Abstract
Transmission of pathogens present in the indoor air can occur through aerosols. This study evaluated the efficacy of an evaporated mix of essential oils to reduce the numbers of culturable aerosolized coronavirus, bacterium and fungus. The essential oil-containing gel was allowed to vaporize inside a glass chamber for 10 or 20 min. Aerosols of a surrogate of SARS-CoV-2, murine hepatitis coronavirus MHV-1, Escherichia coli or Aspergillus flavus spores were produced using a collision nebuliser and passed through the essential oil vapours, then collected on a six-stage Andersen sampler. The six-stages of the impact sampler capture aerosols in sizes ranging from 7 to 0.65 µm. The number of culturable microbes present in the aerosols collected in the different stages were enumerated and compared to the number of culturable microbes in control microbial aerosols that were not exposed to the evaporated essential oils. After 10 and 20 min evaporation, the essential oils reduced the numbers of culturable aerosolized coronavirus by 48% (log10 reduction = 0.3; p = 0.002 vs. control) and 53% (log10 reduction = 0.3; p = 0.001 vs. control), respectively. The essential oils vaporised for 10 min, reduced the number of viable E. coli by 51% (log10 reduction = 0.3; p = 0.032 vs. control). The Aspergillus flavus spores were mostly observed in the larger aerosols (7.00 µm to 2.10 µm) and the essential oils vaporised for 10 min reduced the number of viable spores by 72% (log10 reduction = 0.6; p = 0.008 vs. control). The vapours produced by a gel containing naturally occurring essential oils were able to significantly reduce the viable numbers of aerosolized coronavirus, bacteria and fungal spores. The antimicrobial gel containing the essential oils may be able to reduce aerosol transmission of microbes when used in domestic and workplace settings.
1. Introduction
The majority of the urban population spend up to 90% of their time indoors [1,2]. The indoor environment harbours a diverse microbial population including viruses, bacteria, fungi and protozoa [3,4,5,6] that is referred to as the indoor microbiome. A major component of the indoor microbiome are endogenous microbes shed by human and animal occupants, with a minor constituent being the transient microbiota of the external environment transported inside [7]. Additional sources that can contribute to the indoor microbiome include water from indoor plumbing such as toilets and showers, soil, and heating and ventilation systems such as air-conditioning systems [4,8,9,10,11,12].
Human exposure to the indoor microbiome has been recognised as a factor for the development of respiratory diseases and allergies. Pathogens present in the indoor microbiome can be transmitted to humans either through aerosols or from contaminated surfaces. Key pathogens that are transmitted through aerosols include the bacteria Staphylococcus aureus [13], Mycobacterium tuberculosis [14], the fungus Aspergillus fumigatus [15], and the viruses influenza virus, Ebola and SARS-CoV [16]. While there was considerable speculation regarding the aerosol transmission of the SARS-CoV-2 virus [17,18], current data confirms aerosol transmission of this virus [19,20].
Hospitals and food industries use UV-C irradiation, plasma air ionization and fumigation with disinfectants to reduce air borne pathogens in the indoor air [21,22]. However, these strategies are expensive and may not be suitable in domestic settings. Portable indoor air cleaners/purifiers with HEPA filters are effective in reducing the microbial concentration in aerosols including SARS-CoV-2 in classrooms, offices and hospitals [23,24,25]. Vapours of essential oils have good antimicrobial activity against respiratory pathogens, including influenza virus [26,27] and offer an alternative strategy for disinfecting the indoor air [28,29,30]. The vapours, when dispersed in the air, can significantly reduce the microbial levels indoors [31,32,33]. A study has shown that vapours of the essential oils of cassia (Cinnamomum cassia) and clove (Syzygium aromaticum) can reduce the growth of Salmonella enterica serovar Typhi, Yersinia enterocolitica and Escherichia coli on agar plates [34]. Another study has demonstrated that vapours of the essential oils of red thyme can reduce the growth of fungi on agar plates [35].
Essential oils have been hypothesised to have anti-coronavirus activity, but this is based mostly upon molecular docking studies of the oils with surface spike proteins in SARS-CoV-2 [36]. They may also have effects on the envelop and capsid [37]. One study has found that several diterpenoids, sesquiterpenoids, triterpenoids and lignoids could reduce the cytopathic effect of SARS-CoV when coincubated with the cells and virus [38], probably by inhibiting a protease. Essential oils of Laurus nobilis, when added together with SARS-CoV, could reduce the infective titre of the virus in cell culture [39]. Essential oils from thyme can inhibit the replication of feline coronavirus when added shortly after infection of cells in laboratory studies [40]. Administration by spray of a mixture of oleoresins and essential oils from botanicals two hours prior to infection with the coronavirus avian infectious bronchitis virus decreased signs and symptoms and reduced the viral titre in chickens [41].
The current study aimed to evaluate the antimicrobial efficacy of vapours of an antimicrobial gel containing essential oils for its activity against aerosolised cells of a coronavirus surrogate of SARS-CoV-2, pathogenic bacteria and fungal spores.
2. Results
2.1. Analysis of the Essential Oils within the Gel Vapors
The total volatiles were 53.5 ± 0.3%. As the antimicrobial gel containing the essential oils was a commercial product, the identity of the essential oils it contains was unknown. However, experiments were performed using NMR and GCMS to preliminarily identify the essential oil compounds that were released. The essential oils that vaporized from the gel were preliminarily identified as eucalyptol, myrcene, limonene, terpinene and cymene.
2.2. Activity of the Gel in Solution against Coronavirus
The antimicrobial gel, containing the essential oils as active ingredients, when incubated in media with the coronavirus, reduced the numbers of infectious MHV-1 in a dose-dependent manner. The greatest quantity (50 mg) of the gel reduced the ability of the coronavirus to infect the mouse A9 cells by >99.99% (no viral cells were cultured) within 30 min of incubation compared to control (p < 0.001; Table 1). The smaller quantity (25 mg) of the gel reduced the numbers of coronavirus by 98.6% after 30 min of incubation compared to control (p < 0.001). There was no effect of incubation time for the 25 mg or 50 mg of the gel on virus numbers (p > 0.05).

Table 1.
Effect of different concentrations of essential oil-containing gel against coronavirus in solution at two time points.
2.3. Activity of the Evaporated Essential Oils against Coronavirus Aerosols
The essential oil vapours were active against MHV-1 aerosols. Initial studies using the vaporized essential oils without MHV-1 showed that the collected vapours that had dissolved into the DMEM or DMEM containing 20% BSA had no cytotoxic effect on the A9 cells.
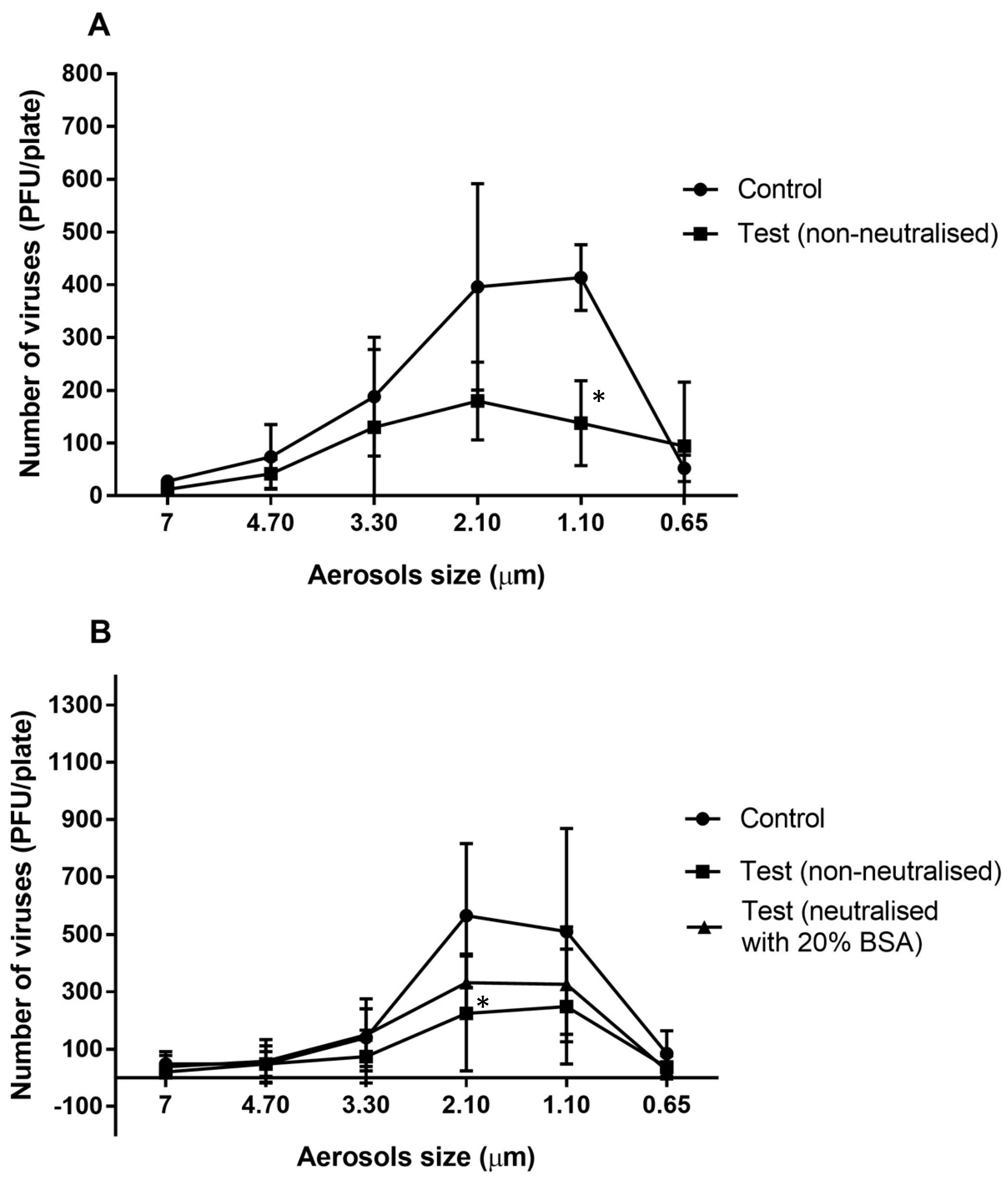
The majority of the viral particles travelled in aerosols of 3.30 to 0.65 µm in the absence of the essential oil vapours (Figure 1A) with most viral particles travelling in the 2.10 and 1.10 µm aerosols (Figure 1A). After allowing the essential oils to vaporize in the chamber for 10 min, the numbers of viral particles that were able to infect the mouse cells were reduced for most aerosol sizes, with a significant reduction of 67% in the 1.10 µm aerosol (p = 0.011; Figure 1A). A slightly greater reduction of 78% was produced in the 2.10 µm aerosols compared to the controls when the essential oils were allowed to vaporize for 20 min (p = 0.011; Figure 1B). Overall, exposure of MHV-1 aerosols to the essential oil vapours (vaporized for 10 min) resulted a significant 48% reduction compared to the untreated control (p = 0.002; Table 2).
Figure 1.
Number of murine hepatitis viral cells (MHV-1) recovered from different aerosol sizes with or without neutralisation of the vaporised essential oils for 10 min (A) or 20 min (B). The antimicrobial gel significantly (*) reduced the ability of viral aerosols to infected A9 cells in aerosols sizes of 2.10 and 1.10 µm compared to the untreated control (p = 0.011). Data points represent the mean (±95% confidence interval) of three independent experiments. BSA = bovine serum albumin.

Table 2.
Ability of vapours of essential oils to reduce the infectivity of aerosolised murine hepatitis virus.
After allowing the essential oils to vaporize for 20 min, there was a significant 53% reduction in the number of viable aerosolized viral particles compared to the control (p = 0.001; Table 2). Following use of 20% BSA, the activity of the vapours of the essential oils was slightly but not significantly (p = 0.078; Table 2) reduced, resulting in a 33% reduction in the viability of viral aerosols compared to control (p = 0.001; Table 2)
2.4. Neutralization of Essential Oils
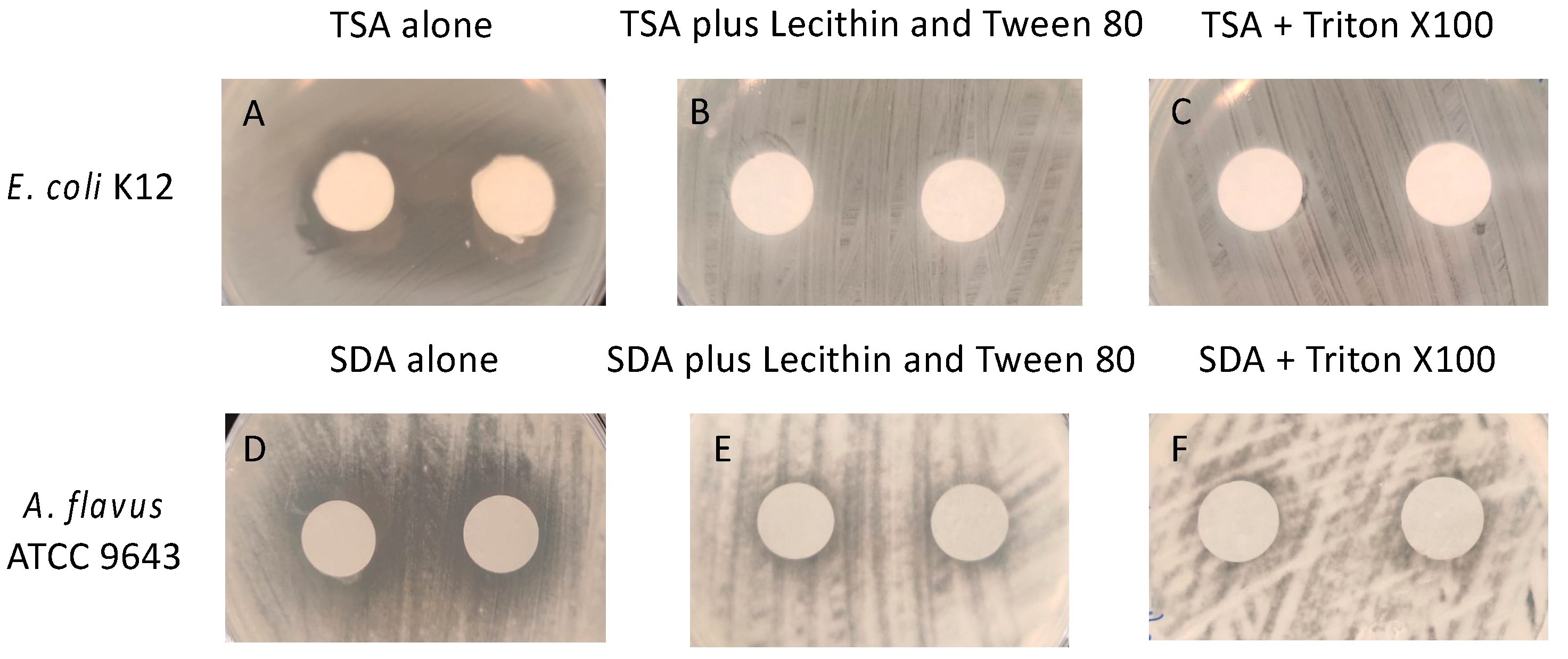
In order to determine whether the essential oils (active ingredients) in the gels were biocidal or biostatic, experiments were conducted to find suitable neutralising agents. Figure 2 shows pictures of the zones of inhibition produced by the essential oils in the gel. Zones of inhibition of the growth of E. coli were produced by the essential oils (Figure 2A) and these were completely absent in the presence of Tween 80 + lecithin (Figure 2B) or Trition X100 (Figure 2C). Zones of inhibition were also seen with A. flavus (Figure 2D), and these were substantially reduced by both Tween 80 + lecithin and Trition X100 (Figure 2E,F).
Figure 2.
Demonstration of neutralization of active ingredients (essential oils) in the gel. (A,D) in the absencde of neutralisers, (B–F) in the presence of neutralisers. TSA = tryptic soy agar; SDA = Sabouraud’s dextrose agar.
2.5. Activity against Aerosols of Bacteria or Fungal Spores
The antimicrobial essential oils from the gel in vaporized form were active against aerosols of E. coli. In the absence of antimicrobial gel, this bacterium mostly travelled in aerosol particle sizes between 3.30 to 1.10 µm (Figure 3). Overall, the vapours of essential oils produced a reduction in bacterial viability of 29% (p = 0.018) when neutralised during bacterial growth and 51% (p = 0.032) when not neutralised during bacterial growth (Table 3). Without neutralising the gel during bacterial growth, the vapours of essential oils reduced the number of live bacteria in the 3.30, 2.10 and 1.10 µm aerosols by 76%, 69% and 64%, respectively (p = 0.001).
Figure 3.
Numbers of E. coli K12 recovered from different aerosol sizes with or without neutralisation of the vapours of essential oils for 10 min. The gel significantly reduced the viability of bacteria in aerosols sizes 3.30, 2.10 and 1.10 µm when essential oils were non-neutralised during bacterial growth compared to the untreated control (*, p = 0.001). Data points represent the mean (±95% confidence interval) of three independent experiments. CFU = colony forming units.

Table 3.
The ability of vapours of essential oils to reduce the numbers of aerosolised E. coli K12.
Similarly, the essential oil vapours were also active against aerosols of Aspergillus flavus spores. The spores mainly travelled in aerosols of between 7.00 µm to 2.10 µm, with significantly (p < 0.001) higher numbers in 2.10 µm than other aerosols sizes (Figure 4). No spores travelled in aerosols of 1.10 µm or 0.65 µm (Figure 4). Overall, the vapours of essential oils reduced the viability of spores of A. flavus by 72% in non-neutralised conditions and 67% when neutralised (p ≤ 0.008; Table 4). Following exposure to the vapours of essential oils, the number of spores in aerosols of 2.10 µm was reduced compared to control by 60% and 73% in neutralised and non-neutralised conditions, respectively (p = 0.001). There was no effect of the addition of neutralising agents on the activity of the vapours of essential oils against A. flavus spores (p ≥ 0.08).
Figure 4.
Number of A. flavus spores recovered from different aerosol sizes with or without neutralisation of the vaporized essential oils for 10 min. The essential oils significantly reduced the viability of spores of A. flavus in aerosols sizes 3.30 and 2.10 µm in both neutralised and non-neutralised conditions compared to the untreated control (*, p < 0.05). Data points represent the mean (±95% confidence interval) of three independent experiments. CFU = colony forming units.

Table 4.
The ability of the antimicrobial gel vaporised for 10 min to reduce the numbers of aerosolised A. flavus spores.
3. Discussion
This study has demonstrated that compounds found in essential oils composed of a mixture of eucalyptol, myrcene, limonene, terpinene and cymene have good antimicrobial activity against aerosols of coronavirus, bacteria and fungal spores. Direct contact with the essential oils in the gel resulted in a complete kill of the coronavirus. The essential oil vapours were able to reduce the numbers of aerosolised the coronavirus MHV-1 and the bacterium E. coli by ≥50% and the reduce the number of aerosolised A. flavus spores that could germinate by ≥66%.
SARS-CoV-2 is mainly spread via aerosols [20], therefore the ability of substances to prevent subsequent growth of the virus from aerosols might be important to reduce the spread of this disease. A previous study has demonstrated that aerosolized tea tree oil (from Melaleuca sp.) or eucalyptus oil can inactivate aerosolized influenza virus in a concentration and time dependent manner, with viral titres being uncountable within 30 min of exposure [27,42]. An aerosol of an essential oil blend of tea tree (Melaleuca sp.), eucalyptus and lemon myrtle in the ratio 4.5:4.5:1 has been shown to reduce the number of viable E. coli in aerosols to < 10% of the initial inoculum in 15 min, the number of aerosolized bacteriophage MS2 to < 10% after 60 min, and the number of Aspergillus niger spores to <10% in 120 min [43]. The analysis of compounds in the gel analysed in the current study revealed that it may contain terpene compounds such as limonene (from citrus), myrcene (from cannabis), cymene (from cumin and thyme) and terpinene (from cardamom and marjoram), as well as terpenoids such as eucalyptol (from eucalyptus). All these terpenes and terpenoids have been shown to posses antibacterial activity [44], and there is evidence that lower doses are needed to disrupt bacterial cell membranes (i.e., a synergistic activity) when used in combination [45].
Similarly to the current study when the essential oils in the gel were directly mixed with viruses in suspension, a nasal spray containing anise oil, eucalyptus oil, levomenthol, myrrh extract, clove oil, peppermint oil, ratanhia root extract and tormentil root extract, reduced the infectivity of SARS-CoV-2 when mixed in suspension [46]. 3β-Friedelanol, when mixed in media and applied to mammalian cells before viral infection, can prevent infection of cells by human CoV-229E [47]. A proprietary mix of essential oils has been shown to reduce the infectivity of surface dried bacteriophage phi 6 [48]. Another study reported that aerosolised influenza virus or bacteriophage M13 exposed to vaporised essential oils of tea tree or eucalyptus for 24 h resulted in approximately 87% reduction in influenza viral titres, but only 25–42% reduction in M13 titres [27]. The use of bacteriophages has been claimed to be a surrogate of coronaviruses; however, bacteriophages are structurally and biochemically very different to enveloped RNA viruses such as coronaviruses as they do not normally possess a lipid envelop that surrounds their capsid. Surface coatings composed of tea–cinnamaldehyde–copper or tea–cinnamaldehyde–silver can prevent infection of cells by the coronavirus MHV-A59 [49].
The essential oil vapours were bacteriostatic as their activity was diminished in the presence of agents that neutralized their antimicrobial compounds, while the viral and fungal activity was unaffected by neutralizers (20% BSA or lecithin/Tween 80, respectively). In vitro studies performed using essential oils have shown that the active compounds in essential oils are bactericidal for E. coli at higher concentrations and bacteriostatic at lower concentrations [50]. Essential oil vapours can affect spore formation in A. fumigatus [51] and can be either fungistatic or fungicidal depending on the active compound [52]. There are several potential chemicals that are recommended by ASTM International as appropriate to test for their ability to neutralize antimicrobial agents (see ASTM E1054-08, 2013 edition), and the list includes lecithin and Tween 80 that can be used to neutralize cresols and parabens that are chemically similar to essential oils present in the ingredients of used in the present study.
This study used a ready-made bacterial filtration efficiency testing rig that is usually used to assess the ability of face masks to filter the bacterium Staphylococcus aureus as specified by standard ASTM F2101-1 [53]. The Andersen impactor has been widely used to sample environmental bacteria and fungi [54] and has the advantage of being able to directly capture the biological aerosols on agar plates which can then be incubated to directly culture the organisms. Neutralizing chemicals can also be incorporated into the agar to inactivate antimicrobial agents present in the aerosols. While viruses can be captured on the agar plates, they had to be recovered from the agar and cultured on susceptible cells. Other researchers have used this method to culture virus in aerosols [55,56]. A major advantage of the Andersen impactor is that it allows differentiation of the size of the aerosols in which microbes travel [57].
Human activities such as speaking, coughing and sneezing generate microbial aerosols in sizes ranging from <1 µm to >100 µm [58,59,60,61]. Larger aerosols or droplets remain airborne for a short time and settle close to the source [62]. Smaller aerosols under 5 µm in size can remain airborne for longer periods and are able to make their way to the lungs [63]. Aerosols of this size are implicated in the airborne transmission of Mycobacterium tuberculosis [64], Aspergillus fumigatus spores [65], and viruses including the influenza virus [66] and SARS-CoV [67]. The present study demonstrated that vapours of essential oils could be active on aerosols of 7–1.1 µm.
The number of viral copies that are needed to cause an infection is expressed as ID50 which denotes the mean dose that causes an infection in 50% of susceptible subjects. While the ID50 for SARS-CoV-2 is not known, the ID50 for SARS-CoV ranged from 16 to 160 viral particles/person [68]. The essential oil vapours produced by the antimicrobial gel were able to significantly reduce the number of viable viral particles in aerosols under 5 µm by 48% within 10 min and the reduction increased to 53% when the gel was allowed to vaporise for 20 min indicating sustained and perhaps increasing antimicrobial activity. Allowing the gel to vaporise for longer durations and reducing the air flow (i.e., increasing the time for the virus and vapour to interact) may result in further reductions in viral numbers and this should be tested in future experiments.
We believe this report is the first to show activity of essential oil vapours against an aerosolized coronavirus. This study also demonstrated that essential oil vapours can significantly reduce the viable numbers of bacteria and fungal spores. Activity was rapid, occurring within the 2 min of air collection through the tube for viruses and fungal spores as the active ingredients in the essential oil vapours were neutralised during collection. Future work should examine the spectrum of activity of essential oil vapours in general, as well as those contained in the product used in the current study. Bioactive compounds in the essential oils at higher concentrations compared to the gel used in this study may impact human health [69]. Whilst the low concentration of the active compounds present in the gel in the current study may not impact human health, this should be examined in future studies. The results of the current study suggest that using essential oil vapours may reduce the transmission of respiratory pathogens, improve indoor air quality and the health of human occupants.
4. Materials and Methods
4.1. Microorganisms and Their Preparation
The mouse hepatitis virus (MHV-1) ATCC/VR261 is an enveloped single-strand RNA virus and an accepted surrogate of the SAR-CoV-2 virus (https://www.tga.gov.au/surrogate-viruses-use-disinfectant-efficacy-tests-justify-claims-against-covid-19; accessed on 7 January 2022) [70]. Viral stock was prepared by growing in A9 mouse fibroblast cells (ATCC/CCL 1.4) in Dulbecco’s minimum essential medium (DMEM, Thermofisher, Macquarie Park, NSW, Australia) containing 10% foetal bovine serum (FBS; Thermofisher), 100 µg/mL streptomycin sulphate and 100 I.U. penicillin G, (Thermofisher). Viral titres (1.0 × 105 to 1.0 × 106 plaque forming units (PFU)/mL) were determined by plaque assay. Aliquots were diluted ten-fold and inoculated into the wells of 12-well plates containing A9 cells and incubated for 1 h at 37 °C in the presence of 5% (v/v) CO2. The plates were gently rocked once every 15 min to prevent the cells from drying out. After incubation, an overlay media containing a 50:50 mix of 2% (w/v) agar (Sigma-Aldrich, Castle Hill, NSW, Australia) and DMEM was added to each well and further incubated for 72 h. Following incubation, the cells were fixed with 4% (v/v) formaldehyde (Sigma-Aldrich) for 2–3 h, the agar overlay removed, and the number of plaques produced by viral particles (PFUs) visualized after staining with 1% (w/v) crystal violet (Sigma-Aldrich).
Escherichia coli K12 (ATCC 10798) was grown overnight in tryptic soy broth (TSB; BD, Sydney, NSW, Australia) to mid-log phase. Following incubation, bacterial cells were collected by centrifuging and were washed once with phosphate buffer saline (PBS; NaCl 8 g L−1, KCl 0.2 g L−1, Na2HPO4 1.15 g L−1, KH2PO4 0.2 g L−1, pH 7.4). Following washing, cells were re-suspended in PBS and the concentration adjusted spectrophotometrically to an optical density of 0.1 at 660 nm which yielded 1.0 × 108 colony forming units (CFU/mL) upon retrospective agar plate counts, then further serially diluted to a final concentration of 1.0 × 104 CFU/mL. E. coli has been previously used in aerosol research as it can be aerosolised during use of toilet facilities [71].
The spores of Aspergillus flavus ATCC 9643 were produced by growth on Sabouraud’s dextrose agar (SDA; Thermofisher) for 10 days at 25 °C. The fungal growth was suspended in sterile deionized water and filtered through sterile 70 µm filters to remove hyphal fragments. Spores were resuspended in sterile deionized water and their concentration adjusted spectrophotometrically to an optical density of 0.2 at 660 nm which yielded 1.0 × 106 CFU/mL, which were then serially diluted to a final concentration of 1.0 × 104 CFU/mL.
4.2. Essential Oil Formulation
The essential oil-containing gel (Mould Gone, SAN-AIR, West Gosford, NSW, Australia) was supplied in sealed containers. The total volatile content of the sample was determined in duplicate in accordance with ASTM D 2369 “Volatile Content of Coatings” by heating the gel in a constant temperature oven held at 110 ± 5 °C. To identify the volatiles, the gel was kept in a closed glass jar and allowed to vaporize for 40 min. To enhance the release of the volatile compounds, the jar was kept in water bath with 50 °C. The vapour that settled on the side of jar was collected in deuterated chloroform (CDCl3) for 1H NMR analysis and compared with a control of CDCl3 alone. The types of volatile organic compounds present in the gel were examined by GC-MS with a modification of ASTM D6886-12 “Method for Low VOC Waterborne Coatings” using an injection temperature of 30 °C, an initial oven temperature of 30 °C that was increased to 80 °C over 10 min, sampling time 1.00 min, pressure 100.0 kPa (hold time 20 min), carrier gas hydrogen, total flow of 50.0 mL/min, column flow at 1.88 mL/min, linear velocity of 49.1 cm/s, and purge flow of 3.0 mL/min. The mass observed in the GC-MS was corelated with the 1H NMR data to give presumptive identification of the essential oils.
4.3. Activity of the Essential Oil-Containing Gel against Coronavirus in Solution
In order to demonstrate that the gel had anti-coronaviral activity, the first experiments incubated aliquots of the gel directly with viral particles in suspension. Cells of MHV-1 (1.0 × 105 (PFU)/mL) were incubated with 25 mg or 50 mg of the antimicrobial gel in DMEM at ambient temperature for 0.5 or 2 h. Following incubation, the DMEM was removed, diluted in 20% (w/v) bovine serum albumin (BSA; Sigma-Aldrich) prepared in PBS and incubated for 10–15 min to neutralize the antimicrobial agents released from the gel. Thereafter, 100 µL aliquots were diluted ten-fold (in 20% BSA) and numbers assayed using the plaque assay as described above. Controls were the viral inoculum incubated in DMEM or PBS without the antimicrobial gel. The percentage reduction in PFU for each quantity of the gel compared to the negative control (PBS) was calculated.
4.4. Activity of the Essential Oils as Vapours against Coronavirus Aerosols
A bacterial filtration efficiency (BFE) test rig (CH Technologies, Westwood, NJ, USA) was used to produce viral aerosols (Figure 5). The antimicrobial gel (10 g in total) was removed from its container and allowed to vaporise into the glass aerosol chamber (60 × 8 cm; 3016 cm3) for 10 min or 20 min prior to the introduction of the virus. The viral inoculum (50 µL; 1.0 × 106 PFU/mL) was aerosolized using a continuous drive syringe pump through a nebulizer with an airflow of 28.3 L min−1 for one minute and allowed to interact with vapours of the antimicrobial gel as they passed through the glass tube. The size of the aerosols produced was approximately 3.0 ± 0.3 µm and these travelled through the glass aerosol chamber into an Anderson sieve sampler and were collected by flowing past 2% (w/v) agar plates. The largest (7 µm) sized aerosols were captured on the agar plate at the top of the Anderson sieve and the smallest (0.65 µm) on the agar plate at the bottom of the device. After one minute, the airflow was stopped to cease aerosol generation, and the vacuum pump was run for a further one minute to collect any residual aerosols from the glass chamber.
Figure 5.
The bacterial filtration efficiency rig containing an Anderson sieve sampler. Aerosols of 3.0 ± 0.3 µm on average of viruses, bacteria or fungal spores were produced in the chamber.
Following this, agar plates were flooded with 1.5 mL of either 20% BSA in DMEM (neutralised samples) or DMEM alone (non-neutralised samples), and viruses were carefully removed using a sterile cell scrapper. Aliquots (100 µL) from each plate were placed in duplicate on A9 cells in 12-well cell culture plates to culture any virus particles. The culture conditions were as described above. Control runs were performed at the beginning of each experiment prior to the addition of the gel in the glass aerosol chamber to collect infectious viruses so that any reduction in the number of infectious viruses could be calculated as a percentage of this control. In addition, controls to examine any cytotoxic effect of the essential oil vapours on the A9 cells were also examined.
This followed the protocol for viral testing. Briefly, the essential oils were allowed to evaporate from the gel for 20 min, after which time sterile DMEM was aerosolised into the chamber with an airflow of 28.3 L min−1 for one minute. After one minute, the airflow was stopped to cease aerosol generation, and the vacuum pump was run for a further one minute to collect any residual aerosols from the glass chamber. The aerosols were collected into the Anderson sieve containing agar plates. The plates were flooded with 1.5 mL of DMEM alone, scrapped to mimic the technique for viral collection, and then 100 μL samples added to A9 cells in 12-well culture plates. After incubation for 1 h at 37 °C in the presence of 5% (v/v) CO2 with rocking, overlay media (50:50 mix of 2% (w/v) agar and DMEM) was added to each well and further incubated for 72 h. Following incubation, the cells were fixed with 4% (v/v) formaldehyde for 2–3 h, the agar overlay removed, the cells stained with 1% (w/v) crystal violet and any cytotoxic effect examined by microscopy.
4.5. Activity Essential Oil Vapours against Bacterial and Fungal Spore Aerosols
Initially, the ability of two different potential neutralising agents were examined to determine which would neutralise the antibacterial and antifungal effects of the essential oil vapours. Tryptic soy agar (TSA; BD, Macquarie Park, NSW, Australia) or SDA was made containing either Tween® 80 (5 mL L−1) and lecithin (0.7 g L−1), or 2% (w/v) Triton X100 (Sigma Aldrich, Castle Hill NSW, Australia). Filter paper discs (3 cm in diameter) were soaked whilst pre-heating (80 °C; 10 g liquified) the San Air gel. This allowed the assay to be performed in the absence of the gel itself, which may have had some activity which would not be present in the gel vapours. Lawns of E. coli (1 × 108 mL−1) or A. flavus (1 × 106 mL−1) were made of TSA or SDA plates, respectively, and then two paper discs soaked in San Air gel applied per plate. The plates were incubated for 16 h at 37 °C for the bacteria and 48 h at 25 °C for the fungi. After incubation, the size of zones of inhibition were compared.
The anti-bacterial activity of the essential oil vapours for 10 min against E. coli and its sporicidal activity against A. flavus spores was determined using a similar method as described for MHV-1, except using 50 µL of E. coli or A. flavus spores (1 × 104 CFU/mL). Bacteria were collected on agar plates composed of tryptic soy agar (TSA; BD, Macquarie Park, NSW, Australia) alone or containing TSA and the neutralizers Tween® 80 (5 mL L−1) and lecithin (0.7 g L−1). Fungal spores were collected on SDA plates alone or containing the same neutralizers. The numbers of viable cells from each of the six plates in the Anderson sieve collector were enumerated following incubation at 37 °C for 24 h for bacteria and at 25 °C for 72 h for fungal spores. Control runs were conducted prior to the addition of the gel in the glass aerosol chamber to collect viable bacteria and fungal spores. Test and control runs were performed in duplicate and repeated twice. The percentage of cells remaining viable after passage through the gel vapours was calculated by comparing numbers in the absence (control) and presence (test) of the gel vapours.
4.6. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7.04 software (GraphPad Software, La Jolla, CA, USA). The concentration and time dependent effect of the antimicrobial gel in solution was determined using two-way ANOVA. The effect of antimicrobial gel vapours at single time points on different aerosols sizes and overall percentage (%) reduction was assessed using Welch’s t-test and one-way ANOVA with Tukey’s test, respectively. Statistical significance was set as p < 0.05.
5. Conclusions
This study has demonstrated that short interactions between aerosolized coronavirus, the bacterium E. coli or spores of the fungus A. flavus and essential oil vapours from Melaleuca genus plants can reduce the number of culturable microbial cells. This may have implications in the control of airborne diseases such as COVID-19. The testing set up can be used in future studies to determine the ability of other essential oil vapours to reduce the viability of other microbes that can be transmitted via aerosols, such as Mycobacterium tuberculosis, Legionella pneumophilia and influenza viruses.
Author Contributions
Conceptualization, M.W. and A.K.V.; methodology, P.K., M.Y., R.K., M.W. and A.K.V.; resources, M.W.; writing—original draft preparation, A.K.V.; writing—review and editing, P.K, M.Y., R.K., M.W. and A.K.V.; supervision, M.W. and A.K.V.; project administration, M.W.; funding acquisition, M.W., A.K.V., P.K. and M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by an Ideas grant from the NHMRC (APP1183597), and a grant from San Air, West Gosford, NSW, Australia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Acknowledgments
We thank San Air for the donation of the Mould Gone product for testing.
Conflicts of Interest
Funding and in-kind contribution for this study was received from San Air. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; or in the writing of the manuscript.
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, P.; Martinac, I. Indoor climate and air quality. Review of current and future topics in the field of ISB study group 10. Int. J. Biometeorol. 1998, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J., II; Garcia, E.B.; Marr, L.C. Total virus and bacteria concentrations in indoor and outdoor air. Environ. Sci. Technol. Lett. 2015, 2, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Stephens, B. Microbiology of the built environment. Nat. Rev. Microbiol. 2018, 16, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. The diversity and distribution of fungi on residential surfaces. PLoS ONE 2013, 8, e78866. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Lindow, S.E.; Taylor, J.W.; Bruns, T.D. Airborne bacterial communities in residences: Similarities and differences with fungi. PLoS ONE 2014, 9, e91283. [Google Scholar] [CrossRef]
- Prussin, A.J., II; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef] [Green Version]
- Meadow, J.F.; Altrichter, A.E.; Kembel, S.W.; Kline, J.; Mhuireach, G.; Moriyama, M.; Northcutt, D.; O’Connor, T.K.; Womack, A.M.; Brown, G.Z.; et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air 2014, 24, 41–48. [Google Scholar] [CrossRef]
- Kelley, S.T.; Gilbert, J.A. Studying the microbiology of the indoor environment. Genome Biol. 2013, 14, 202. [Google Scholar] [CrossRef] [Green Version]
- Leung, M.H.; Lee, P.K. The roles of the outdoors and occupants in contributing to a potential pan-microbiome of the built environment: A review. Microbiome 2016, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.I.; Bhangar, S.; Pasut, W.; Arens, E.A.; Taylor, J.W.; Lindow, S.E.; Nazaroff, W.W.; Bruns, T.D. Chamber bioaerosol study: Outdoor air and human occupants as sources of indoor airborne microbes. PLoS ONE 2015, 10, e0128022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettleson, E.M.; Adhikari, A.; Vesper, S.; Coombs, K.; Indugula, R.; Reponen, T. Key determinants of the fungal and bacterial microbiomes in homes. Environ. Res. 2015, 138, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dancer, S.J. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: The case for hospital cleaning. Lancet Infect. Dis. 2008, 8, 101–113. [Google Scholar] [CrossRef]
- Clark, S.O.; Hall, Y.; Kelly, D.L.; Hatch, G.J.; Williams, A. Survival of Mycobacterium tuberculosis during experimental aerosolization and implications for aerosol challenge models. J. Appl. Microbiol. 2011, 111, 350–359. [Google Scholar] [CrossRef]
- McCormick, A.; Loeffler, J.; Ebel, F. Aspergillus fumigatus: Contours of an opportunistic human pathogen. Cell. Microbiol. 2010, 12, 1535–1543. [Google Scholar] [CrossRef]
- Jones, R.M.; Brosseau, L.M. Aerosol transmission of infectious disease. J. Occup. Environ. Med. 2015, 57, 501–508. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Asadi, S.; Bouvier, N.; Wexler, A.S.; Ristenpart, W.D. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol. Sci. Technol. 2020, 54, 635–638. [Google Scholar] [CrossRef] [Green Version]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Ananda-Rajah, M.R. Scientific evidence supports aerosol transmission of SARS-CoV-2. Antimicrob. Resist. Infect. Control 2020, 9, 202. [Google Scholar] [CrossRef]
- Ijaz, M.K.; Zargar, B.; Wright, K.E.; Rubino, J.R.; Sattar, S.A. Generic aspects of the airborne spread of human pathogens indoors and emerging air decontamination technologies. Am. J. Infect. Control 2016, 44, S109–S120. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Tiwari, B.K.; Duffy, G. Emerging technologies for aerial decontamination of food storage environments to eliminate microbial cross-contamination. Foods 2020, 9, 1779. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, W.; Russell, G.; Willard, E.; Stehle, J., Jr. Impact of a novel mobile high-efficiency particulate air-ultraviolet air recirculation system on the bacterial air burden during routine care. Am. J. Infect. Control 2019, 47, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Curtius, J.; Granzin, M.; Schrod, J. Testing mobile air purifiers in a school classroom: Reducing the airborne transmission risk for SARS-CoV-2. Aerosol Sci. Technol. 2021, 55, 586–599. [Google Scholar] [CrossRef]
- Guo, J.; Xiong, Y.; Kang, T.; Xiang, Z.; Qin, C. Bacterial community analysis of floor dust and HEPA filters in air purifiers used in office rooms in ILAS, Beijing. Sci. Rep. 2020, 10, 6417. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Usachev, E.V.; Pyankov, O.V.; Usacheva, O.V.; Agranovski, I.E. Antiviral activity of tea tree and eucalyptus oil aerosol and vapour. J. Aerosol Sci. 2013, 59, 22–30. [Google Scholar] [CrossRef]
- Karpinski, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Maczka, W.; Duda-Madej, A.; Gorny, A.; Grabarczyk, M.; Winska, K. Can eucalyptol replace antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca genus as antimicrobial agents: From Farm to pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef]
- Sato, K.; Krist, S.; Buchbauer, G. Antimicrobial effect of vapours of geraniol, (R)-(–)-linalool, terpineol,γ-terpinene and 1,8-cineole on airborne microbes using an airwasher. Flavour Fragr. J. 2007, 22, 435–437. [Google Scholar] [CrossRef]
- Lanzerstorfer, A.; Hackl, M.; Schlomer, M.; Rest, B.; Deutsch-Grasl, E.; Lanzerstorfer, C. The influence of air-dispersed essential oils from lemon (Citrus limon) and silver fir (Abies alba) on airborne bacteria and fungi in hospital rooms. J. Environ. Sci. Health Part A 2019, 54, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, F.; Belotti, L.; Vecchi, S.; Testa, C.; Beretta, G. Air dispersed essential oils combined with standard sanitization procedures for environmental microbiota control in nosocomial hospitalization rooms. Complement. Med. 2016, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, J.K.; Morri, S.; Shetty, P.H. Assessment and modelling the antibacterial efficacy of vapours of cassia and clove essential oils against pathogens causing foodborne illness. LWT-Food Sci. Technol. 2021, 150, 112076. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Logrieco, A.F.; Baruzzi, F. Unravelling the Antifungal Effect of Red Thyme Oil (Thymus vulgaris L.) Compounds in Vapor Phase. Molecules 2020, 25, 4761. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and antiviral effects of Thymus vulgaris essential oil on feline coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Rosenbloom, R.; Petteruti, M.; Hilt, D.A.; McCall, A.W.; Williams, S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010, 149, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Pyankov, O.; Usachev, E.V.; Pyankova, O.; Agranovski, I.E. Inactivation of airborne influenza virus by tea tree and eucalyptus oils. Aerosol Sci. Technol. 2012, 46, 1295–1302. [Google Scholar] [CrossRef]
- Mirskaya, E.; Agranovski, I.E. Control of airborne microorganisms by essential oils released by VaxiPod. Atmosphere 2021, 12, 1418. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [Green Version]
- Meister, T.L.; Todt, D.; Bruggemann, Y.; Steinmann, J.; Banava, S.; Brill, F.H.H.; Steinmann, J.; Pfaender, S.; Steinmann, E. Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2. J. Hosp. Infect. 2021, 120, 9–13. [Google Scholar] [CrossRef]
- Chang, F.R.; Yen, C.T.; Ei-Shazly, M.; Lin, W.H.; Yen, M.H.; Lin, K.H.; Wu, Y.C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 2012, 7, 1415–1417. [Google Scholar] [CrossRef] [Green Version]
- Bailey, E.S.; Curcic, M.; Biros, J.; Erdogmus, H.; Bac, N.; Sacco, A., Jr. Essential oil disinfectant efficacy against SARS-CoV-2 microbial surrogates. Front. Public Health 2021, 9, 783832. [Google Scholar] [CrossRef]
- Cox, H.J.; Sharples, G.J.; Badyal, J.P.S. Tea-essential oil-metal hybrid nanocoatings for bacterial and viral inactivation. ACS Appl. Nano Mater. 2021, 4, 12619–12628. [Google Scholar] [CrossRef]
- Puvaca, N.; Milenkovic, J.; Galonja Coghill, T.; Bursic, V.; Petrovic, A.; Tanaskovic, S.; Pelic, M.; Ljubojevic Pelic, D.; Miljkovic, T. Antimicrobial activity of selected essential oils against selected pathogenic bacteria: In vitro study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef]
- Inouye, S.; Watanabe, M.; Nishiyama, Y.; Takeo, K.; Akao, M.; Yamaguchi, H. Antisporulating and respiration-inhibitory effects of essential oils on filamentous fungi. Mycoses 1998, 41, 403–410. [Google Scholar] [CrossRef]
- Inouye, S.; Tsuruoka, T.; Watanabe, M.; Takeo, K.; Akao, M.; Nishiyama, Y.; Yamaguchi, H. Inhibitory effect of essential oils on apical growth of Aspergillus fumigatus by vapour contact. Mycoses 2000, 43, 17–23. [Google Scholar] [CrossRef]
- ASTM F2101-19; Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus aureus. ASTM International: West Conshohocken, PA, USA, 2019.
- Xu, Z.; Yao, M. Monitoring of bioaerosol inhalation risks in different environments using a six-stage Andersen sampler and the PCR-DGGE method. Environ. Monit Assess 2013, 185, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; de Meulder, D.; Bestebroer, T.M.; Mulders, A.; Fouchier, R.A.M.; Herfst, S. Comparison of three air samplers for the collection of four nebulized respiratory viruses—Collection of respiratory viruses from air. Indoor Air 2021, 31, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Aarnink, A.J.; Wang, W.; Fabri, T.; Groot Koerkamp, P.W.; de Jong, M.C. Airborne virus sampling: Efficiencies of samplers and their detection limits for infectious bursal disease virus (IBDV). Ann. Agric. Environ. Med. 2014, 21, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C.Y.H.; Li, Y.; Katoshevski, D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009, 40, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.Y.H.; Wan, M.P.; Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Li, Y.; Xie, X.; et al. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J. Aerosol Sci. 2009, 40, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.R.; Morawska, L.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Chao, C.Y.H.; Wan, M.P.; Li, Y.; Xie, X.; Katoshevski, D.; et al. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011, 42, 839–851. [Google Scholar] [CrossRef]
- Papineni, R.S.; Rosenthal, F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997, 10, 105–116. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Chwang, A.T.; Ho, P.L.; Seto, W.H. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air 2007, 17, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.K.; Ensor, D.S.; Sparks, L.E. Airborne particle sizes and sources found in indoor air. Atmos. Environ. Part A Gen. Top. 1990, 26, 2149–2162. [Google Scholar] [CrossRef]
- Cole, E.C.; Cook, C.E. Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. Am. J. Infect. Control 1998, 26, 453–464. [Google Scholar] [CrossRef]
- Morris, G.; Kokki, M.H.; Anderson, K.; Richardson, M.D. Sampling of Aspergillus spores in air. J. Hosp. Infect. 2000, 44, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Alford, R.H.; Kasel, J.A.; Gerone, P.J.; Knight, V. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 1966, 122, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Wilson, P.; Shetty, N.; Noakes, C.J. Aerosol-transmitted infections-a new consideration for public health and infection control teams. Curr. Treat. Options Infect. Dis. 2015, 7, 176–201. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Bartrand, T.A.; Weir, M.H.; Omura, T.; Haas, C.N. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010, 30, 1129–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo Milhem, S.; Verriele, M.; Nicolas, M.; Thevenet, F. Does the ubiquitous use of essential oil-based products promote indoor air quality? A critical literature review. Environ. Sci. Pollut. Res. Int. 2020, 27, 14365–14411. [Google Scholar] [CrossRef]
- Ahmed, W.; Bertsch, P.M.; Bibby, K.; Haramoto, E.; Hewitt, J.; Huygens, F.; Gyawali, P.; Korajkic, A.; Riddell, S.; Sherchan, S.P.; et al. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020, 191, 110092. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.C.K.; Tan, T.F.; Li, W.S.; Ip, D.K.M. Emission strength of airborne pathogens during toilet flushing. Indoor Air 2018, 28, 73–79. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).