementation of ration on performance, egg quality, serum cholesterol, and total phenol content of egg yolk of laying quails. Rev. Bras. de Zootec. 2018, 47. [Google Scholar] [CrossRef] [Green Version]
- Abuk, M.; Bozkurt, M.; Alcicek, A.H.M.E.T.; Çatli, A.U.; Baser, K.H.C. Effect of a dietary essential oil mixture on per-formance of laying hens in the summer season. South Afr. J. Anim. Sci. 2006, 36, 215–221. [Google Scholar]
- Saleh, L.; Pal Singh, R.; Nagar, S. Efficacy of Foeniculum vulgare seeds powder on growth performance in broiler. Int. J. Food Sci. Nutr. 2018, 3, 167–170. [Google Scholar]
- Cabuk, M.; Bozkurt, M.; Alcicek, A.; Akbaþ, Y.; Küçükyýlmaz, K. Effect of a herbal essential oil mixture on growth and internal organ weight of broilers from young and old breeder flocks. South. Afr. J. Anim. Sci. 2006, 36, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Cengiz, S.S.; Yesilbag, D.; Eren, M.; Cetin, I.; Meral, Y.; Biricik, H.A.K.A.N. Effects of volatile oil additives on growth, carcass performances, and calcium and phosphorus concentrations in serum and bone of broilers. Rev. Med. Vet. 2016, 167, 230–239. [Google Scholar]
- Mohammed, A.A.; Abbas, R.J. The Effect of Using Fennel Seeds (Foeniculum vulgare L.) on Productive Performance of Broiler Chickens. Int. J. Poult. Sci. 2009, 8, 642–644. [Google Scholar] [CrossRef] [Green Version]
- Nasiroleslami, M.; Torki, M. Including essential oils of fennel (Foeniculum vulgare) and ginger (Zingiber officinale) to diet and evaluating performance of laying hens, white blood cell count and egg quality characteristics. Adv. Environ. Biol. 2010, 4, 341–345. [Google Scholar]
- Safaei-Cherehh, A.; Rasouli, B.; Alaba, P.A.; Seidavi, A.; Hernández, S.R.; Salem, A.Z.M. Effect of dietary Foeniculum vulgare Mill. extract on growth performance, blood metabolites, immunity and ileal microflora in male broilers. Agrofor. Syst. 2018, 94, 1269–1278. [Google Scholar] [CrossRef]
- Kazemi-Fard, M.; Kermanshahi, H.; Rezaei, M. Effect of Different Levels of Fennel Extract and Vitamin D3 on Post Molt Broiler Breeder Performance. Res. Anim. Prod. (Sci. Res.) 2013, 4, 15–34. [Google Scholar]
- Reza, T.; Ghiasi, H.; Ebrahimi, M. The Effect of Using Fennel on Plasma Estrogen and Performance of Laying Hens. J. Biochem. Tech. 2018, 115–122, Spesial Issue (2). [Google Scholar] [CrossRef] [Green Version]
- Kazemi-Fard, M.; Kermanshahi, H.; Rezaei, M. Effect of different levels of fennel extract and vitamin D3 on performance, hatchability and immunity in post molted broiler breeders. J. Anim. Sci. 2012, 3, 733–745. [Google Scholar]
- Özek, K.; Wellmann, K.; Ertekin, B.; Tarım, B. Effects of dietary herbal essential oil mixture and organic acid preparation on laying traits, gastrointestinal tract characteristics, blood parameters and immune response of laying hens in a hot summer season. J. Anim. Feed Sci. 2011, 20, 575–586. [Google Scholar] [CrossRef]
- Bozkurt, M.; Küçükyilmaz, K.; Uğur Çatli, A.; Özyildiz, Z.; Çinar, M.; Çabuk, M.; Çoven, F. Influences of an essential oil mixture supplementation to corn versus wheat-based practical diets on growth, organ size, intestinal morphology and immune response of male and female broilers. Ital. J. Anim. Sci. 2012, 11, e54. [Google Scholar] [CrossRef]
- Gharaghani, H.; Shariatmadari, F.; Torshizi, K. Comparison of oxidative quality of meat of chickens feed corn or wheat based diets with fennel (Foeniculum vulgare Mill), antibiotic and probiotic as feed additive, under different storage con-ditions. Arch. Fur Geflugelkd. 2013, 77, 199–205. [Google Scholar]
- Hodgson, I.; Stewart, J.; Fyfe, L. Inhibition of Bacteria and Yeast by Oil of Fennel and Paraben: Development of Synergistic Antimicrobial Combinations. J. Essent. Oil Res. 1998, 10, 293–297. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Studies on the influence of dietary spices on food transit time in experimental rats. Nutr. Res. 2001, 21, 1309–1314. [Google Scholar] [CrossRef]
- Elgayyar, M.; Draughon, F.A.; Golden, D.A.; Mount, J.R. Antimicrobial Activity of Essential Oils from Plants against Se-lected Pathogenic and Saprophytic Microorganisms. J. Food Prot. 2001, 64, 1019–1024. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Esteban, J.; Sanz, J. Comparison of the Volatile Composition of Wild Fennel Samples (Foeniculum vulgare Mill.) from Central Spain. J. Agric. Food Chem. 2006, 54, 6814–6818. [Google Scholar] [CrossRef]
- Premavalli, K.; Omprakash, A.V. Effect of dietary supplementation of fennel seeds (Foeniculum vulgare Mill.) on production performance of Japanese quail (Coturnix japonica). J. Ent. Zool Stud. 2020, 8, 1588–1590. [Google Scholar]
- Abdel-Latif, H.M.; Abdel-Daim, M.M.; Shukry, M.; Nowosad, J.; Kucharczyk, D. Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: A review. Aquaculture 2021, 547, 737369. [Google Scholar] [CrossRef]
- Tollba, A.; Hassan, M.S.H. Using some natural additives to improve physiological and productive performance of broiler chicken under high temperature conditions. Egypt Poult. Sci. J. 2003, 23, 313–326. [Google Scholar]
- Murray, R.K.; Granner, D.K.; Mayes, P.A.; Rodwell, V.W. The Text Book of Harper’s Biochemistry, 22nd ed.; Appletone: Altos, CA, USA, 1991. [Google Scholar]
- El-Deek, A.; Attia, Y.A.; Hannfy, M.M. Effects of anise, ginger, and fennel and their mixture on performance of broilers. Arch. Geflugelk 2003, 67, 92–96. [Google Scholar]
- Akdemir, F.; Sahin, K. Genistein supplementation to the quail: Effects on egg production and egg yolk genistein, daidzein, and lipid peroxidation levels. Poult. Sci. 2009, 88, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Bollengier-Lee, S.; Mitchell, M.; Utomo, D.; Williams, P.; Whitehead, C. Influence of high dietary vitamin E supplemen-tation on egg production and plasma characteristics in hens subjected to heat stress. Br. Poult. Sci. 1998, 39, 106–112. [Google Scholar] [CrossRef]
- Vakili, R.; Majidzadeh Heravi, R. Performance and egg quality of laying hens fed diets supplemented with herbal extracts and flaxseed. Poult. Sci. J. 2016, 4, 107–116. [Google Scholar]
- Wahab, F.; Chand, N.; Khan, R.U.; Ahmad, N.; Parvez, U.; Rehman, Z.U.; Naz, S. Dietary Supplementation of Fenugreek (Trigonella foenum graecum) on the Egg Quality Characteristics of Rhode Island Red Spent Layers. Pak. J. Zoöl. 2019, 51. [Google Scholar] [CrossRef]
- Souza, A.V.; Morais, M.V.M.; Rocha, M.C.; Souza, R.M.; Valentim, J.K.; Pietramale, R.T.R.; Silva, N.E.M.; Moraleco, D.D.; Lima, H.J.D. Influence of fennel in Japanese Quail Diet over egg quality and behavior aspects. Bol. de Indústria Anim. 2020, 77, 1–13. [Google Scholar] [CrossRef]
- Yazarlou, M.; Sharifi, S.; Melaki, M.; Zahedi, M.; Bahmani, K. The Effect of Fennel Seed Levels on the Physical and Qualitative Properties of Japanese Quail’s Egg”, the First National Seminar on the Management of Raising Poultry and Domestic Animals in the Tropical Regions; Shahid Bahonar University: Kerman, Branch, 2012; p. 948. [Google Scholar]
- Cantore, P.L.; Iacobellis, N.S.; De Marco, A.; Capasso, F.; Senatore, F. Antibacterial Activity of Coriandrum sativum L. and Foeniculum vulgare Miller Var. vulgare (Miller) Essential Oils. J. Agric. Food Chem. 2004, 52, 7862–7866. [Google Scholar] [CrossRef]
- Özcan, M.M.; Chalchat, J.-C.; Arslan, D.; Ateş, A.; Ünver, A. Comparative Essential Oil Composition and Antifungal Effect of Bitter Fennel (Foeniculum vulgare ssp. piperitum) Fruit Oils Obtained During Different Vegetation. J. Med. Food 2006, 9, 552–561. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Kujundžić, S.; Soković, M.; Couladis, M. Essential oil composition and antifungal activity of Foeniculum vulgare Mill. obtained by different distillation conditions. Phytother Res. 2003, 17, 368–371. [Google Scholar]
- Barrahi, M.; Esmail, A.; Elhartiti, H.; Chahboun, N.; Benali, A.; Amiyare, R.; Lakhrissi, B.; Rhaiem, N.; Zarrouk, A.; Ouhssine, M. Chemical composition and evaluation of antibacterial activity of fennel (Foeniculum vulgare Mill) seed essential oil against some pathogenic bacterial strains. Glob. J. Environ. Sci. 2020, 18, 295–307. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.; Colombatto, D.; McAllister, T.A.; Beauchemin, K. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2007, 145, 209–228. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.-M.; Amaral, L.; Bolla, J.-M. Geraniol Restores Antibiotic Activities against Multidrug-Resistant Isolates from Gram-Negative Species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannenas, I. How to use plant extracts and phytogenics in animal diets. In World Nutrition Forum, The Future of Animal Nutrition; Binder, E.M., Schatzmayr, G., Eds.; Nottingham University Press: Nottingham, UK, 2008; pp. 111–129. [Google Scholar]
- Deying, M.; Shan, A.; Chen, Z.; Du, J.; Song, K.; Li, J.; Xu, Q. Effect of Ligustrum lucidum and Schisandra chinensis on the egg production, antioxidant status and immunity of laying hens during heat stress. Arch. Anim. Nutr. 2005, 59, 439–447. [Google Scholar]
- Shawky, S.M.; Fathalla, S.I.; Zahran, I.S.; Gaafar, K.M.; Hussein, M.K.; Abu-Alya, I.S. Immunological Stimulant Effect of Linseed Oil and Fennel Oil Supplemented Diet on Broilers. Adv. Anim. Vet. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Akbarian, A. Alleviating Some Physiological Responses to High Ambient Temperatures in Finishing Broilers by Dietary Plant Extracts Rich in Phenolic Compounds. PhD Research. 2014. Available online: https://www.semanticscholar.org/paper/Alleviating-some-physiological-responses-to-high-in-Akbarian/9ea133f9bbccc256a454abf9217989ac9fa764cd (accessed on 31 December 2014).
- Samadi, Z.N.; Hadjzadeh, M.A.R.; Marjaneh, R.M.; Rad, A.K. The hepatoprotective effects of fennel seeds extract and trans anethole in streptozotocin-induced liver injury in rats. Food Sci. Nutr. 2020, 9, 1121–1131. [Google Scholar] [CrossRef]
- Nahid, S.; Montaseri, A.; Najafpour, A.; Dolatkhah, H.; Rajabzadeh, A.; Khaki, A.A. Study of Foeniculum vulgare (Fennel) Seed Extract Effects on Serum Level of Oxidative Stress. Crescent. J. Med. Biol. Sci. 2015, 2, 2. [Google Scholar]
- Khan, R.U.; Rahman, Z.U.; Nikousefat, Z.; Javdani, M.; Laudadio, V.; Tufarelli, V. Vitamin E: Pharmaceutical role in avian male fecundity. World’s Poult. Sci. J. 2012, 68, 63–70. [Google Scholar] [CrossRef]
- Chithra, V.; Leelamma, S. Foeniculum vulgare (fennel) changes the levels of peroxides and activity of antioxidant enzymes in experimental animals. Indian J. Biochem. Biophys 1999, 36, 59–61. [Google Scholar]
- Chang, S.H.; Bassiri, A.; Jalali, H. Evaluation of antioxidant activity of fennel (Foeniculum vulgare) seed extract on oxidative stability of olive oil. J. Chem. Health Risk 2013, 3, 53–61. [Google Scholar]
- Yang, C.; Chowdhury, M.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M. Effects of various feed additives on the methane emissions from beef cattle. Int. Congr. Ser. 2006, 1293, 152–155. [Google Scholar] [CrossRef]

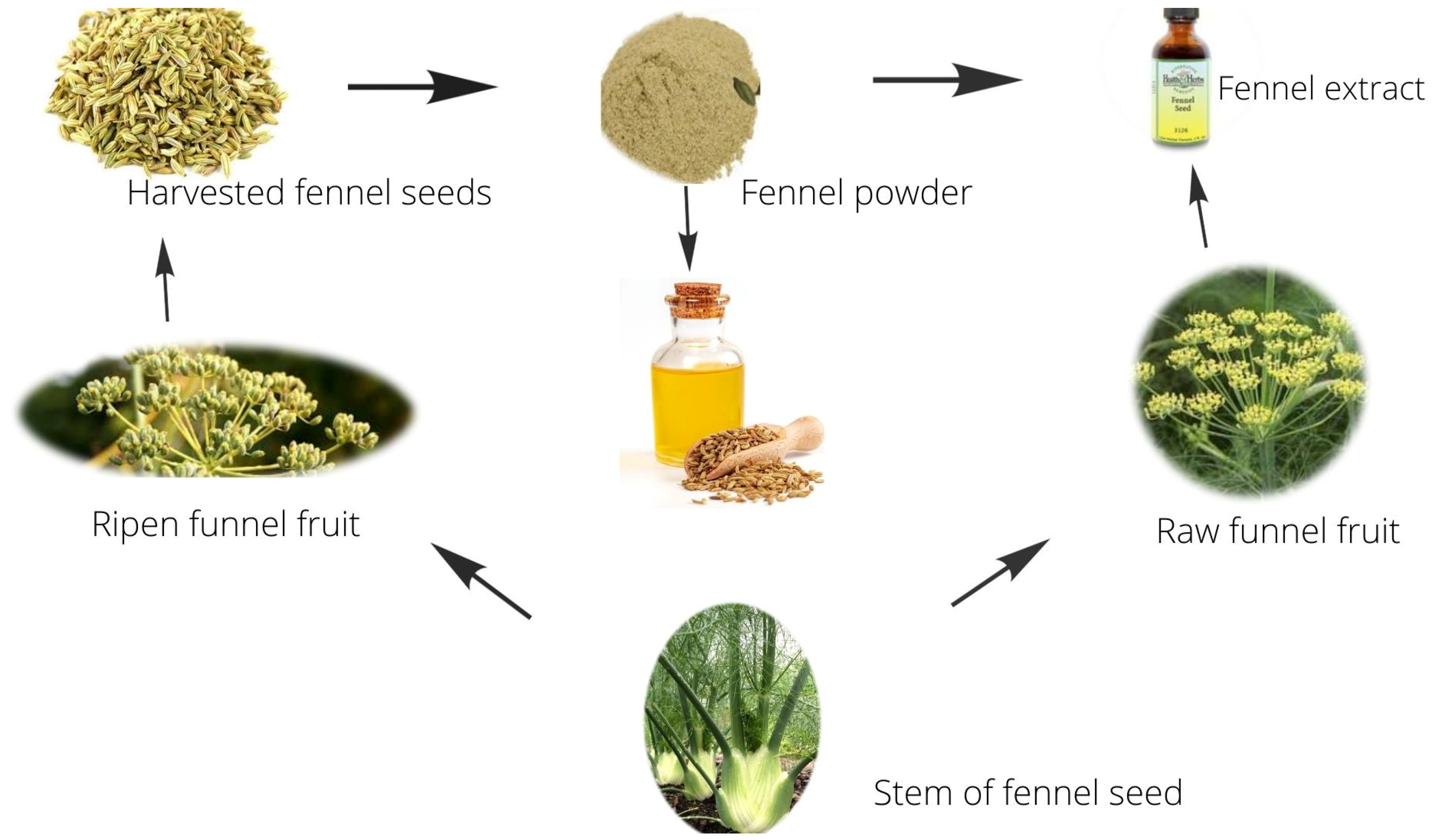
| Kingdom | Plantae | Plant family | Apiacea | Sowing time | March April |
| Division | Magnoliophyta | Plant height approx. | 40–200 cm | Best germination temperature | 15–20 °C |
| Class | Magnoliopsida | Flowering time | July August September | Germination time in days | 7–14 |
| Order | Apiales | Flower color | Yellow | Planting distance | 20–60 cm |
| Family | Apiaceae (Umbelliferae) | Root system | Taproot | Bad intercropping partner | Dill |
| Genus | Foeniculum | Lifecycle | Biennial Perennial Vivacious | ||
| Species | Foeniculum vulgare | Sunlight | Full sun |
| Nutrient Composition | Quantity/100 g | Minerals | Concentration, mg | Vitamins | Concentration |
|---|---|---|---|---|---|
| Moisture | 90.21 | Calcium, Ca | 49 | Vitamin C | 12 mg |
| Energy | 31 kcal | Iron, Fe | 0.73 | Thiamin B-1 | 0.01 mg |
| Protein | 1.24 | Magnesium, Mg | 17 | Riboflavin B-2 | 0.032 mg |
| Total lipid (fat) | 0.2 | Phosphorus, P | 50 | Niacin B-3 | 0.64 mg |
| Carbohydrate | 7.3 | Potassium, K | 414 | Vitamin B-6 | 0.047 mg |
| Total dietary fiber | 3.1 | Sodium, Na | 52 | Folate | 27 µg |
| Sugars | 3.93 | Zinc, Zn | 0.2 | Vitamin A | 48 µg |
| Lipids | Vitamin E | 0.58 mg | |||
| Fatty acids, total saturated | 0.09 | Essential amino acids | Concentration, mg | Nonessential amino acid | Concentration, mg |
| Fatty acid, total monounsaturated | 0.068 | Leucine | 0.63 | Glycine | 0.55 |
| Fatty acids, total polyunsaturated | 0.169 | Isoleucine | 0.73 | Proline | 0.53 |
| Phenylalanine | 0.45 | ||||
| Tryptophane | 0.53 | ||||
| Essential oils (% of total oil) | |||||
| Monoterpenes | Oxygenated monoterpene | ||||
| α-thujene | 0.14 | 1,8-cineol | 0.17 | ||
| α-pinene | 0.37 | Fenchone | 10.99 | ||
| Camphene | 0.08 | Linalool | 0.11 | ||
| Sabinene | 0.14 | Fenchyl alcohol | 0.04 | ||
| β-pinene | 0.05 | α–thujone | 0.04 | ||
| β-myrcene | 0.81 | Camphor | 0.47 | ||
| α-phellandrene | 0.18 | Estragole | 7.17 | ||
| Parameters | Dose | Source | Poultry Species | Effect | Reference |
|---|---|---|---|---|---|
| Feed Intake | 1.2 and 3.2% | Fennel seed pow-der | Broilers | Increased | Al-Sagon et al. [61] |
| 0.15 and 0.25 g/kg | Fennel essential oil | Broilers | Increased | Gharehsheikhlou et al. [62] | |
| 1 and 2% | Fennel seed | Broiler | Increased | Ragab [63] | |
| 40 mg/kg | Fennel extract | Laying hens | Increased | Vakili [64] | |
| 0.25 and 0.5% | Fennel seed Pow-der | Broilers | Increased | Saki et al. [65] | |
| 0.25, 0.50 and 0.75 g/kg | Fennel seed meal | Japanese quail | Increased | Henda et al. [66] | |
| 1.0% | Fennel seed | Japanese quail | Increased | Ragab [67] | |
| 5 g/kg | Fennel seed | Laying hens | Decreased | Abou-Elkhair et al. [68] | |
| 2.5% | Fennel seed pow-der | Broilers | Decreased | Zahira Abul-Jabbar et al. [69] | |
| 10 and 20 g/kg | Fennel seed fruit | Laying hens | No effect | Gharghani et al. [70] | |
| 0.25 to 1.5 g/kg | Fennel seed | Broilers | No effect | Soltan et al. [71] | |
| 200 mg/kg | Fennel essential oil | Broilers | Increased | A. R. Ghiasvand et al. [72] | |
| 5–10% | Fennel seed | Broilers | Increased | Milica et al. [73] | |
| 0.3, 0.6 and 0.9% | Fennel seeds | Broiler | No effect | Bugdaycı et al. [74] | |
| 24 mg/kg | Essential oil | Laying hens | No effect | Cabuk et al. [75] | |
| 250 to 750 g/50 kg | Fennel seed | Broilers | Increased | Saleh Lamarb et al. [76] | |
| Feed Efficiency | 24 mg/kg | Fennel essential oil | Broilers | No effect | Cabuk et al. [77] |
| 1.2 and 3.2% | Fennel seed pow-der | Broilers | Improved | Al-Sagan et al. [61] | |
| 100 mg/kg | Fennel essential oil | Broilers | Improved | Cengiz et al. [78] | |
| 5% | Fennel seed pow-der | Broilers | Improved | Zahira Abul-Jabbar et al. [69] | |
| 0.25 and 0.5% | Fennel seed pow-der | Broilers | Improved | Saki et al. [65] | |
| 1, 2 and 3 g/kg | Fennel seed | Broilers | Improved | Abdullah and Abbas [79] | |
| 5%, 10% or 15% | MOL | Japanese quail | Improved | Ragab [67] | |
| 300 mg | Fennel essential oil | Laying hens | No effect | Nasiroleslami et al. [80] | |
| 0.3, 0.6 and 0.9% | Fennel seed | Laying quails | No effect | Bugdaycı et al. [74] | |
| 5 g/kg | Fennel seed | Laying hens | Improved | Abou-Elkhair et al. [68] | |
| 0.25 and 0.5% | Fennel seed pow-der | Broilers | Improved | Saki et al. [65] | |
| 1, 2 and 3 g/kg | Fennel seed | Broilers | No effect | Abdullah and Abbas [79] | |
| 100 to 400 ppm | Fennel extract | Broilers | Not effected | Ali Safaei et al. [81] | |
| 250 to 750 g/50 kg | Fennel seed | Broilers | Improved | Saleh Lamarb et al. [76] | |
| Body Weight | 24 mg/kg | Essential oil | Laying hens | Improved | Cabuk et al. [77] |
| 0.3, 0.6 and 0.9% | Fennel seed | Laying quails | No effect | Bugdaycı et al. [74] | |
| 1, 2 and 3 g/kg | Fennel seed | Broilers | Increased | Abdullah and Abbas [79] | |
| 1% | Fennel seed with kemzyme dry and CP | Japanese quails | Increased | Ragab [67] | |
| 10 and 20 g/kg | Fennel fruit | Laying hens | Increased | Gharaghani et al. [70] | |
| 0.25, 0.5 and 0.75 g/kg | Fennel seed meal | Japanese quails | Increased | Henda et al. [66] | |
| 0.5, 1.0, and 1.5% | Fennel seed pow-der | Japanese quails | Increased | Premavalli et al. [81] | |
| 5 g/kg | Fennel seed | Laying hens | No effect | Abou-Al-khair et al. [68] | |
| 100 to 400 ppm | Fennel extract | Broilers | Increased | Ali Safaei et al. [81] | |
| Growth Performance | 250 to 750 g/50 kg | Fennel seed | Broilers | Increased | Saleh Lamarb et al. [76] |
| 1.2 and 3.2% | Fennel seed pow-der | Broilers | Increased | Al-Sagon et al. [61] | |
| 1% | Fennel seed | Japanese quails | Increased | Ragab [67] | |
| 0.15 and 0.25 g/kg | Fennel essential oil | Broilers | Increased | Gharehsheikhlou et al. [62] | |
| 300 mg/kg | Fennel essential oil | Laying hens | No effect | Nasiroleslami et al. [80] | |
| 250, 500 and 750 g/50 kg | Fennel seed | Broilers | Improved | Saleh Lamarb et al. [76] | |
| Carcass Traits/Dressing Percentage | 10 and 20 g/kg | Fennel fruit | Laying hens | Increased | Gharghani et al. [70] |
| 100 mg/kg | Fennel essential oil | Broilers | Improved | Cengis et al. [78] | |
| 1.2 and 3.2% | Fennel seed pow-der | Broilers | Improved | Al-Sagan et al. [61] | |
| 1, 2 and 3 g/kg | Fennel seed | Broilers | No effect except Pancreas and stomach weight percentage | Abdullah and Abbas [79] | |
| 0.5 and 1% | Fennel seed | Japanese quails | Improved | Ragab et al. [67] | |
| 0.25, 0.50 and 0.75 g/kg | Fennel Seed Meal | Japanese quails | Improved | Henda et al. [66] | |
| 100 mg/kg | Fennel oil | Broilers | No effect | Cengis et al. [78] | |
| 200 mg/kg | Fennel essential oil | Broilers | No effect | A. R. Ghiasvand et al. [72] | |
| Egg Production and Quality | 0.15 and 0.25 g/kg | Fennel essential oil | Broilers | Improved | Gharehsheikhlou et al. [62] |
| 50 mg/kg | Fennel Extract | Broiler breeder | Improved | Kazemi et al. [82] | |
| 10 mg/kg | Fennel seed ex-tract | Laying hens | Improved | Raza et al. [83] | |
| 300 mg/kg | Fennel essential oil | Laying hens | No effect on egg index and yolk index, improved egg shell weight and thickness Haugh unit decreased | Nasiroleslami et al. [80] | |
| 24 mg/kg | Fennel essential oil | Laying hens | Improved | Cabuk et al. [75] | |
| 0.3, 0.6 and 0.9% | Fennel seed | Laying quails | No effect | Bugdaycı et al. [74] | |
| Immunity | 24 mg/kg | Fennel essential oil | Laying hens | Improved | Cabuk et al. [77] |
| 50 mg/kg | Fennel extract | Broiler breeder | Improved | Kazemi et al. [84] | |
| 36 mg/kg | Fennel seed | Laying hens | Improved | K-Ozek [85] | |
| Fennel essential oil | Broilers | Ghiasvand et al. [72] | |||
| 48 mg/kg | Essential oil | Broilers | No effect on antibody titer against IBD and ND | Bozkurt et al. [86] | |
| Relative Weight of Lymphoid Organs | 100, 200, 300 and 400 ppm | Fennel extract | Broilers | ND, IBD titer improved | Ali Safaei et al. [81] |
| 60–120 ml/liter | Fennel seed meal | Japanese quails | Improved | Henda et al. [66] | |
| 0.5 and 1% | Fennel seed | Japanese quails | Improved | Ragab [67] | |
| 0.3 ml of fennel oil/kg | Fennel essential oil | Broilers | Improved | Zahira Abul-Jabbar et al. [69] | |
| 48 mg/kg | Essential oil | Broilers | No effect on relative weight of liver and Bursa | Bozkurt et al. [86] | |
| 1 and 2% | Fennel seed | Broilers | Improved | Ragab [63] | |
| 10 mg/kg | Fennel seed ex-tract | Laying hens | Improved | Raza et al. [83] | |
| 1, 2 and 3 g/kg | Fennel seed | Broilers | Improved | Abdullah and Abbas [79] | |
| Antioxidant Activity | 1.2 and 3.2% | Fennel seed pow-der | Broilers | Decreased MDA concentration | Al-Sagan et al. [61] |
| 5 g/kg | Fennel seed | Laying hens | Decreased MDA concentration | Abou-Al-Khair et al. [68] | |
| 10 and 20 g/kg | Fennel fruit | Laying hens | Decreased MDA concentration | Gharaghani et al. [70] | |
| Blood Biochemistry | 1% | Ground Fennel seed | Broilers | Decreased MDA concentration | Gharaghani et al. [87] |
| 1, 2 and 3 g/kg | Fennel seed | Broilers | Higher RBC count, Hb and PCV | Abdullah and Abbas [79] | |
| 0.5 and 1% | Fennel seed | Japanese quails | Higher contents of serum glucose, tri-glycerides, aspartate aminotransferase, alanine aminotransferase, total protein and albumin | Ragab.S et al [67] | |
| 1 and 2% | Fennel seed | Broilers | Improved leukocyte count | Ragab [63] | |
| 5% | Fennel seed pow-der | Broilers | Lower concentration of glucose, tri-glycerides and uric acid | Zahira Abul-Jabbar et al. [69] | |
| 10 mg/kg | Fennel seed ex-tract | Laying hens | No effect on cholesterol and triglyceride | Raza et al. [83] | |
| 100, 200, 300 and 400 ppm | Fennel extract | Broilers | No effect on concentration of glucose, triglyceride, LDL and alkaline phos-phatase while HDL increased, and uric acid decreased | Ali Safaei et al. [81] | |
| 0.25, 0.5 and 0.75 g/kg | Fennel seed meal | Japanese quails | Non-significant increase in serum total protein albumin and globulin | Henda et al. [66] | |
| Economics Efficiency | 0.15 and 0.25 g/kg | Fennel essential oil | Broilers | Improved the total cholesterol/HDL ratio and LDL/HDL ratio | Gharehsheikhlou et al. [62] |
| 200 mg/kg | Fennel essential oil | Broilers | No effect on blood lymphocyte and heterophil percentages and heterophil to lymphocyte ratio | A. R. Ghiasvand et al. [72] | |
| 3.2% | Fennel seed pow-der | Broilers | Increased net profit | Al-Sagon et al. [61] | |
| 0.25, 0.5 and 0.75 g/kg | Fennel seed meal | Japanese quails | Improved | Henda et al. [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.U.; Fatima, A.; Naz, S.; Ragni, M.; Tarricone, S.; Tufarelli, V. Perspective, Opportunities and Challenges in Using Fennel (Foeniculum vulgare) in Poultry Health and Production as an Eco-Friendly Alternative to Antibiotics: A Review. Antibiotics 2022, 11, 278. https://doi.org/10.3390/antibiotics11020278
Khan RU, Fatima A, Naz S, Ragni M, Tarricone S, Tufarelli V. Perspective, Opportunities and Challenges in Using Fennel (Foeniculum vulgare) in Poultry Health and Production as an Eco-Friendly Alternative to Antibiotics: A Review. Antibiotics. 2022; 11(2):278. https://doi.org/10.3390/antibiotics11020278
Chicago/Turabian StyleKhan, Rifat Ullah, Adia Fatima, Shabana Naz, Marco Ragni, Simona Tarricone, and Vincenzo Tufarelli. 2022. "Perspective, Opportunities and Challenges in Using Fennel (Foeniculum vulgare) in Poultry Health and Production as an Eco-Friendly Alternative to Antibiotics: A Review" Antibiotics 11, no. 2: 278. https://doi.org/10.3390/antibiotics11020278
APA StyleKhan, R. U., Fatima, A., Naz, S., Ragni, M., Tarricone, S., & Tufarelli, V. (2022). Perspective, Opportunities and Challenges in Using Fennel (Foeniculum vulgare) in Poultry Health and Production as an Eco-Friendly Alternative to Antibiotics: A Review. Antibiotics, 11(2), 278. https://doi.org/10.3390/antibiotics11020278









