Distribution of Carbapenemase Genes among Carbapenem-Non-Susceptible Acinetobacter baumanii Blood Isolates in Indonesia: A Multicenter Study
Abstract
:1. Introduction
2. Results
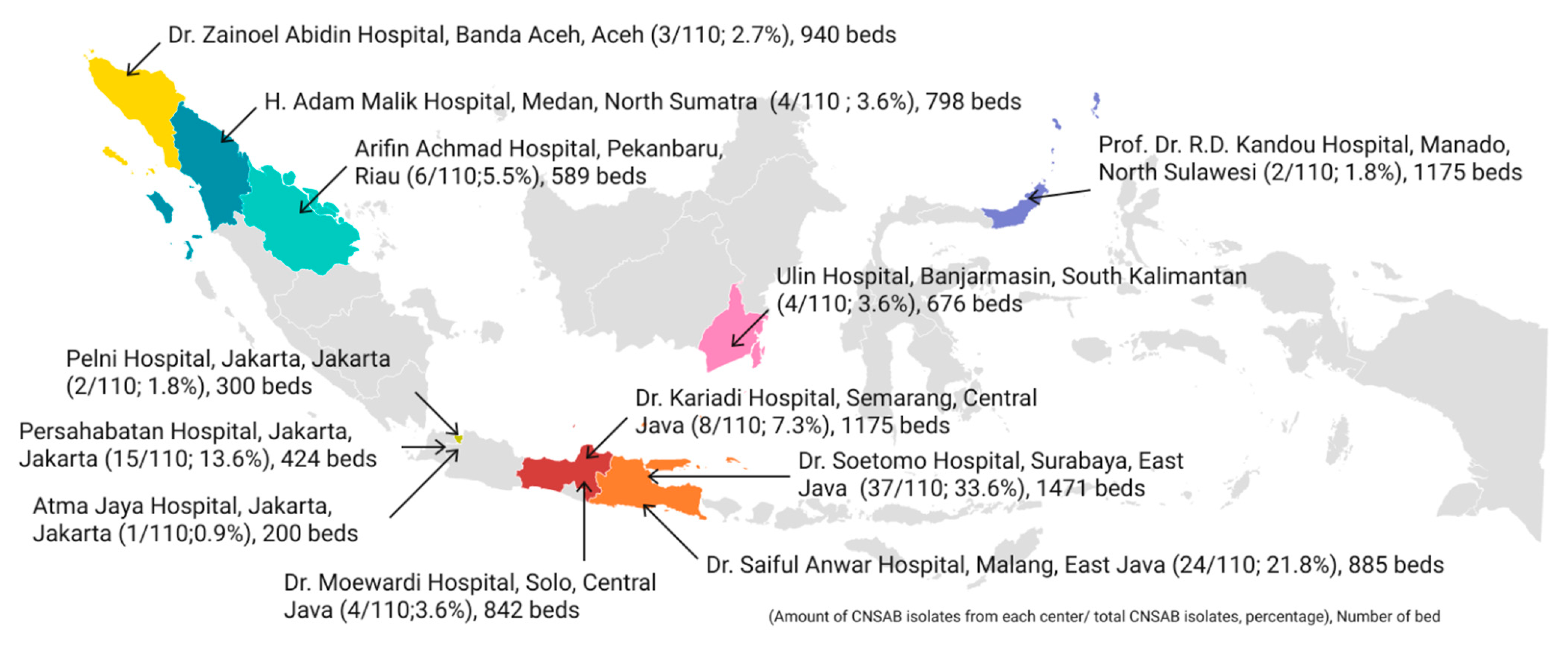
2.1. Distribution of CNSAB Isolates and Patients’ Characteristics
2.2. Antibiotic Resistance Test Results
2.3. Distribution of the Carbapenemase Genes
2.4. Association between the Presence of Carbapenemase Genes and Antibiotic Resistance Tests
3. Discussion
4. Materials and Methods
4.1. Study Setting
4.2. Bacterial Isolates
4.3. Confirmatory Test for A. baumannii
4.4. Detection of Carbapenemase Genes
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowak, P.; Paluchowska, P. Acinetobacter baumannii: Biology and drug resistance—Role of carbapenemases. Folia Histochem. Cytobiol. 2016, 54, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Durante-Mangoni, E.; Zarrilli, R. Global spread of drug-resistant Acinetobacter baumannii: Molecular epidemiology and management of antimicrobial resistance. Future Microbiol. 2011, 6, 407–422. [Google Scholar] [CrossRef]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Montefour, K.; Frieden, J.; Hurst, S.; Helmich, C.; Headley, D.; Martin, M.; Boyle, D.A. Acinetobacter baumannii: An emerging multidrug-resistant pathogen in critical care. Crit. Care Nurse 2008, 28, 15–25; quiz 26. [Google Scholar] [CrossRef]
- Sebeny, P.J.; Riddle, M.S.; Petersen, K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin. Infect. Dis. 2008, 47, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Esterly, J.S.; Griffith, M.; Qi, C.; Malczynski, M.; Postelnick, M.J.; Scheetz, M.H. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob. Agents Chemother. 2011, 55, 4844–4849. [Google Scholar] [CrossRef] [Green Version]
- Doi, Y.; Murray, G.L.; Peleg, A.Y. Acinetobacter baumannii: Evolution of antimicrobial resistance-treatment options. Semin. Respir. Crit. Care Med. 2015, 36, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Rossi, I.; Royer, S.; Ferreira, M.L.; Campos, P.A.; Fuga, B.; Melo, G.N.; Machado, L.G.; Resende, D.S.; Batistao, D.; Urzedo, J.E.; et al. Incidence of infections caused by carbapenem-resistant Acinetobacter baumannii. Am. J. Infect. Control 2019, 47, 1431–1435. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Apisarnthanarak, A.; Khan, E.; Suwantarat, N.; Ghafur, A.; Tambyah, P.A. Carbapenem-Resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin. Microbiol. Rev. 2017, 30, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Karuniawati, A.; Saharman, Y.R.; Lestari, D.C. Detection of carbapenemase encoding genes in Enterobacteriace, Pseudomonas aeruginosa, and Acinetobacter baumanii isolated from patients at Intensive Care Unit Cipto Mangunkusumo Hospital in 2011. Acta Med. Indones. 2013, 45, 101–106. [Google Scholar]
- Kim, U.J.; Kim, H.K.; An, J.H.; Cho, S.K.; Park, K.H.; Jang, H.C. Update on the Epidemiology, Treatment, and Outcomes of Carbapenem-resistant Acinetobacter infections. Chonnam. Med. J. 2014, 50, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Anggraini, D.; Santosaningsih, D.; Dwi Endraswari, P.; Moehario, L.; Riezke, C.V.; Enty, E.; Marindra, F.; Verbrugh, H.A. Epidemiology study of Acinetobacter spp. isolated from blood culture in Indonesia. Int. J. Infect. Dis. 2020, 101, 62–63. [Google Scholar] [CrossRef]
- Eichenberger, E.M.; Thaden, J.T. Epidemiology and Mechanisms of Resistance of Extensively Drug Resistant Gram-Negative Bacteria. Antibiotics 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Evans, B.A.; Amyes, S.G. OXA beta-lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [Green Version]
- Jeannot, K.; Diancourt, L.; Vaux, S.; Thouverez, M.; Ribeiro, A.; Coignard, B.; Courvalin, P.; Brisse, S. Molecular epidemiology of carbapenem non-susceptible Acinetobacter baumannii in France. PLoS ONE 2014, 9, e115452. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Shibl, A.M.; Ali, M.S.; Khubnani, H.; Radwan, H.H.; Livermore, D.M. Distribution of β-lactamases in carbapenem-non-susceptible Acinetobacter baumannii in Riyadh, Saudi Arabia. J. Glob. Antimicrob. Resist. 2014, 2, 17–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, F.; Luo, Y.; Fan, B.; Tao, Z.; Li, Y.; Gu, D. Distribution pattern of carbapenemases and solitary contribution to resistance in clinical strains of Acinetobacter baumannii. Ann. Palliat. Med. 2021, 10, 9184–9191. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, B.; Luo, Y.; Tao, Z.; Nie, Y.; Wang, Y.; Ding, F.; Li, Y.; Gu, D. Comparative analysis of carbapenemases, RND family efflux pumps and biofilm formation potential among Acinetobacter baumannii strains with different carbapenem susceptibility. BMC Infect. Dis. 2021, 21, 841. [Google Scholar] [CrossRef]
- Endo, S.; Yano, H.; Hirakata, Y.; Arai, K.; Kanamori, H.; Ogawa, M.; Shimojima, M.; Ishibashi, N.; Aoyagi, T.; Hatta, M.; et al. Molecular epidemiology of carbapenem-non-susceptible Acinetobacter baumannii in Japan. J. Antimicrob. Chemother. 2012, 67, 1623–1626. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, H.; Klugman, K.P.; Walsh, T.; Ramon-Pardo, P.; Sigauque, B.; Khan, W.; Laxminarayan, R.; Heddini, A.; Stelling, J. A framework for global surveillance of antibiotic resistance. Drug Resist. Update 2011, 14, 79–87. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; De Pascale, G.; Manno, D.; Spanu, T.; Cambieri, A.; Antonelli, M.; Sanguinetti, M.; Fadda, G.; Cauda, R. Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J. Antimicrob. Chemother. 2008, 62, 1130–1137. [Google Scholar] [CrossRef]
- Saharman, Y.R.; Karuniawati, A.; Sedono, R.; Aditianingsih, D.; Sudarmono, P.; Goessens, W.H.F.; Klaassen, C.H.W.; Verbrugh, H.A.; Severin, J.A. Endemic carbapenem-nonsusceptible Acinetobacter baumannii-calcoaceticus complex in intensive care units of the national referral hospital in Jakarta, Indonesia. Antimicrob. Resist. Infect. Control 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Tjoa, E.; Moehario, L.H.; Rukmana, A.; Rohsiswatmo, R. Acinetobacter baumannii: Role in Blood Stream Infection in Neonatal Unit, Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia. Int. J. Microbiol. 2013, 2013, 180763. [Google Scholar] [CrossRef] [Green Version]
- Tsiatsiou, O.; Iosifidis, Ε.; Katragkou, A.; Dimou, V.; Sarafidis, K.; Karampatakis, T.; Antachopoulos, C.; Orfanou, A.; Tsakris, A.; Drossou-Agakidou, V.; et al. Successful management of an outbreak due to carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. Eur. J. Pediatr. 2015, 174, 65–74. [Google Scholar] [CrossRef]
- Maciel, W.G.; da Silva, K.E.; Croda, J.; Cayô, R.; Ramos, A.C.; de Sales, R.O.; de Almeida de Souza, G.H.; Bampi, J.V.B.; Limiere, L.C.; Casagrande, J.C.; et al. Clonal spread of carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. J. Hosp. Infect. 2018, 98, 300–304. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef] [Green Version]
- Piperaki, E.T.; Tzouvelekis, L.S.; Miriagou, V.; Daikos, G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019, 25, 951–957. [Google Scholar] [CrossRef]
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69 (Suppl. S7), S565–S575. [Google Scholar] [CrossRef] [Green Version]
- AlAmri, A.M.; AlQurayan, A.M.; Sebastian, T.; AlNimr, A.M. Molecular Surveillance of Multidrug-Resistant Acinetobacter baumannii. Curr. Microbiol. 2020, 77, 335–342. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vardakas, K.Z.; Roussos, N.S. Trimethoprim/sulfamethoxazole for Acinetobacter spp.: A review of current microbiological and clinical evidence. Int. J. Antimicrob. Agents 2015, 46, 231–241. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Fleming-Dutra, K.E.; Roberts, R.M.; Hicks, L.A. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm. Rep. 2016, 65, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Raz-Pasteur, A.; Liron, Y.; Amir-Ronen, R.; Abdelgani, S.; Ohanyan, A.; Geffen, Y.; Paul, M. Trimethoprim-sulfamethoxazole vs. colistin or ampicillin-sulbactam for the treatment of carbapenem-resistant Acinetobacter baumannii: A retrospective matched cohort study. J. Glob. Antimicrob. Resist. 2019, 17, 168–172. [Google Scholar] [CrossRef]
- Nirwati, H.; Hakim, M.S.; Darma, S.; Mustafa, M.; Nuryastuti, T. Detection of blaoxa genes and identification of biofilm-producing capacity of Acinetobacter baumannii in a tertiary teaching hospital, Klaten, Indonesia. Med. J. Malaysia 2018, 73, 291–296. [Google Scholar]
- Pournaras, S.; Gogou, V.; Giannouli, M.; Dimitroulia, E.; Dafopoulou, K.; Tsakris, A.; Zarrilli, R. Single-locus-sequence-based typing of blaOXA-51-like genes for rapid assignment of Acinetobacter baumannii clinical isolates to international clonal lineages. J. Clin. Microbiol. 2014, 52, 1653–1657. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Choi, J.Y.; Kim, H.W.; Kim, S.H.; Chung, D.R.; Peck, K.R.; Thamlikitkul, V.; So, T.M.; Yasin, R.M.; Hsueh, P.R.; et al. Spread of carbapenem-resistant Acinetobacter baumannii global clone 2 in Asia and AbaR-type resistance islands. Antimicrob. Agents Chemother. 2013, 57, 5239–5246. [Google Scholar] [CrossRef] [Green Version]
- Kazi, M.; Nikam, C.; Shetty, A.; Rodrigues, C. Dual-tubed multiplex-PCR for molecular characterization of carbapenemases isolated among Acinetobacter spp. and Pseudomonas spp. J. Appl. Microbiol. 2015, 118, 1096–1102. [Google Scholar] [CrossRef]
- Goic-Barisic, I.; Kovacic, A.; Medic, D.; Jakovac, S.; Petrovic, T.; Tonkic, M.; Novak, A.; Rubic, Z.; Radic, M.; Milosavljevic, B.; et al. Endemicity of OXA-23 and OXA-72 in clinical isolates of Acinetobacter baumannii from three neighbouring countries in Southeast Europe. J. Appl. Genet. 2021, 62, 353–359. [Google Scholar] [CrossRef]
- Ruiz, M.; Marti, S.; Fernandez-Cuenca, F.; Pascual, A.; Vila, J. High prevalence of carbapenem-hydrolysing oxacillinases in epidemiologically related and unrelated Acinetobacter baumannii clinical isolates in Spain. Clin. Microbiol. Infect. 2007, 13, 1192–1198. [Google Scholar] [CrossRef] [Green Version]
- Quinteira, S.; Grosso, F.; Ramos, H.; Peixe, L. Molecular epidemiology of imipenem-resistant Acinetobacter haemolyticus and Acinetobacter baumannii isolates carrying plasmid-mediated OXA-40 from a Portuguese hospital. Antimicrob. Agents Chemother. 2007, 51, 3465–3466. [Google Scholar] [CrossRef] [Green Version]
- Hoang Quoc, C.; Nguyen Thi Phuong, T.; Nguyen Duc, H.; Tran Le, T.; Tran Thi Thu, H.; Nguyen Tuan, S.; Phan Trong, L. Carbapenemase Genes and Multidrug Resistance of Acinetobacter baumannii: A Cross Sectional Study of Patients with Pneumonia in Southern Vietnam. Antibiotics 2019, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [Green Version]
- CEFOBID1. Cefoperazone Package Insert; Pfizer: New York, NY, USA, 2006. [Google Scholar]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2021. [Google Scholar]
- Garcia, L.S. Clinical Microbiology Procedures Handbook; American Society for Microbiology Press: Washington DC, USA, 2010; Volume 1. [Google Scholar]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, M.H. Heat Treatment of Bacteri: A Simple Method of DNA Extraction for Molecular Techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Higgins, P.G.; Wisplinghoff, H.; Krut, O.; Seifert, H. A PCR-based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin. Microbiol. Infect. 2007, 13, 1199–1201. [Google Scholar] [CrossRef] [Green Version]
- Higgins, P.G.; Lehmann, M.; Wisplinghoff, H.; Seifert, H. gyrB multiplex PCR to differentiate between Acinetobacter calcoaceticus and Acinetobacter genomic species 3. J. Clin. Microbiol. 2010, 48, 4592–4594. [Google Scholar] [CrossRef] [Green Version]
- Nishio, H.; Komatsu, M.; Shibata, N.; Shimakawa, K.; Sueyoshi, N.; Ura, T.; Satoh, K.; Toyokawa, M.; Nakamura, T.; Wada, Y.; et al. Metallo-β-Lactamase-Producing Gram-Negative Bacilli: Laboratory-Based Surveillance in Cooperation with 13 Clinical Laboratories in the Kinki Region of Japan. J. Clin. Microbiol. 2004, 42, 5256–5263. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, P.; Poirel, L. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 2002, 8, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, Y.; Wilharm, G.; Zander, E.; Wichelhaus, T.A.; Gottig, S.; Hunfeld, K.P.; Seifert, H.; Witte, W.; Higgins, P.G. Molecular characterization of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J. Antimicrob. Chemother. 2011, 66, 1998–2001. [Google Scholar] [CrossRef] [Green Version]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endraputra, P.N.; Kuntaman, K.; Wasito, E.B.; Shirakawa, T.; Raharjo, D.; Setyarini, W. Profile variation of bla genes among non-lactose fermenting Gram-negative bacilli between clinical and environmental isolates of Dr. Soetomo Hospital, Surabya, Indonesia. Biodiversitas 2021, 22, 5047–5054. [Google Scholar] [CrossRef]


| Characteristics | Group | Frequency (%) |
|---|---|---|
| Sex | Male | 66 (60.0) |
| Female | 44 (40.0) | |
| Age (years) | <1 | 17 (15.5) |
| 1–14 | 10 (9.1) | |
| 15–64 | 69 (62.7) | |
| 65–70 | 10 (9.1) | |
| >70 | 4 (3.6) | |
| Unit | Intensive care unit | 84 (76.3) |
| Non-intensive care unit | 26 (24.7) |
| Gene Distribution | Frequency (%) |
|---|---|
| blaOXA-51-like | 110 (100.0) |
| blaOXA-23-like | 92 (83.6) |
| blaOXA-24-like | 41 (37.3) |
| blaOXA-48-like | 0 (0.0) |
| blaNDM-1 | 5 (4.5) |
| blaVIM | 1 (0.9) |
| blaIMP-1 | 1 (0.9) |
| Gene combinations | |
| One gene | 83 (75.5) |
| blaOXA-23-like | 66 (60.9) |
| blaOXA-24-like | 17 (15.5) |
| Two-gene combinations | 25 (22.7) |
| blaOXA-23-like/blaOXA-24-like | 21 (19.1) |
| blaOXA-23-like/blaNDM-1 | 2 (1.8) |
| blaOXA-24-like/blaNDM-1 | 1 (0.9) |
| blaOXA-23-like/blaIMP-1 | 1 (0.9) |
| Three-gene combinations | 2 (1.8) |
| blaOXA-23-like/blaOXA-24-like/blaNDM-1 | 1 (0.9) |
| blaOXA-23-like/blaOXA-24-like/blaVIM | 1 (0.9) |
| Carbapenemase Gene | Java (n = 116) | Outside Java (n = 24) |
|---|---|---|
| Frequency (%) | Frequency (%) | |
| blaOXA-23-like | 75 (82.4) | 17 (89.5) |
| blaOXA-24-like | 38 (41.8) | 3 (15.8) |
| blaNDM-1 | 2 (2.2) | 3 (15.8) |
| blaVIM | 1 (1.1) | 0 (0.0) |
| blaIMP-1 | 0 (0.0) | 1 (5.3) |
| Name of Antibiotic | Antibiotic Susceptibility Result | blaOXA-23-like | p-Value | blaOXA-24-like | p-Value | ||
|---|---|---|---|---|---|---|---|
| Detected | Not Detected | Detected | Not Detected | ||||
| Ampicillin-sulbactam | Susceptible | 2 (2.2) | 0 (0.0) | 1.000 | 0 (0.0) | 2 (2.9) | 0.528 |
| Non-susceptible | 90 (97.8) | 18 (100) | 41 (100) | 67 (97.1) | |||
| Piperacillin-tazobactam | Susceptible | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Non-susceptible | 92 (100) | 18 (100) | 41 (100) | 68 (100) | |||
| Cefazoline | Susceptible | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Non-susceptible | 92 (100) | 18 (100) | 41 (100) | 69 (100) | |||
| Ceftriaxone | Susceptible | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Non-susceptible | 92 (100) | 18 (100) | 41 (100) | 69 (100) | |||
| Ceftazidime | Susceptible | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Non-susceptible | 92 (100) | 18 (100) | 41 (100) | 69 (100) | |||
| Cefepime | Susceptible | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Non-susceptible | 92 (100) | 18 (100) | 41 (100) | 69 (100) | |||
| Gentamicin | Susceptible | 3 (3.3) | 1 (5.6) | 0.516 | 1 (2.4) | 3 (4.3) | 1.000 |
| Non-susceptible | 89 (96.7) | 17 (94.4) | 40 (97.6) | 66 (95.7) | |||
| Amikacin | Susceptible | 22 (23.9) | 2 (11.1) | 0.352 | 3 (7.3) | 21 (30.4) | 0.005 * |
| Non-susceptible | 70 (76.1) | 16 (88.9) | 38 (92.7) | 48 (69.6) | |||
| Ciprofloxacin | Susceptible | 4 (4.3) | 0 (0.0) | 1.000 | 0 (0.0) | 4 (5.8) | 0.295 |
| Non-susceptible | 88 (95.7) | 18 (100) | 41 (100) | 65 (94.2) | |||
| Levofloxacin | Susceptible | 3 (3.3) | 0 (0) | 1.000 | 0 (0) | 3 (4.3) | 0.292 |
| Non-susceptible | 89 (96.7) | 18 (100) | 41 (100) | 66 (95.7) | |||
| Tigecycline | Susceptible | 41(44.6) | 9 (50.0) | 0.672 | 11 (26.8) | 39 (56.5) | 0.002 * |
| Non-susceptible | 51 (55.4) | 9 (50.0) | 30 (73.2) | 30 (43.5) | |||
| Trimethoprim-sulfamethoxazole | Susceptible | 41 (44.6) | 1 (5.6) | 0.002 * | 7 (17.1) | 35 (50.7) | <0.001 ** |
| Non-susceptible | 51 (55.4) | 17 (94.4) | 34 (82.9) | 34 (49.3) | |||
| Fosfomycin | Susceptible | 11 (12.0) | 2 (11.1) | 1.000 | 6 (14.6) | 7 (10.1) | 0.547 |
| Non-susceptible | 81 (88.0) | 16 (88.9) | 35 (85.4) | 62 (89.9) | |||
| Cefoperazone-sulbactam | Susceptible | 1 (1.1) | 6 (33.3) | <0.001 ** | 6 (14.6) | 1 (1.4) | 0.010 * |
| Non-susceptible | 91 (98.9) | 12 (66.7) | 35 (85.4) | 68 (98.6) | |||
| Target Gene | Primer Sequence (5′–3′) | Amplicon (Base Pair) | Reference |
|---|---|---|---|
| gyrB | Sp2F: GTTCCTGATCCGAAATTCTCG | 490 | [48,49] |
| Sp4R: AACGGAGCTTGTCAGGGTTA | |||
| Sp4F: CACGCCGTAAGAGTGCATTA | 294 | [48,49] | |
| Sp4R: AACGGAGCTTGTCAGGGTTA | |||
| blaOXA-51-like | F: ATGAACATTAAAGCACTCTTAC | 825 | [36] |
| R: CTATAAAATACCTAATTGTTCT | |||
| blaIMP-1 | F: CTACCGCAGCAGAGTCTTTG | 587 | [50] |
| R: AACCAGTTTTGCCTTACCAT | |||
| blaVIM | F: TGGGCCATTCAGCCAGAT C | 510 | [50,51] |
| R: ATGGTGTTTGGTCGCATATC | |||
| blaNDM-1 | F: CTGAGCACCGCATTAGCC | 754 | [52] |
| R: GGGCCGTATGAGTGATTGC | |||
| blaOXA-23-like | F: GATCGGATTGGAGAACCA GA | 501 | [53] |
| R: ATTCTTGACCGCATTTCCAT | |||
| blaOXA-24-like | F: GGTTAGTTGGCCCCCTTAAA | 246 | [53] |
| R: AGTTGAGCGAAAAGG GGATT | |||
| blaOXA-48-like | F: TTGGTGGCATCGATTATCGG | 744 | [54] |
| R: GAGCACTTCTTTTGTGATGGC | |||
| R: CCCCTCTGCGCTCTACATAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anggraini, D.; Santosaningsih, D.; Saharman, Y.R.; Endraswari, P.D.; Cahyarini, C.; Saptawati, L.; Hayati, Z.; Farida, H.; Siregar, C.; Pasaribu, M.; et al. Distribution of Carbapenemase Genes among Carbapenem-Non-Susceptible Acinetobacter baumanii Blood Isolates in Indonesia: A Multicenter Study. Antibiotics 2022, 11, 366. https://doi.org/10.3390/antibiotics11030366
Anggraini D, Santosaningsih D, Saharman YR, Endraswari PD, Cahyarini C, Saptawati L, Hayati Z, Farida H, Siregar C, Pasaribu M, et al. Distribution of Carbapenemase Genes among Carbapenem-Non-Susceptible Acinetobacter baumanii Blood Isolates in Indonesia: A Multicenter Study. Antibiotics. 2022; 11(3):366. https://doi.org/10.3390/antibiotics11030366
Chicago/Turabian StyleAnggraini, Dewi, Dewi Santosaningsih, Yulia Rosa Saharman, Pepy Dwi Endraswari, Cahyarini Cahyarini, Leli Saptawati, Zinatul Hayati, Helmia Farida, Cherry Siregar, Munawaroh Pasaribu, and et al. 2022. "Distribution of Carbapenemase Genes among Carbapenem-Non-Susceptible Acinetobacter baumanii Blood Isolates in Indonesia: A Multicenter Study" Antibiotics 11, no. 3: 366. https://doi.org/10.3390/antibiotics11030366
APA StyleAnggraini, D., Santosaningsih, D., Saharman, Y. R., Endraswari, P. D., Cahyarini, C., Saptawati, L., Hayati, Z., Farida, H., Siregar, C., Pasaribu, M., Homenta, H., Tjoa, E., Jasmin, N., Sarassari, R., Setyarini, W., Hadi, U., & Kuntaman, K. (2022). Distribution of Carbapenemase Genes among Carbapenem-Non-Susceptible Acinetobacter baumanii Blood Isolates in Indonesia: A Multicenter Study. Antibiotics, 11(3), 366. https://doi.org/10.3390/antibiotics11030366






