Influence of Sub-Inhibitory Dosage of Cefotaxime on Multidrug Resistant Staphylococcus haemolyticus Isolated from Sick Neonatal Care Unit
Abstract
:1. Introduction
2. Results
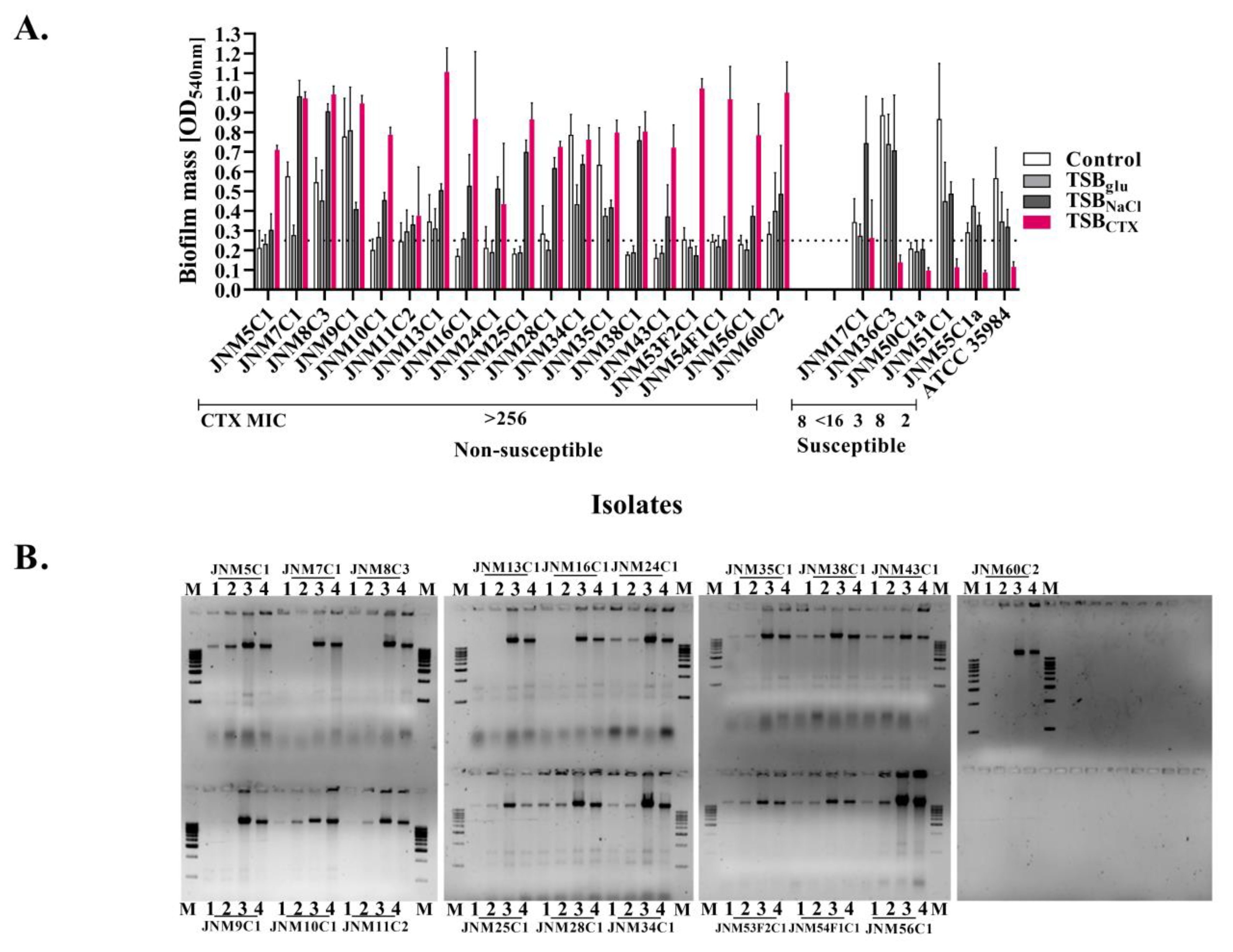
2.1. Susceptibility Profiling
2.2. Biofilm Enhancement and eDNA Release among the Isolates
2.3. Whole Genome Sequencing and Resistome Mapping of Ancestral Populations
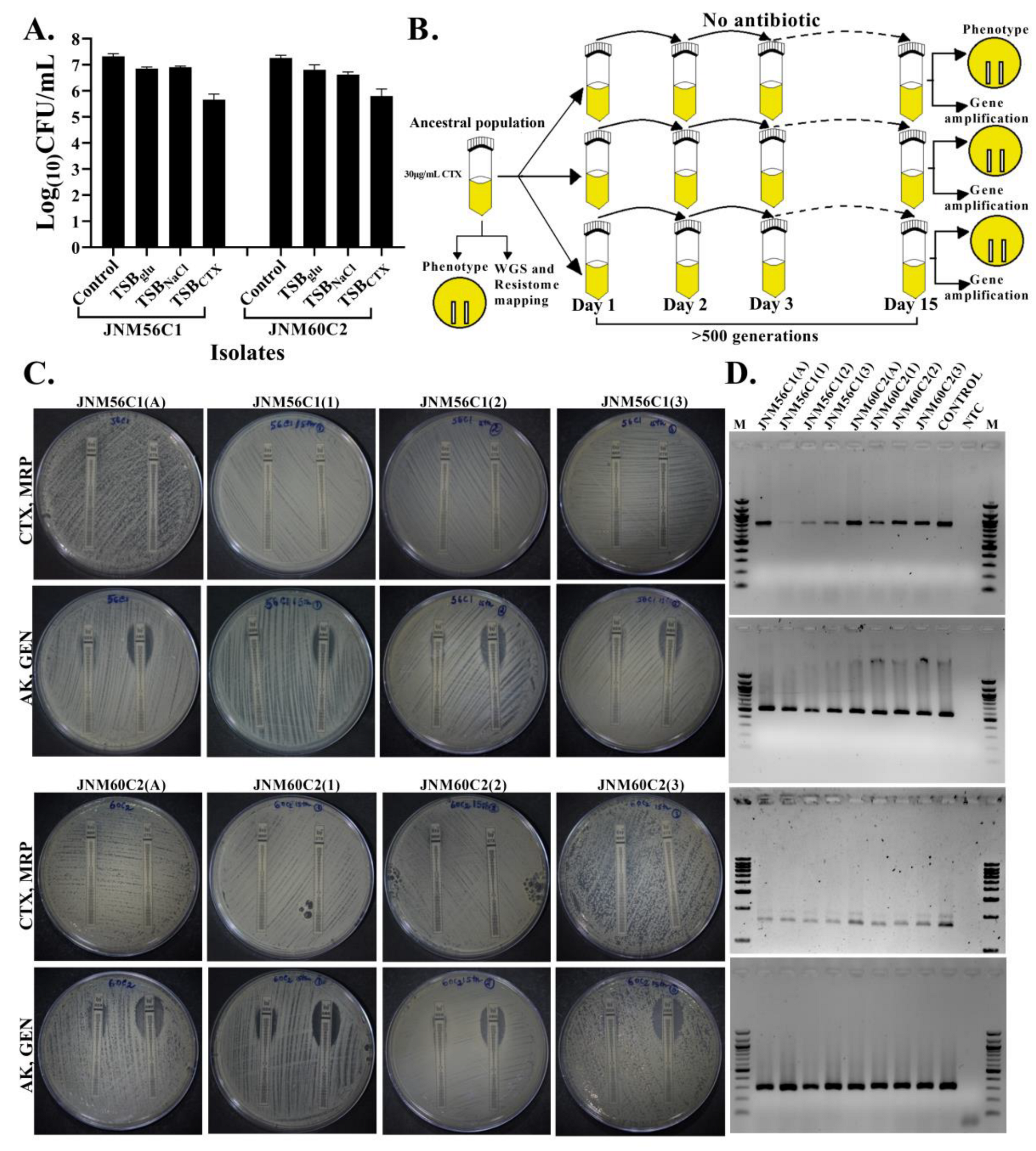
2.4. No Change in Susceptibility in the Absence of Antibiotic Selection
3. Discussion
4. Materials and Methods
4.1. Minimum Inhibitory Concentration (MIC) Determination of S. haemolyticus Isolates
4.2. Quantification of Biofilms
4.3. Extracellular DNA (eDNA) Quantification
4.4. Genomic DNA Isolation and Whole-Genome Sequencing
4.5. Short-Term Evolution Experiment
4.6. Amplification of Genes
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, V.C.; Seixas, M.D.L.; Guimarães, L.C.; Ferreira, D.C.; Da Cunha, D.C.; Nouér, S.A.; Dos Santos, K.R.N. High rate of neonates colonized by methicillin-resistant Staphylococcus species in an Intensive Care Unit. J. Infect. Dev. Ctries. 2019, 13, 810–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaha, D.C.; Zdrinca, M.M.; Vesa, C.M.; Daina, L.G.; Daina, M.C. A six-year evaluation of sepsis in neonates. Rom. Biotechnol. Lett. 2020, 25, 1892–1898. [Google Scholar] [CrossRef]
- Czekaj, T.; Ciszewski, M.; Szewczyk, E.M. Staphylococcus haemolyticus—An emerging threat in the twilight of the antibiotics age. Microbiology 2015, 161, 2061–2068. [Google Scholar] [CrossRef]
- Cavanagh, J.P.; Hjerde, E.; Holden, M.T.; Kahlke, T.; Klingenberg, C.; Flægstad, T.; Parkhill, J.; Bentley, S.D.; Sollid, J.U. Whole-genome sequencing reveals clonal expansion of multiresistant Staphylococcus haemolyticus in European hospitals. J. Antimicrob. Chemother. 2014, 69, 2920–2927. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Ribeiro, P.M.; Sued-Karam, B.R.; Faria, Y.V.; Nogueira, B.A.; Colodette, S.S.; Fracalanzza, S.E.; Duarte, J.L.; Júnior, R.H.; Mattos-Guaraldi, A.L. Influence of antibiotics on biofilm formation by different clones of nosocomial Staphylococcus haemolyticus. Future Microbiol. 2019, 14, 789–799. [Google Scholar] [CrossRef]
- Giormezis, N.; Kolonitsiou, F.; Foka, A.; Drougka, E.; Liakopoulos, A.; Makri, A.; Papanastasiou, A.D.; Vogiatzi, A.; Dimitriou, G.; Marangos, M.; et al. Coagulase-negative staphylococcal bloodstream and prosthetic-device-associated infections: The role of biofilm formation and distribution of adhesin and toxin genes. J. Med. Microbiol. 2014, 63, 1500–1508. [Google Scholar] [CrossRef]
- Pereira, P.M.; Binatti, V.B.; Sued, B.P.; Ramos, J.N.; Peixoto, R.S.; Simões, C.; de Castro, E.A.; Duarte, J.L.; Vieira, V.V.; Hirata, R.; et al. Staphylococcus haemolyticus disseminated among neonates with bacteremia in a neonatal intensive care unit in Rio de Janeiro, Brazil. Diagn. Microbiol. Infect. Dis. 2014, 78, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Neonatal Mortality-UNICEF Data. Available online: https://data.unicef.org/topic/child-survival/neonatal-mortality/ (accessed on 23 February 2022).
- Jain, K.; Sankar, M.J.; Nangia, S.; Ballambattu, V.B.; Sundaram, V.; Ramji, S.; Plakkal, N.; Kumar, P.; Jain, A.; Sivanandan, S.; et al. Causes of death in preterm neonates (<33 weeks) born in tertiary care hospitals in India: Analysis of three large prospective multicentric cohorts. J. Perinatol. 2019, 39, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Wattal, C.; Kler, N.; Oberoi, J.K.; Fursule, A.; Kumar, A.; Thakur, A. Neonatal Sepsis: Mortality and Morbidity in Neonatal Sepsis due to Multidrug-Resistant (MDR) Organisms: Part 1. Indian J. Pediatr. 2020, 87, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Investigators of the Delhi Neonatal Infection Study (DeNIS) Collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: A cohort study. Lancet Glob. Health 2016, 4, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Nazir, A. Neonatal sepsis due to coagulase negative Staphylococci: A study from Kashmir valley, India. Int. J. Contemp. Pediatr. 2019, 6, 650. [Google Scholar] [CrossRef]
- Härtel, C.; Faust, K.; Fortmann, I.; Humberg, A.; Pagel, J.; Haug, C.; Kühl, R.; Bohnhorst, B.; Pirr, S.; Viemann, D.; et al. Sepsis related mortality of extremely low gestational age newborns after the introduction of colonization screening for multi-drug resistant organisms. Antimicrob. Resist. Infect. Control 2020, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Kranjec, C.; Kristensen, S.S.; Bartkiewicz, K.T.; Brønner, M.; Cavanagh, J.P.; Srikantam, A.; Mathiesen, G.; Diep, D.B. A bacteriocin-based treatment option for Staphylococcus haemolyticus biofilms. Sci. Rep. 2021, 11, 13909. [Google Scholar] [CrossRef] [PubMed]
- Al-Mousawi, A.H.; Al-Kaabi, S.J.; Albaghdadi, A.J.H.; Almulla, A.F.; Raheem, A.; Algon, A.A.A. Effect of Black Grape Seed Extract (Vitis vinifera) on Biofilm Formation of Methicillin-Resistant Staphylococcus aureus and Staphylococcus haemolyticus. Curr. Microbiol. 2020, 77, 238–245. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- de Oliveira, A.; Cataneli Pereira, V.; Pinheiro, L.; Moraes Riboli, D.F.; Benini Martins, K.; Ribeiro de Souza da Cunha, M. Antimicrobial Resistance Profile of Planktonic and Biofilm Cells of Staphylococcus aureus and Coagulase-Negative Staphylococci. Int. J. Mol. Sci. 2016, 17, 1423. [Google Scholar] [CrossRef] [Green Version]
- Martins, K.B.; Ferreira, A.M.; Pereira, V.C.; Pinheiro, L.; de Oliveira, A.; da Cunha, M. In vitro Effects of Antimicrobial Agents on Planktonic and Biofilm Forms of Staphylococcus saprophyticus Isolated From Patients with Urinary Tract Infections. Front. Microbiol. 2019, 10, 40. [Google Scholar] [CrossRef]
- Alexander, H.K.; MacLean, R.C. Stochastic bacterial population dynamics restrict the establishment of antibiotic resistance from single cells. Proc. Natl. Acad. Sci. USA 2020, 117, 19455–19464. [Google Scholar] [CrossRef]
- Song, T.; Duperthuy, M.; Wai, S.N. Sub-Optimal Treatment of Bacterial Biofilms. Antibiotics 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicaksono, W.A.; Erschen, S.; Krause, R.; Müller, H.; Cernava, T.; Berg, G. Enhanced survival of multi-species biofilms under stress is promoted by low-abundant but antimicrobial-resistant keystone species. J. Hazard. Mater. 2022, 422, 126836. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Sun, F.J.; Wang, Q.; Liu, Y.; Xiong, L.R.; Xia, P.Y. Erythromycin resistance features and biofilm formation affected by subinhibitory erythromycin in clinical isolates of Staphylococcus epidermidis. J. Microbiol. Immunol. Infect. 2016, 49, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. mBio 2012, 3, e00198-12. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.; Epstein, S.B.; Callahan, M.T.; Piotrowski, B.O.; Simon, G.L.; Roberts, A.D.; Keiser, J.F.; Kaplan, J.B. Induction of MRSA Biofilm by Low-Dose β-Lactam Antibiotics: Specificity, Prevalence and Dose-Response Effects. Dose-Response 2013, 12, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Hallinen, K.M.; Wood, K.B. Interplay between Antibiotic Efficacy and Drug-Induced Lysis Underlies Enhanced Biofilm Formation at Subinhibitory Drug Concentrations. Antimicrob. Agents Chemother. 2017, 62, e01603-17. [Google Scholar] [CrossRef] [Green Version]
- Tahrioui, A.; Duchesne, R.; Bouffartigues, E.; Rodrigues, S.; Maillot, O.; Tortuel, D.; Hardouin, J.; Taupin, L.; Groleau, M.C.; Dufour, A.; et al. Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation. NPJ Biofilms Microbiomes 2019, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Cadavid, E.; Robledo, S.M.; Quiñones, W.; Echeverri, F. Induction of Biofilm Formation in Klebsiella pneumoniae ATCC 13884 by Several Drugs: The Possible Role of Quorum Sensing Modulation. Antibiotics 2018, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, S.; Biswal, N.; Bethou, A.; Mathai, B. Comparison of two empiric antibiotic regimen in late onset neonatal sepsis--a randomized controlled trial. J. Trop. Pediatr. 2014, 60, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Yen, P.; Papin, J.A. History of antibiotic adaptation influences microbial evolutionary dynamics during subsequent treatment. PLoS Biol. 2017, 15, e2001586. [Google Scholar] [CrossRef] [Green Version]
- Dorado-Morales, P.; Garcillán-Barcia, M.P.; Lasa, I.; Solano, C. Fitness Cost Evolution of Natural Plasmids of Staphylococcus aureus. mBio 2021, 12, e03094-20. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.P.; Klingenberg, C.; Hanssen, A.M.; Fredheim, E.A.; Francois, P.; Schrenzel, J.; Flægstad, T.; Sollid, J.E. Core genome conservation of Staphylococcus haemolyticus limits sequence based population structure analysis. J. Microbiol. Methods 2012, 89, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pain, M.; Hjerde, E.; Klingenberg, C.; Cavanagh, J.P. Comparative Genomic Analysis of Staphylococcus haemolyticus Reveals Key to Hospital Adaptation and Pathogenicity. Front. Microbiol. 2019, 10, 2096. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.; Sivanandan, S.; Agarwal, R.; Ellis, S.; Sharland, M.; Sankar, M.J. Neonatal sepsis in South Asia: Huge burden and spiralling antimicrobial resistance. BMJ 2019, 364, k5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brochmann, P.R.; Hesketh, A.; Jana, B.; Brodersen, G.H.; Guardabassi, L. Transcriptome analysis of extended-spectrum β-lactamase-producing Escherichia coli and methicillin-resistant Staphylococcus aureus exposed to cefotaxime. Sci. Rep. 2018, 8, 16076. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Sato, Y.; Unno, Y.; Ubagai, T.; Ono, Y. Sub-minimum inhibitory concentrations of colistin and polymyxin B promote Acinetobacter baumannii biofilm formation. PLoS ONE 2018, 13, e0194556. [Google Scholar] [CrossRef] [Green Version]
- Teh, A.; Lee, S.M.; Dykes, G.A. Growth in the presence of specific antibiotics induces biofilm formation by a Campylobacter jejuni strain sensitive to them but not in resistant strains. J. Glob. Antimicrob. Resist. 2019, 18, 55–58. [Google Scholar] [CrossRef]
- Vinod Kumar, K.; Lall, C.; Vimal Raj, R.; Vedhagiri, K.; Sunish, I.P.; Vijayachari, P. Can Subminimal Inhibitory Concentrations of Antibiotics Induce the Formation of Biofilm in Leptospira? Microb. Drug Resist. 2018, 24, 1040–1042. [Google Scholar] [CrossRef]
- Lorian, V. Some effects of subinhibitory concentrations of antibiotics on bacteria. Bull. N. Y. Acad. Med. 1975, 51, 1046–1055. [Google Scholar]
- Panda, S.; Jena, S.; Sharma, S.; Dhawan, B.; Nath, G.; Singh, D.V. Identification of Novel Sequence Types among Staphylococcus haemolyticus Isolated from Variety of Infections in India. PLoS ONE 2016, 11, e0166193. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Singh, D.V. Biofilm Formation by ica-Negative Ocular Isolates of Staphylococcus haemolyticus. Front. Microbiol. 2018, 9, 2687. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, A.H.; Wong, A.; Kassen, R. The fitness costs of antibiotic resistance mutations. Evol. Appl. 2015, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Rasouly, A.; Shamovsky, Y.; Epshtein, V.; Tam, K.; Vasilyev, N.; Hao, Z.; Quarta, G.; Pani, B.; Li, L.; Vallin, C.; et al. Analysing the fitness cost of antibiotic resistance to identify targets for combination antimicrobials. Nat. Microbiol. 2021, 6, 1410–1423. [Google Scholar] [CrossRef]
- Cao, S.; Huseby, D.L.; Brandis, G.; Hughes, D. Alternative Evolutionary Pathways for Drug-Resistant Small Colony Variant Mutants in Staphylococcus aureus. mBio 2017, 8, e00358-17. [Google Scholar] [CrossRef] [Green Version]
- Lamrabet, O.; Martin, M.; Lenski, R.E.; Schneider, D. Changes in Intrinsic Antibiotic Susceptibility during a Long-Term Evolution Experiment with Escherichia coli. mBio 2019, 10, e00189-19. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI M100-S27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, B.; Bardhan, T.; Chakraborty, M.; Basu, M. Resistance profiles and resistome mapping of multidrug resistant carbapenem-hydrolyzing Klebsiella pneumoniae strains isolated from the nares of preterm neonates. Int. J. Antimicrob. Agents 2019, 53, 535–537. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, M.; Bardhan, T.; Basu, M.; Bhattacharjee, B. Influence of Sub-Inhibitory Dosage of Cefotaxime on Multidrug Resistant Staphylococcus haemolyticus Isolated from Sick Neonatal Care Unit. Antibiotics 2022, 11, 360. https://doi.org/10.3390/antibiotics11030360
Chakraborty M, Bardhan T, Basu M, Bhattacharjee B. Influence of Sub-Inhibitory Dosage of Cefotaxime on Multidrug Resistant Staphylococcus haemolyticus Isolated from Sick Neonatal Care Unit. Antibiotics. 2022; 11(3):360. https://doi.org/10.3390/antibiotics11030360
Chicago/Turabian StyleChakraborty, Madhurima, Taniya Bardhan, Manjari Basu, and Bornali Bhattacharjee. 2022. "Influence of Sub-Inhibitory Dosage of Cefotaxime on Multidrug Resistant Staphylococcus haemolyticus Isolated from Sick Neonatal Care Unit" Antibiotics 11, no. 3: 360. https://doi.org/10.3390/antibiotics11030360






