What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections?
Abstract
:1. Introduction
2. Results
2.1. Demographics
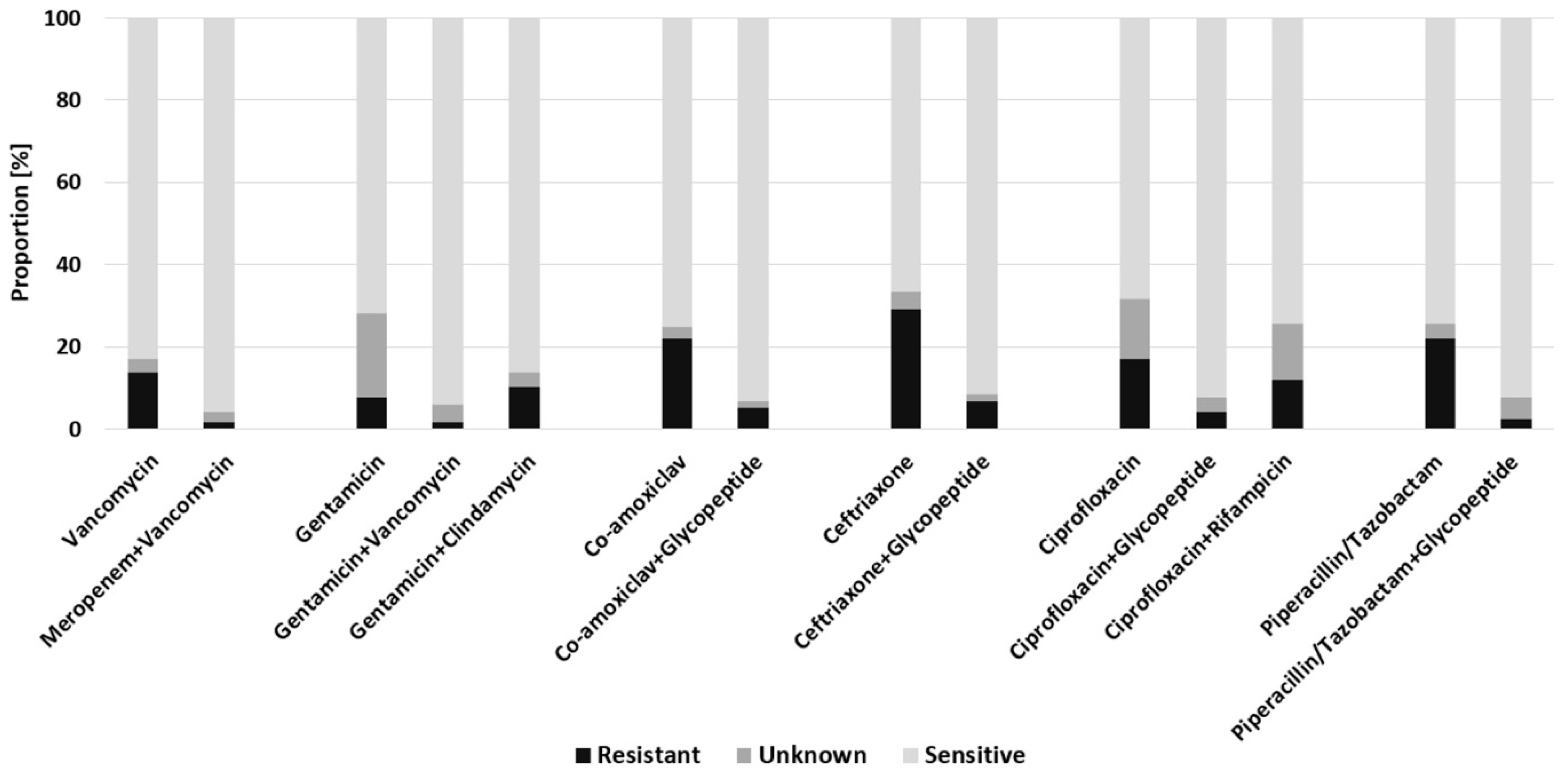
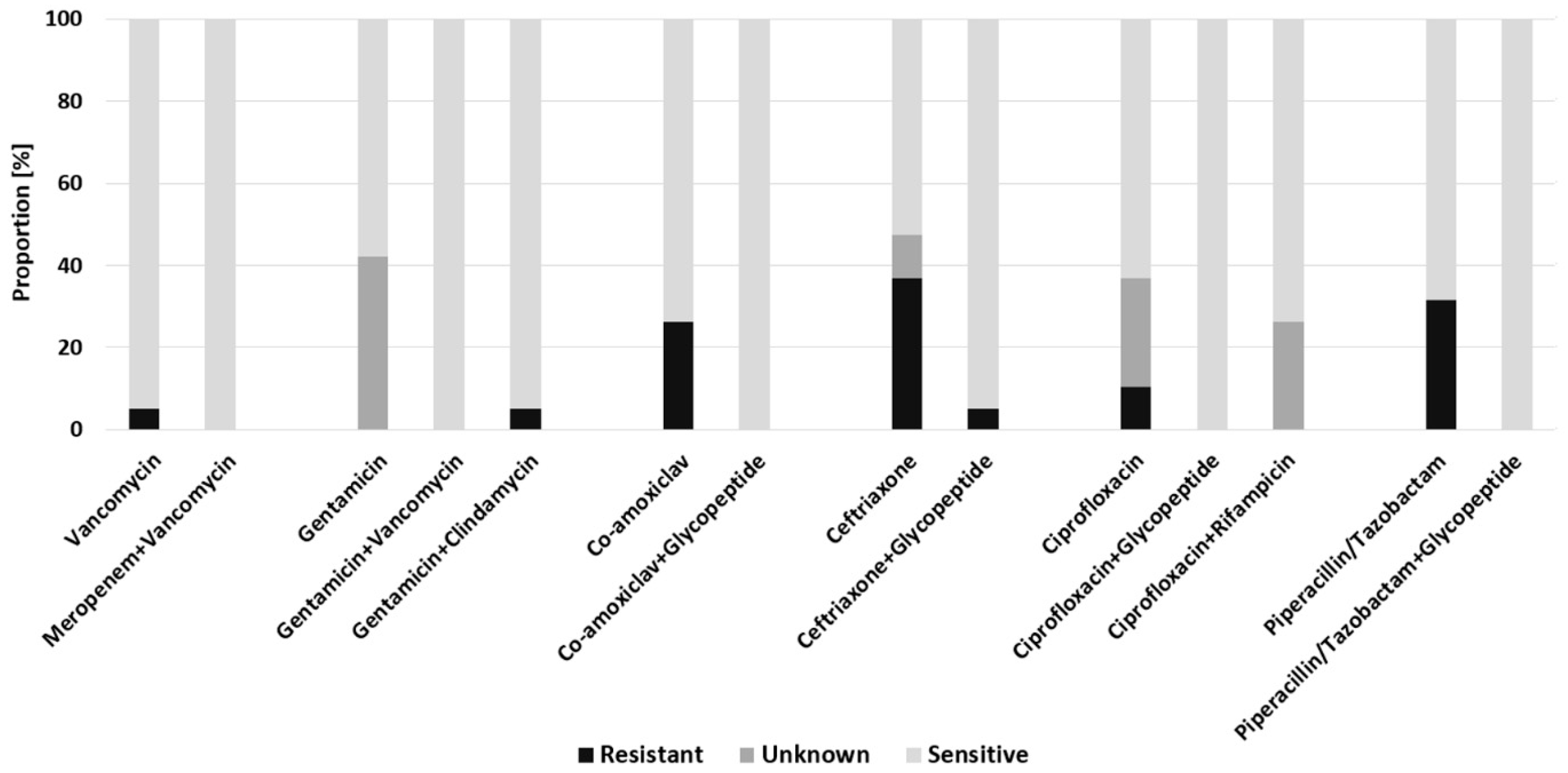
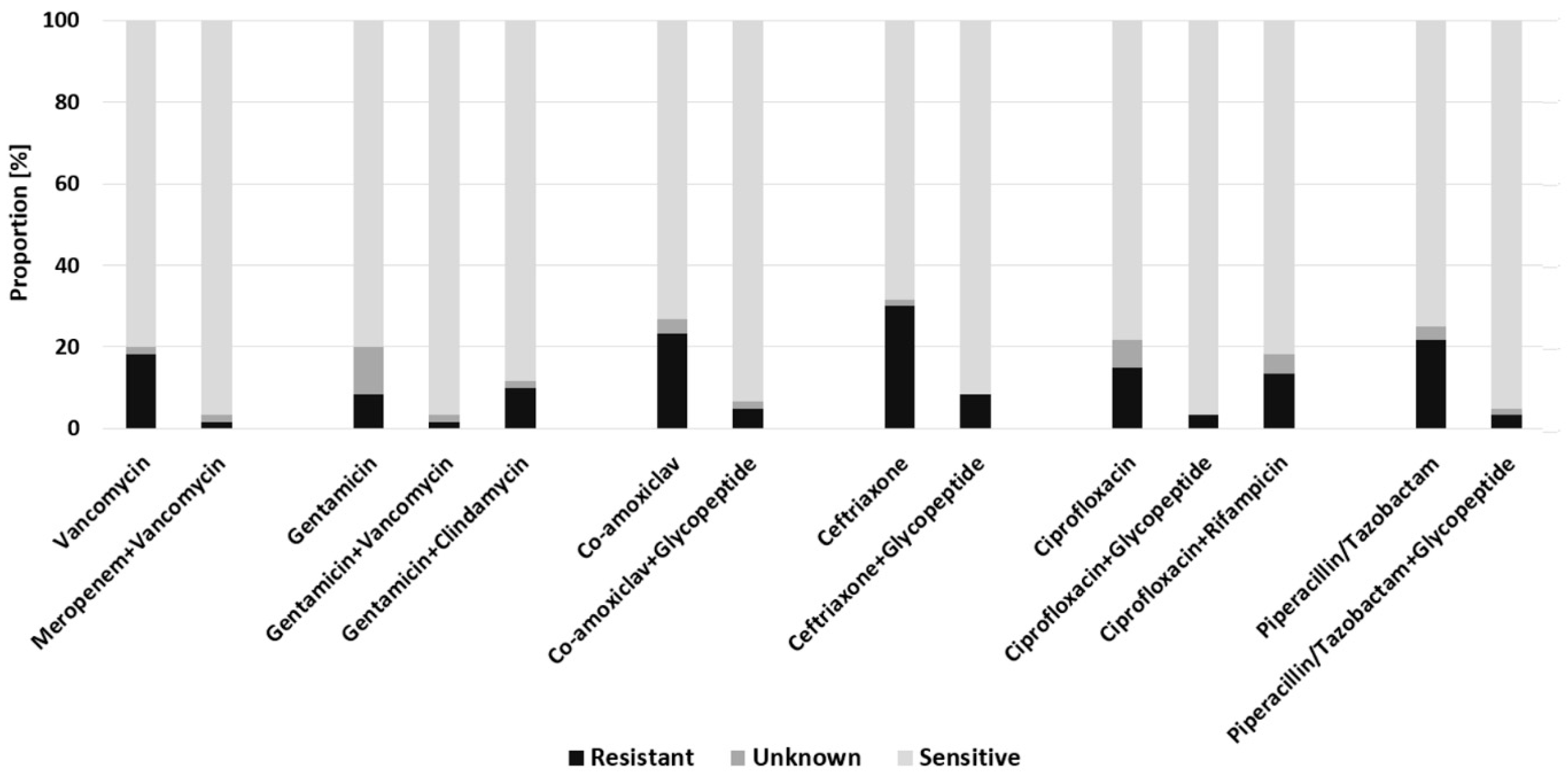
2.2. Empiric Antimicrobial Regimes in FRI
2.3. Empiric Antimicrobial Regimes in Early FRI
2.4. Empiric Antimicrobial Regimes in Delayed FRI
2.5. Empiric Antimicrobial Regimes in Late-Onset FRI
3. Discussion
3.1. Empirical Antibiotic Combination Therapy Is Warranted in FRI
3.2. Downside of Antibiotic Therapy—Development of Multidrug-Resistant Pathogens
3.3. Side Effects of Antibiotic Therapy
3.4. Local Antibiotic Therapy
3.5. Limitations
4. Materials and Methods
4.1. Patient Identification
4.2. Data Collection
4.3. Microbiology
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37 (Suppl. 2), S59–S66. [Google Scholar] [CrossRef] [PubMed]
- Ktistakis, I.; Giannoudi, M.; Giannoudis, P.V. Infection rates after open tibial fractures: Are they decreasing? Injury 2014, 45, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Court-Brown, C.M.; McQueen, M.M. Global Forum: Fractures in the Elderly. J. Bone Jt. Surg. Am. 2016, 98, e36. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.; Rupp, M.; Lang, S.; Alt, V. The epidemiology of fracture-related infections in Germany. Sci. Rep. 2021, 11, 10443. [Google Scholar] [CrossRef]
- Rupp, M.; Walter, N.; Pfeifer, C.; Lang, S.; Kerschbaum, M.; Krutsch, W.; Baumann, F.; Alt, V. The Incidence of Fractures Among the Adult Population of Germany-an Analysis From 2009 through 2019. Dtsch. Arztebl. Int. 2021, 118, 665–669. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Bezstarosti, H.; van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; Obremskey, W.; McNally, M.A.; Metsemakers, W.J.; Verhofstad, M.H.J. Insights into treatment and outcome of fracture-related infection: A systematic literature review. Arch. Orthop. Trauma Surg. 2019, 139, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Bose, D.; Kugan, R.; Stubbs, D.; McNally, M. Management of infected nonunion of the long bones by a multidisciplinary team. Bone Jt. J. 2015, 97-B, 814–817. [Google Scholar] [CrossRef]
- Alt, V.; Giannoudis, P.V. Musculoskeletal infections—A global burden and a new subsection in Injury. Injury 2019, 50, 2152–2153. [Google Scholar] [CrossRef]
- Walter, N.; Rupp, M.; Hierl, K.; Pfeifer, C.; Kerschbaum, M.; Hinterberger, T.; Alt, V. Long-term patient-related quality of life after fracture-related infections of the long bones. Bone Jt. Res. 2021, 10, 321–327. [Google Scholar] [CrossRef]
- Willenegger, H.; Roth, B. Behandlungstaktik und Spätergebnisse bei Frühinfekt nach Osteosynthese. Unfallchirurgie 1986, 12, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupp, M.; Bärtl, S.; Lang, S.; Walter, N.; Alt, V. Frakturassoziierte Infektionen nach Marknagelosteosynthese: Diagnostik und Therapie. Unfallchirurg 2021, 125, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.-J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef]
- Depypere, M.; Kuehl, R.; Metsemakers, W.-J.; Senneville, E.; McNally, M.A.; Obremskey, W.T.; Zimmerli, W.; Atkins, B.L.; Trampuz, A. Recommendations for Systemic Antimicrobial Therapy in Fracture-Related Infection: A Consensus From an International Expert Group. J. Orthop. Trauma 2020, 34, 30–41. [Google Scholar] [CrossRef]
- Walter, N.; Baertl, S.; Engelstaedter, U.; Ehrenschwender, M.; Hitzenbichler, F.; Alt, V.; Rupp, M. Letter in response to article in journal of infection: “The microbiology of chronic osteomyelitis: Changes over ten years”. J. Infect. 2021, 83, 709–737. [Google Scholar] [CrossRef]
- Foster, A.L.; Moriarty, T.F.; Trampuz, A.; Jaiprakash, A.; Burch, M.A.; Crawford, R.; Paterson, D.L.; Metsemakers, W.-J.; Schuetz, M.; Richards, R.G. Fracture-related infection: Current methods for prevention and treatment. Expert Rev. Anti Infect. Ther. 2020, 18, 307–321. [Google Scholar] [CrossRef]
- Rupp, M.; Baertl, S.; Walter, N.; Hitzenbichler, F.; Ehrenschwender, M.; Alt, V. Is There a Difference in Microbiological Epidemiology and Effective Empiric Antimicrobial Therapy Comparing Fracture-Related Infection and Periprosthetic Joint Infection? A Retrospective Comparative Study. Antibiotics 2021, 10, 921. [Google Scholar] [CrossRef]
- Dudareva, M.; Hotchen, A.J.; Ferguson, J.; Hodgson, S.; Scarborough, M.; Atkins, B.L.; McNally, M.A. The microbiology of chronic osteomyelitis: Changes over ten years. J. Infect. 2019, 79, 189–198. [Google Scholar] [CrossRef]
- Malizos, K.; Sarma, J.; Seaton, R.A.; Militz, M.; Menichetti, F.; Riccio, G.; Gaudias, J.; Trostmann, U.; Pathan, R.; Hamed, K. Daptomycin for the treatment of osteomyelitis and orthopaedic device infections: Real-world clinical experience from a European registry. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Telles, J.P.; Cieslinski, J.; Tuon, F.F. Daptomycin to bone and joint infections and prosthesis joint infections: A systematic review. Braz. J. Infect. Dis. 2019, 23, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Uçkay, I.; Vaudaux, P.; François, P.; Schrenzel, J.; Harbarth, S.; Laurent, F.; Bernard, L.; Vandenesch, F.; Etienne, J.; et al. Risk factors for treatment failure in orthopedic device-related methicillin-resistant Staphylococcus aureus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.-A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Valour, F.; Karsenty, J.; Bouaziz, A.; Ader, F.; Tod, M.; Lustig, S.; Laurent, F.; Ecochard, R.; Chidiac, C.; Ferry, T. Antimicrobial-related severe adverse events during treatment of bone and joint infection due to methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Triffault-Fillit, C.; Valour, F.; Guillo, R.; Tod, M.; Goutelle, S.; Lustig, S.; Fessy, M.-H.; Chidiac, C.; Ferry, T. Prospective Cohort Study of the Tolerability of Prosthetic Joint Infection Empirical Antimicrobial Therapy. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Mruk, A.L.; Record, K.E. Antimicrobial options in the treatment of adult staphylococcal bone and joint infections in an era of drug shortages. Orthopedics 2012, 35, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Challagundla, S.R.; Knox, D.; Hawkins, A.; Hamilton, D.; Flynn, R.W.V.; Robertson, S.; Isles, C. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol. Dial. Transplant. 2013, 28, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Torkington, M.S.; Davison, M.J.; Wheelwright, E.F.; Jenkins, P.J.; Anthony, I.; Lovering, A.M.; Blyth, M.; Jones, B. Bone penetration of intravenous flucloxacillin and gentamicin as antibiotic prophylaxis during total hip and knee arthroplasty. Bone Jt. J. 2017, 99-B, 358–364. [Google Scholar] [CrossRef]
- Hellebrekers, P.; Verhofstad, M.H.J.; Leenen, L.P.H.; Varol, H.; van Lieshout, E.M.M.; Hietbrink, F. The effect of early broad-spectrum versus delayed narrow-spectrum antibiotic therapy on the primary cure rate of acute infection after osteosynthesis. Eur. J. Trauma Emerg. Surg. 2020, 46, 1341–1350. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, M.; Vallejo, A.; McNally, M.A.; Moriarty, T.F.; Ferguson, J.Y.; Nijs, S.; Metsemakers, W.J. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Jt. Res. 2018, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Svensson Malchau, K.; Tillander, J.; Zaborowska, M.; Hoffman, M.; Lasa, I.; Thomsen, P.; Malchau, H.; Rolfson, O.; Trobos, M. Biofilm properties in relation to treatment outcome in patients with first-time periprosthetic hip or knee joint infection. J. Orthop. Translat. 2021, 30, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Frew, N.M.; Cannon, T.; Nichol, T.; Smith, T.J.; Stockley, I. Comparison of the elution properties of commercially available gentamicin and bone cement containing vancomycin with ‘home-made’ preparations. Bone Jt. J. 2017, 99-B, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Meyer, C. Antibiotic Elution from Hip and Knee Acrylic Bone Cement Spacers: A Systematic Review. Biomed. Res. Int. 2017, 2017, 4657874. [Google Scholar] [CrossRef] [Green Version]
- Rupp, M.; Kern, S.; Weber, T.; Menges, T.D.; Schnettler, R.; Heiß, C.; Alt, V. Polymicrobial infections and microbial patterns in infected nonunions—A descriptive analysis of 42 cases. BMC Infect. Dis. 2020, 20, 667. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Steiner, O.; Fasshold, L.; Goessler, W.; Holl, A.-M.; Kühn, K.-D. The stability of carbapenems before and after admixture to PMMA-cement used for replacement surgery caused by Gram-negative bacteria. Eur. J. Med. Res. 2020, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Alt, V. Antimicrobial coated implants in trauma and orthopaedics-A clinical review and risk-benefit analysis. Injury 2017, 48, 599–607. [Google Scholar] [CrossRef]
- Wasko, M.K.; Borens, O. Antibiotic cement nail for the treatment of posttraumatic intramedullary infections of the tibia: Midterm results in 10 cases. Injury 2013, 44, 1057–1060. [Google Scholar] [CrossRef]
- Jorge-Mora, A.; Amhaz-Escanlar, S.; Fernandez-Pose, S.; García-Iglesias, A.; Mandia-Mancebo, F.; Franco-Trepat, E.; Guillán-Fresco, M.; Pino-Minguez, J. Commercially available antibiotic-laden PMMA-covered locking nails for the treatment of fracture-related infections—A retrospective case analysis of 10 cases. J. Bone Jt. Infect. 2019, 4, 155–162. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Morgenstern, M.; McNally, M.A.; Moriarty, T.F.; McFadyen, I.; Scarborough, M.; Athanasou, N.A.; Ochsner, P.E.; Kuehl, R.; Raschke, M.; et al. Fracture-related infection: A consensus on definition from an international expert group. Injury 2018, 49, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Metsemakers, W.-J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]

| Characteristic | All (n = 117) | Early (n = 19) | Delayed (n = 60) | Late (n = 38) |
|---|---|---|---|---|
| Demographic data | ||||
| Sex (male) | 85 (72.6%) | 13 (68.4%) | 43 (71.7%) | 29 (76.3%) |
| Age (years) | 55.5 ± 16.8 | 58.1 ± 18.7 | 55.4 ± 17.4 | 54.4 ± 15.1 |
| BMI (kg/m2) | 27.4 ± 5.2 | 28.0 ± 5.3 | 27.4 ± 5.2 | 27.2 ± 5.4 |
| ASA score (range) | 2 (1–4) | 2 (1–3) | 2 (1–4) | 2 (1–4) |
| CCI (range) | 1 (0–6) | 1 (0–4) | 1 (0–5) | 1 (0–6) |
| Site | ||||
| Femur | 17 (14.5%) | 0 | 12 (20.0%) | 5 (13.2%) |
| Shoulder | 7 (6.0%) | 3 (15.8%) | 2 (3.3%) | 2 (5.2%) |
| Forearm | 4 (3.4%) | 2 (10.5%) | 2 (3.3%) | 0 |
| Hand | 1 (0.9%) | 0 | 0 | 1 (2.6%) |
| Tibia | 46 (39.3%) | 9 (47.4%) | 20 (33.3%) | 17 (44.7%) |
| Ankle | 22 (18.8%) | 3 (15.8%) | 13 (21.7%) | 6 (15.8%) |
| Foot | 16 (13.7%) | 1 (5.3%) | 10 (16.7%) | 5 (13.2%) |
| Spine | 4 (3.4%) | 1 (5.3%) | 1 (1.7%) | 2 (5.3%) |
| Chronology of infection | ||||
| Delay from initial fracture care to symptoms (weeks) | 34.5 ± 93.5 | 1.3 ± 0.5 | 4.8 ± 2.2. | 98.1 ± 145.7 |
| Delay from symptoms to surgical treatment for FRI (weeks) | 1.3 ± 2.5 | 1.6 ± 4.3 | 1.1 ± 2.0 | 1.5 ± 2.1 |
| Microbiologic documentation | ||||
| Negative culture | 11 (9.4%) | 0 | 9 (15.0%) | 2 (5.3%) |
| Polymicrobial infection | 10 (8.6%) | 3 (15.8%) | 6 (10.0%) | 1 (2.6%) |
| Pathogen | All (n = 116) | Early (n = 22) | Delayed (n = 56) | Late (n = 38) |
|---|---|---|---|---|
| Staphylococcus aureus (MSSA) Staphylococcus aureus (MRSA) | 46 (39.7%) 1 (0.9%) | 9 (40.9%) | 22 (39.3%) | 15 (39.51%) 1 (2.6%) |
| Staphylococcus epidermidis | 20 (17.2%) | 4 (18.2%) | 9 (16.1%) | 7 (18.4%) |
| Other Staphylococcus species | 11 (9.5%) | 3 (13.6%) | 4 (7.1%) | 4 (10.5%) |
| Streptococcus species | 7 (6.0%) | 1 (4.6%) | 3 (5.4%) | 3 (7.9%) |
| Enterococcus species | 6 (5.2%) | 2 (9.0%) | 3 (5.4%) | 1 (2.6%) |
| Gram-negative bacteria | 19 (16.4%) | 1 (4.6%) | 13 (23.2%) | 5 (13.2%) |
| Other | 6 (5.2%) | 2 (9.0%) | 2 (3.6%) | 2 (5.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baertl, S.; Walter, N.; Engelstaedter, U.; Ehrenschwender, M.; Hitzenbichler, F.; Alt, V.; Rupp, M. What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections? Antibiotics 2022, 11, 287. https://doi.org/10.3390/antibiotics11030287
Baertl S, Walter N, Engelstaedter U, Ehrenschwender M, Hitzenbichler F, Alt V, Rupp M. What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections? Antibiotics. 2022; 11(3):287. https://doi.org/10.3390/antibiotics11030287
Chicago/Turabian StyleBaertl, Susanne, Nike Walter, Ulrike Engelstaedter, Martin Ehrenschwender, Florian Hitzenbichler, Volker Alt, and Markus Rupp. 2022. "What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections?" Antibiotics 11, no. 3: 287. https://doi.org/10.3390/antibiotics11030287
APA StyleBaertl, S., Walter, N., Engelstaedter, U., Ehrenschwender, M., Hitzenbichler, F., Alt, V., & Rupp, M. (2022). What Is the Most Effective Empirical Antibiotic Treatment for Early, Delayed, and Late Fracture-Related Infections? Antibiotics, 11(3), 287. https://doi.org/10.3390/antibiotics11030287







