Terazosin Interferes with Quorum Sensing and Type Three Secretion System and Diminishes the Bacterial Espionage to Mitigate the Salmonella Typhimurium Pathogenesis
Abstract
:1. Introduction
2. Results
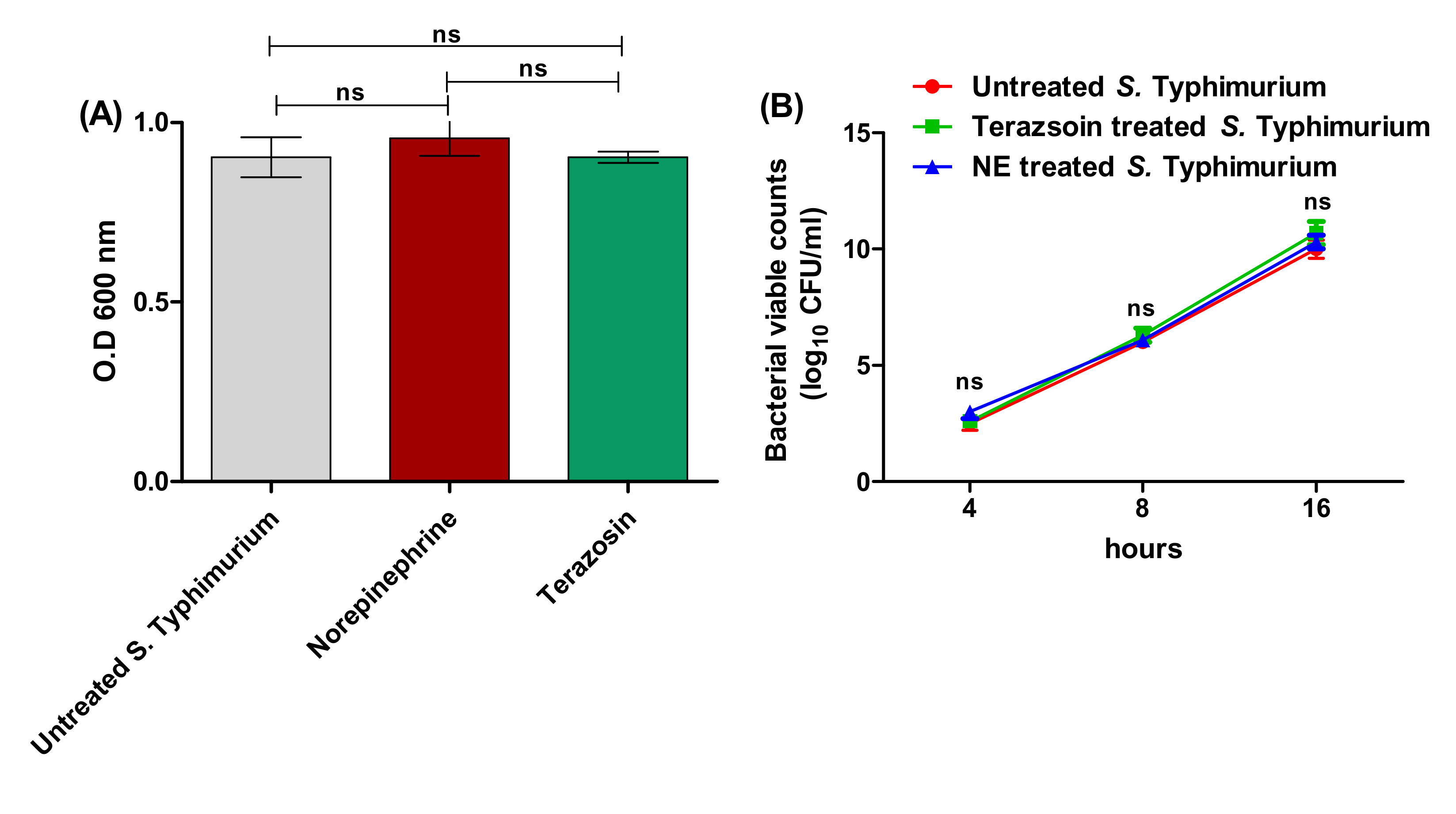
2.1. Detection of the Minimum Inhibitory Concentration (MIC) of Terazosin against S. Typhimurium
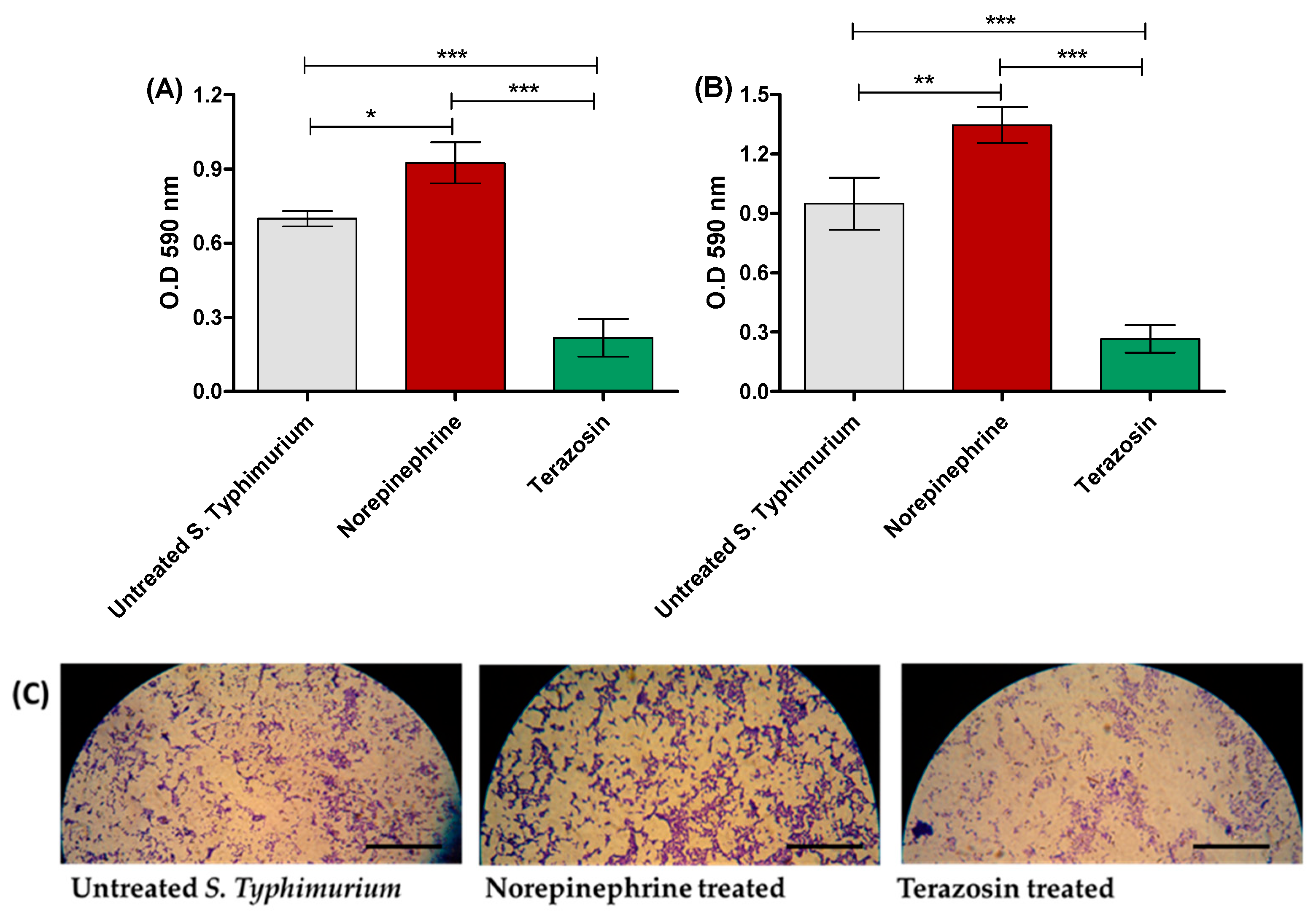
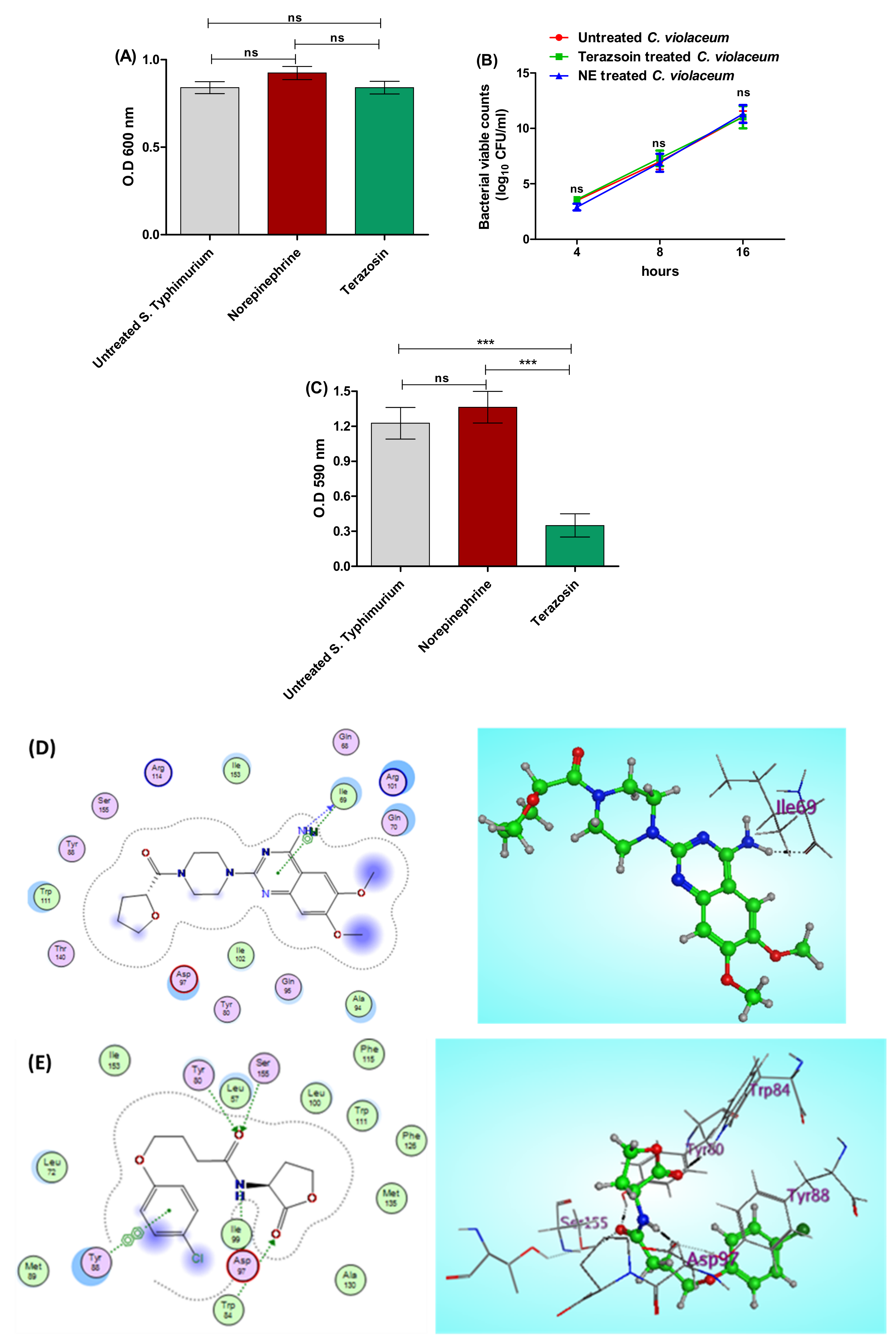
2.2. Terazosin Diminishes S. Typhimurium Adhesion and Biofilm Production
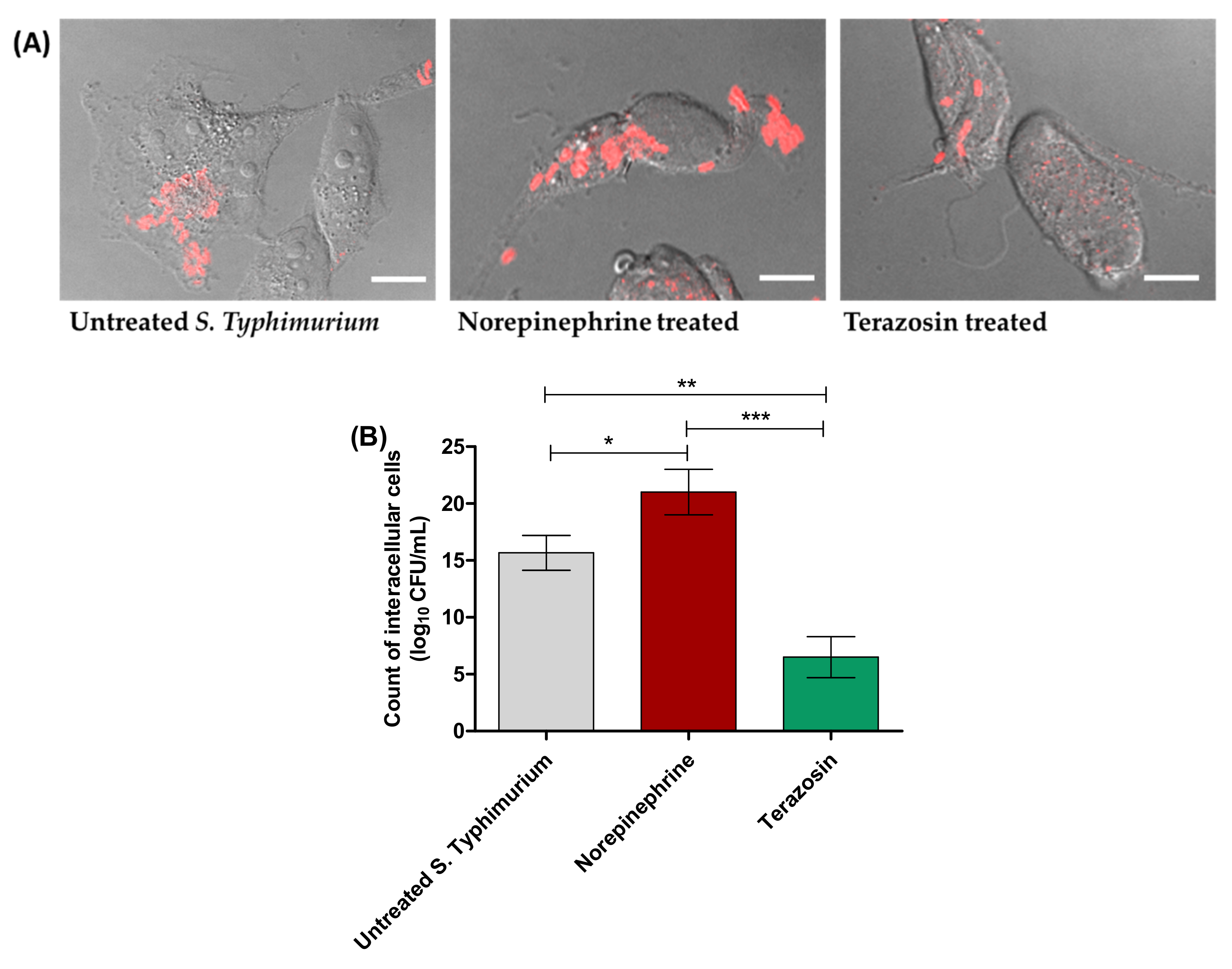
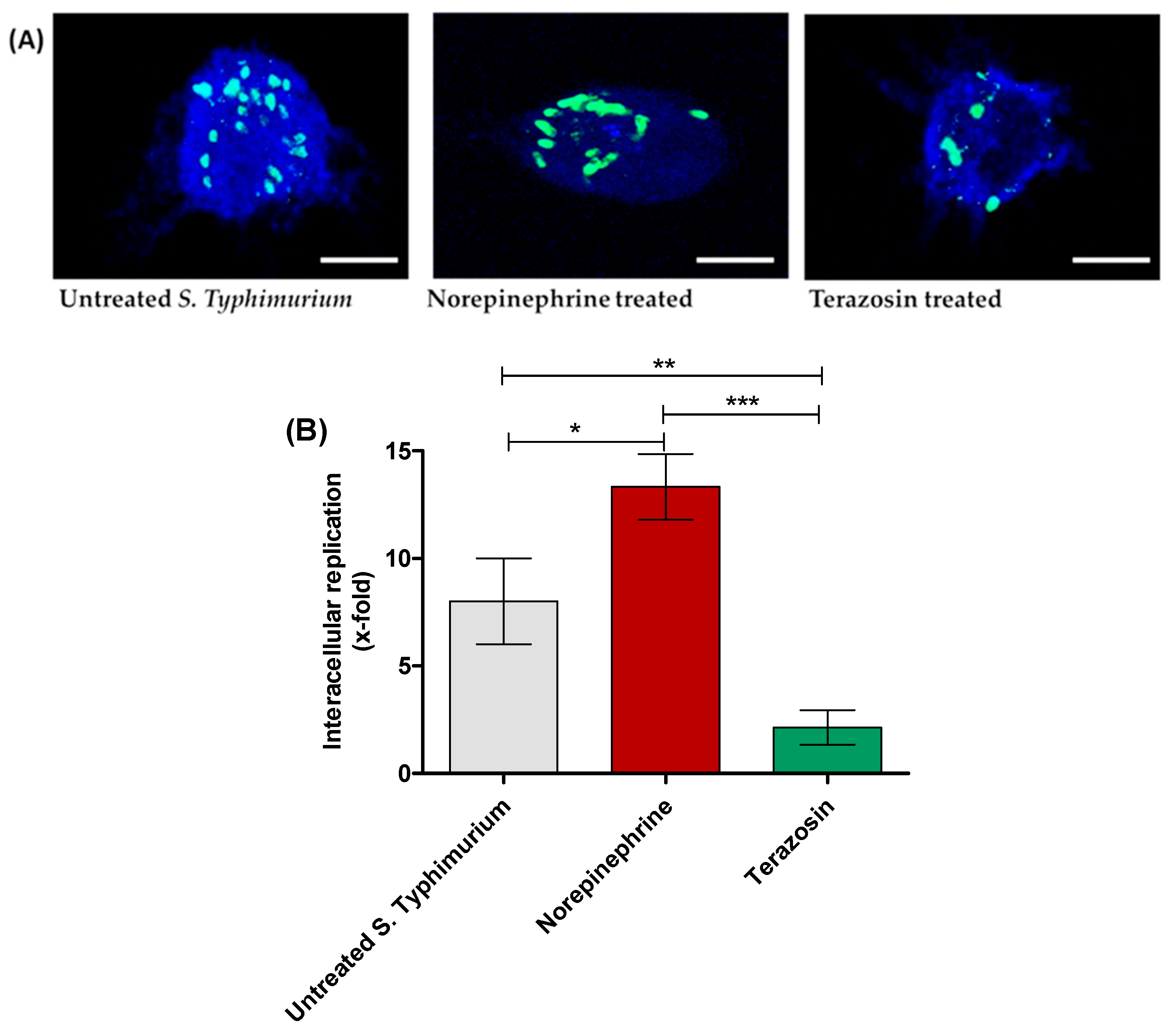
2.3. Terazosin Reduces S. Typhimurium Invasion and Intracellular Replication
2.4. Terazosin In Vivo Mitigates the S. Typhimurium Pathogenesis
2.5. Terazosin Anti-Virulence Activity
2.5.1. Anti-QS Activity
Terazosin Reduces the Production of QS-Controlled C. violaceum Pigment in Comparison to Norepinephrine
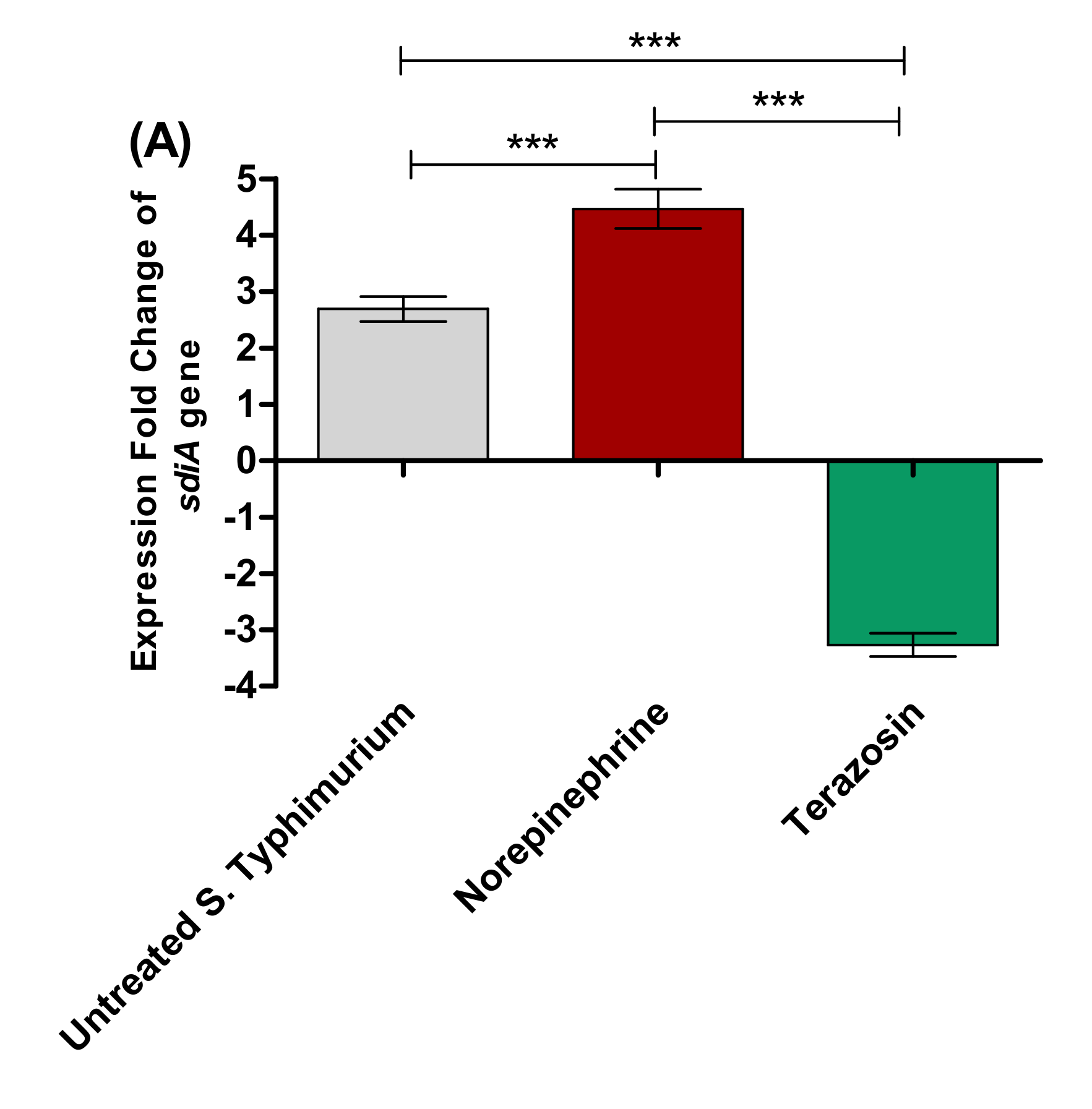
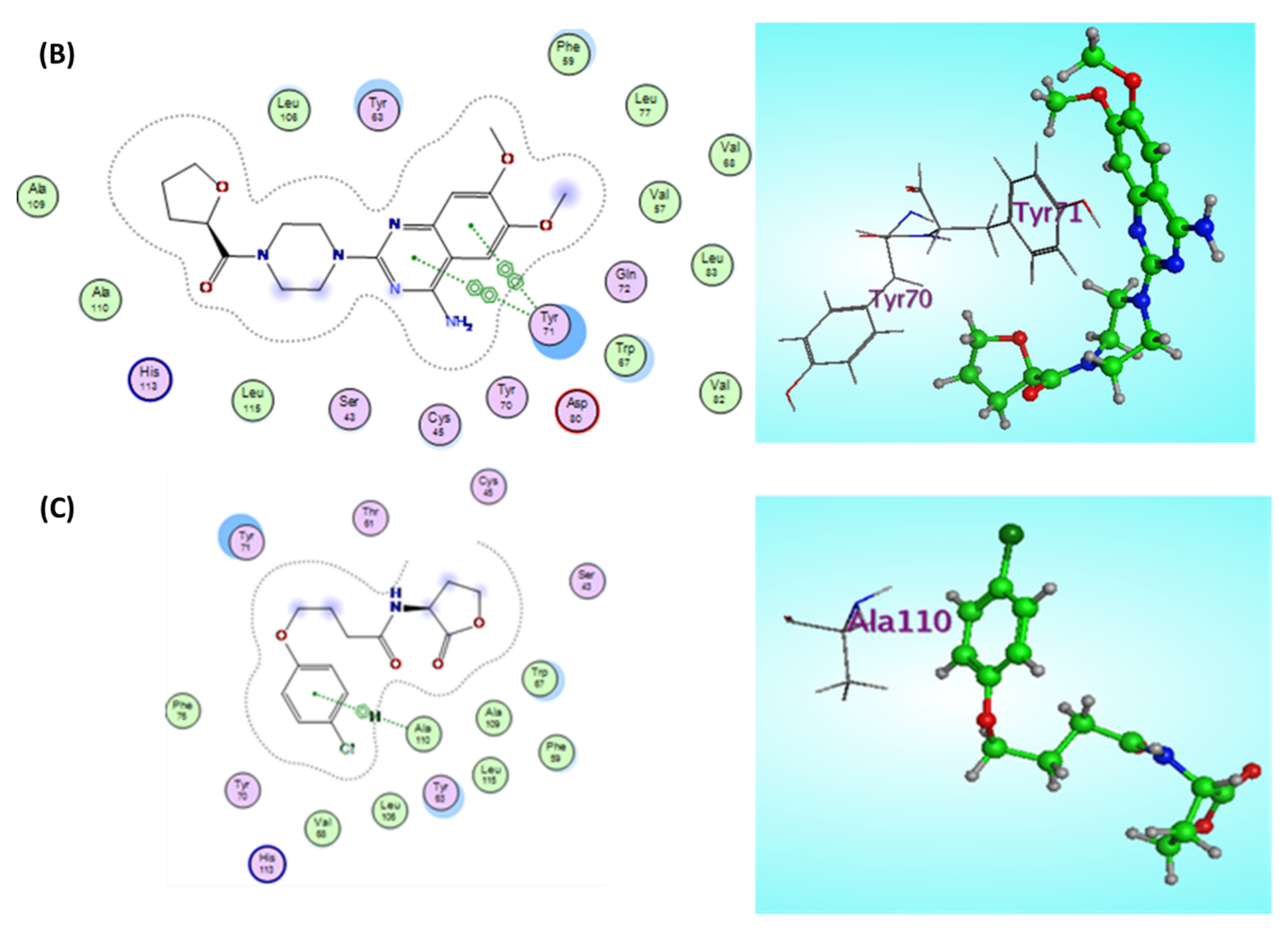
Terazosin Effect on the LuxR Homolog SdiA
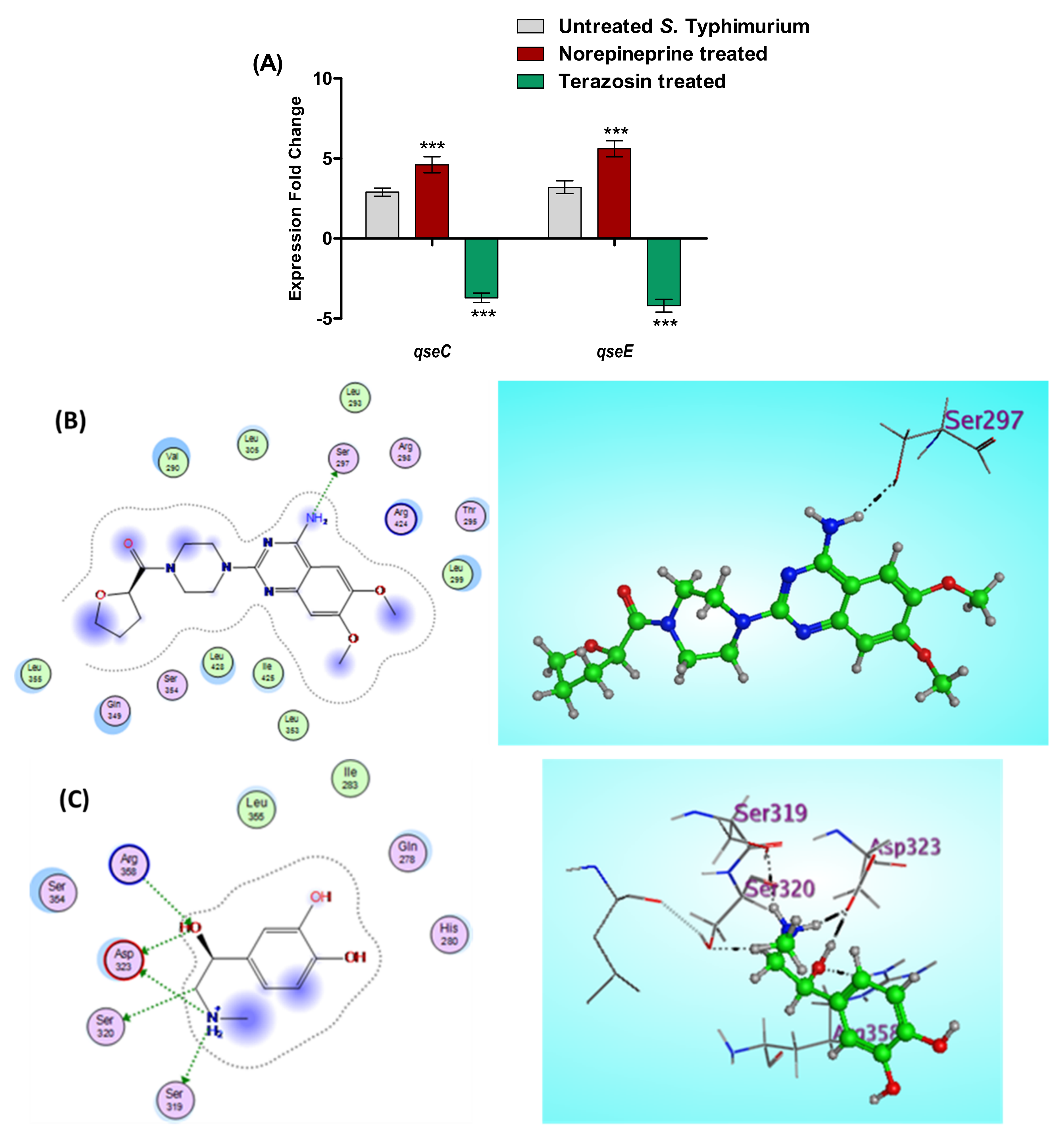
2.5.2. Terazosin Diminishes S. Typhimurium Espionage
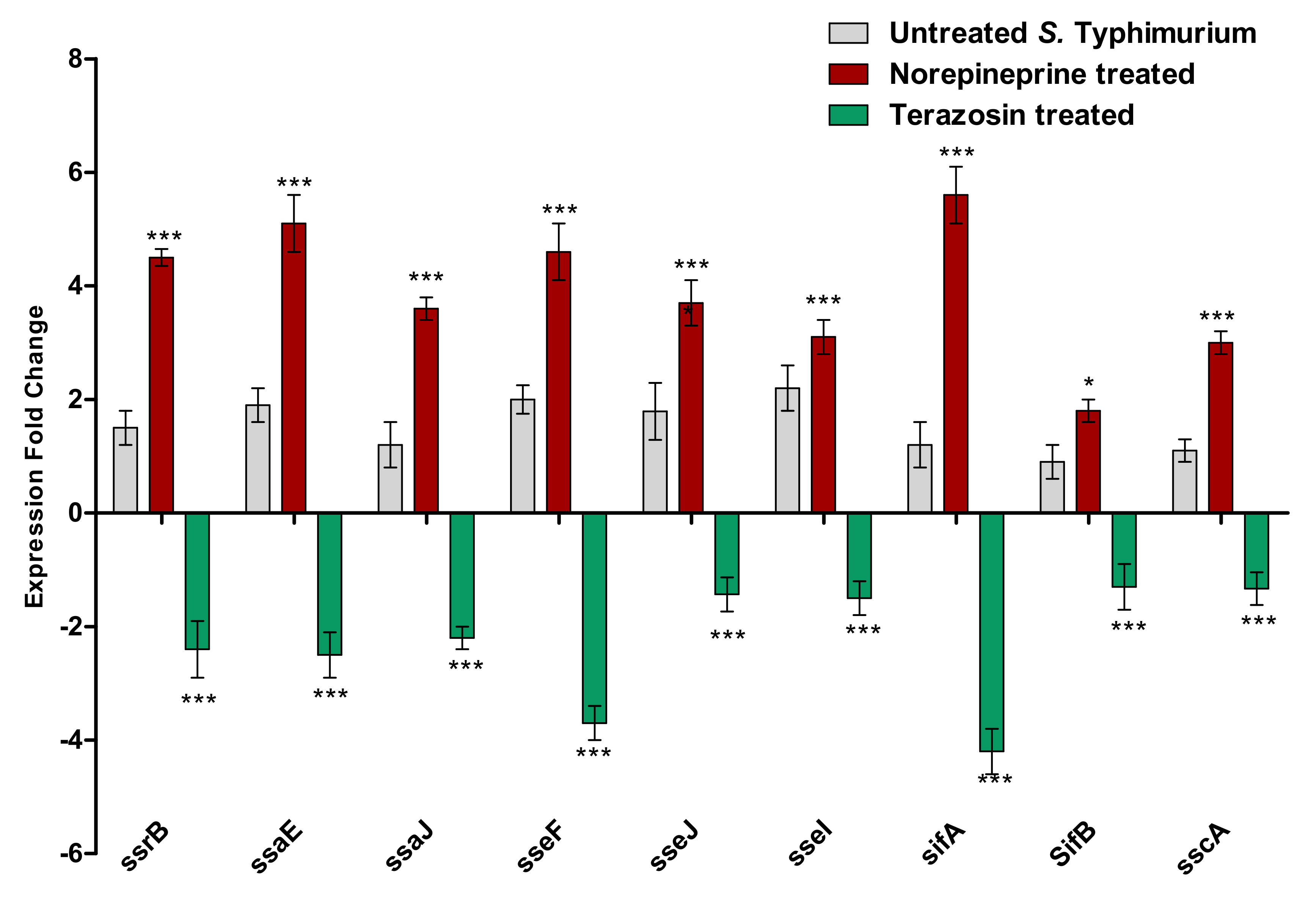
2.5.3. Terazosin Down-Regulates the Expression of T3SS Encoding Genes
3. Discussion
4. Materials and Methods
4.1. Microbiological Media, Chemicals, and Bacterial Strains
4.2. Determination of Terazosin MIC
4.3. Terazosin Effect at the Sub-MIC on Bacterial Growth
4.4. Evaluation of Terazosin Effect on S. Typhimurium Adhesion and Biofilm Formation
4.5. Evaluation of Terazosin Effect on S. Typhimurium Invasion and Intracellular Replication
4.6. Evaluation of the Effect of Terazosin on the Production of QS-Controlled Violacein Pigment
4.7. In Vivo Evaluation of Terazosin Effect on S. Typhimurium Pathogenesis
4.8. Evaluation of Terazosin on the Expression of S. Typhimurium Virulence Genes
4.9. Target Preparation and Ligand Construction for Docking Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Mohr, K.I. History of Antibiotics Research. Curr. Top. Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Khayat, M.T.; Ibrahim, T.S.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms 2020, 8, 1285. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Abbas, H.A.; Khayat, M.T.; Shaldam, M.A.; Askoura, M.; Asfour, H.Z.; Khafagy, E.-S.; Abu Lila, A.S.; Allam, A.N.; Hegazy, W.A.H. Secnidazole Is a Promising Imidazole Mitigator of Serratia marcescens Virulence. Microorganisms 2021, 9, 2333. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, A.N.; Hegazy, W.A.H.; Shaldam, M.A.; Mosbah, R.; Almalki, A.J.; Ibrahim, T.S.; Khayat, M.T.; Khafagy, E.S.; Soliman, W.E.; Abbas, H.A. Xylitol Inhibits Growth and Blocks Virulence in Serratia marcescens. Microorganisms 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Saqr, A.A.; Aldawsari, M.F.; Khafagy, E.-S.; Shaldam, M.A.; Hegazy, W.A.H.; Abbas, H.A. A Novel Use of Allopurinol as A Quorum-Sensing Inhibitor in Pseudomonas aeruginosa. Antibiotics 2021, 10, 1385. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef] [Green Version]
- Muhlen, S.; Dersch, P. Anti-virulence Strategies to Target Bacterial Infections. Curr. Top. Microbiol. Immunol. 2016, 398, 147–183. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Vishwa, B.; Moin, A.; Gowda, D.V.; Rizvi, S.M.D.; Hegazy, W.A.H.; Abu Lila, A.S.; Khafagy, E.S.; Allam, A.N. Pulmonary Targeting of Inhalable Moxifloxacin Microspheres for Effective Management of Tuberculosis. Pharmaceutics 2021, 13, 79. [Google Scholar] [CrossRef]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Contreras, R. Is Quorum Sensing Interference a Viable Alternative to Treat Pseudomonas aeruginosa Infections? Front. Microbiol. 2016, 7, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.T.; Shih, P.C. Effects of quorum sensing signal molecules on the hydrogen peroxide resistance against planktonic Pseudomonas aeruginosa. J. Microbiol. Immunol. Infect. 2000, 33, 154–158. [Google Scholar] [PubMed]
- Jiang, T.; Li, M. Quorum sensing inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 867–894. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef] [Green Version]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Michael, B.; Smith, J.N.; Swift, S.; Heffron, F.; Ahmer, B.M. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J. Bacteriol. 2001, 183, 5733–5742. [Google Scholar] [CrossRef] [Green Version]
- Stevens, A.M.; Dolan, K.M.; Greenberg, E.P. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc. Natl. Acad. Sci. USA 1994, 91, 12619–12623. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, W.A.; Hensel, M. Salmonella enterica as a vaccine carrier. Future Microbiol. 2012, 7, 111–127. [Google Scholar] [CrossRef]
- Askoura, M.; Hegazy, W.A.H. Ciprofloxacin interferes with Salmonella Typhimurium intracellular survival and host virulence through repression of Salmonella pathogenicity island-2 (SPI-2) genes expression. Pathog. Dis. 2020, 78. [Google Scholar] [CrossRef]
- Halatsi, K.; Oikonomou, I.; Lambiri, M.; Mandilara, G.; Vatopoulos, A.; Kyriacou, A. PCR detection of Salmonella spp. using primers targeting the quorum sensing gene sdiA. FEMS Microbiol. Lett. 2006, 259, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraga, A.; Ohlson, M.B.; Miller, S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008, 6, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P. Process of protein transport by the type III secretion system. Microbiol. Mol. Biol. Rev. 2004, 68, 771–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach, R.G.; Hensel, M. Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics resistance of Salmonella enterica. Berl. Munch. Tierarztl. Wochenschr. 2007, 120, 317–327. [Google Scholar]
- Askoura, M.; Almalki, A.J.; Lila, A.S.A.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Ibrahim, T.S.; Hegazy, W.A.H. Alteration of Salmonella enterica Virulence and Host Pathogenesis through Targeting sdiA by Using the CRISPR-Cas9 System. Microorganisms 2021, 9, 2564. [Google Scholar] [CrossRef]
- Smith, J.N.; Ahmer, B.M. Detection of other microbial species by Salmonella: Expression of the SdiA regulon. J. Bacteriol. 2003, 185, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.N.; Dyszel, J.L.; Soares, J.A.; Ellermeier, C.D.; Altier, C.; Lawhon, S.D.; Adams, L.G.; Konjufca, V.; Curtiss, R., 3rd; Slauch, J.M.; et al. SdiA, an N-acylhomoserine lactone receptor, becomes active during the transit of Salmonella enterica through the gastrointestinal tract of turtles. PLoS ONE 2008, 3, e2826. [Google Scholar] [CrossRef]
- Hughes, D.T.; Sperandio, V. Inter-kingdom signalling: Communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Rasko, D.A.; Moreira, C.G.; Li de, R.; Reading, N.C.; Ritchie, J.M.; Waldor, M.K.; Williams, N.; Taussig, R.; Wei, S.; Roth, M.; et al. Targeting QseC signaling and virulence for antibiotic development. Science 2008, 321, 1078–1080. [Google Scholar] [CrossRef] [Green Version]
- Moreira, C.G.; Sperandio, V. Interplay between the QseC and QseE bacterial adrenergic sensor kinases in Salmonella enterica serovar Typhimurium pathogenesis. Infect. Immun. 2012, 80, 4344–4353. [Google Scholar] [CrossRef] [Green Version]
- Kendall, M.M.; Sperandio, V. What a Dinner Party! Mechanisms and Functions of Interkingdom Signaling in Host-Pathogen Associations. mBio 2016, 7, e01748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karavolos, M.H.; Winzer, K.; Williams, P.; Khan, C.M. Pathogen espionage: Multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol. Microbiol. 2013, 87, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Almalki, A.J.; Ibrahim, T.S.; Elhady, S.S.; Darwish, K.M.; Hegazy, W.A.H. Repurposing α-Adrenoreceptor Blockers as Promising Anti-Virulence Agents in Gram-Negative Bacteria. Antibiotics 2022, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Almalki, A.J.; Ibrahim, T.S.; Elhady, S.S.; Hegazy, W.A.H.; Darwish, K.M. Computational and Biological Evaluation of β-Adrenoreceptor Blockers as Promising Bacterial Anti-Virulence Agents. Pharmaceuticals 2022, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug. Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Rajab, A.A.H.; Abu Lila, A.S.; Abbas, H.A. Anti-diabetics and antimicrobials: Harmony of mutual interplay. World J. Diabetes 2021, 12, 1832–1855. [Google Scholar] [CrossRef]
- El-Hamid, M.I.A.; El-Naenaeey, E.-S.Y.; Kandeel, T.M.; Hegazy, W.A.H.; Mosbah, R.A.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 667. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A.H. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Khafagy, E.S.; Saqr, A.A.; Alalaiwe, A.; Abbas, H.A.; Shaldam, M.A.; Hegazy, W.A.H.; Goda, R.M. Tackling Virulence of Pseudomonas aeruginosa by the Natural Furanone Sotolon. Antibiotics 2021, 10, 871. [Google Scholar] [CrossRef]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.H.; Awad, N.F.S.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics 2020, 10, 25. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Khayat, M.T.; Ibrahim, T.S.; Youns, M.; Mosbah, R.; Soliman, W.E. Repurposing of antidiabetics as Serratia marcescens virulence inhibitors. Braz. J. Microbiol. 2021, 52, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, A.N.; Abbas, H.A.; Mohamed, M.F.A.; Asfour, H.Z.; Khayat, M.T.; Ibrahim, T.S.; Youns, M.; Khafagy, E.-S.; Abu Lila, A.S.; Safo, M.K.; et al. Not Only Antimicrobial: Metronidazole Mitigates the Virulence of Proteus mirabilis Isolated from Macerated Diabetic Foot Ulcer. Appl. Sci. 2021, 11, 6847. [Google Scholar] [CrossRef]
- Abbas, H.A.; Hegazy, W.A.H. Targeting the virulence factors of Serratia marcescens by ambroxol. Roum. Arch. Microbiol. Immunol. 2017, 76, 27–32. [Google Scholar]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Brothers, K.M.; Stella, N.A.; Romanowski, E.G.; Kowalski, R.P.; Shanks, R.M. EepR Mediates Secreted-Protein Production, Desiccation Survival, and Proliferation in a Corneal Infection Model. Infect. Immun. 2015, 83, 4373–4382. [Google Scholar] [CrossRef] [Green Version]
- Aldawsari, M.F.; Alalaiwe, A.; Khafagy, E.S.; Al Saqr, A.; Alshahrani, S.M.; Alsulays, B.B.; Alshehri, S.; Abu Lila, A.S.; Danish Rizvi, S.M.; Hegazy, W.A.H. Efficacy of SPG-ODN 1826 Nanovehicles in Inducing M1 Phenotype through TLR-9 Activation in Murine Alveolar J774A.1 Cells: Plausible Nano-Immunotherapy for Lung Carcinoma. Int. J. Mol. Sci. 2021, 22, 6833. [Google Scholar] [CrossRef]
- Askoura, M.; Abbas, H.A.; Al Sadoun, H.; Abdulaal, W.H.; Abu Lila, A.S.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Ibrahim, T.S.; Hegazy, W.A.H. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens 2022, 11, 57. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Henaway, M. Hepatitis C virus pathogenesis: Serum IL-33 level indicates liver damage. Afr. J. Microbiol. Res. 2015, 9, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Hegazy, W.A.H. Diclofenac inhibits virulence of Proteus mirabilis isolated from diabetic foot ulcer. Afr. J. Microbiol. Res. 2016, 10, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Sarma, J.V.; Day, D.E.; Lentsch, A.B.; Huber-Lang, M.S.; Ward, P.A. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS ONE 2009, 4, e4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, A.M.; Soby, S.D. Reclassification of Chromobacterium violaceum ATCC 31532 and its quorum biosensor mutant CV026 to Chromobacterium subtsugae. AMB Express 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.; Xu, X.; Metelitsa, L.; Hensel, M. Evaluation of Salmonella enterica type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect. Immun. 2012, 80, 1193–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, H.A.; Hegazy, W.A.H. Repurposing anti-diabetic drug “Sitagliptin” as a novel virulence attenuating agent in Serratia marcescens. PLoS ONE 2020, 15, e0231625. [Google Scholar] [CrossRef] [Green Version]
- Agha, K.A.; Abo-Dya, N.E.; Ibrahim, T.S.; Abdel-Aal, E.H.; Hegazy, W.A. Benzotriazole-Mediated Synthesis and Antibacterial Activity of Novel N-Acylcephalexins. Sci. Pharm. 2016, 84, 484–496. [Google Scholar] [CrossRef] [Green Version]
- Methner, U.; Rabsch, W.; Reissbrodt, R.; Williams, P.H. Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: Role of catecholate siderophore precursors and degradation products. Int. J. Med. Microbiol. 2008, 298, 429–439. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Abbas, H.A. Evaluation of the role of SsaV Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr. J. Biotechnol. 2017, 16, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Ahmer, B.M. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol. Microbiol. 2004, 52, 933–945. [Google Scholar] [CrossRef]
- Pacheco, A.R.; Sperandio, V. Inter-kingdom signaling: Chemical language between bacteria and host. Curr. Opin. Microbiol. 2009, 12, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Holzer, S.U.; Hensel, M. Divergent roles of Salmonella pathogenicity island 2 and metabolic traits during interaction of S. enterica serovar typhimurium with host cells. PLoS ONE 2012, 7, e33220. [Google Scholar] [CrossRef]
- Xu, X.; Hegazy, W.A.; Guo, L.; Gao, X.; Courtney, A.N.; Kurbanov, S.; Liu, D.; Tian, G.; Manuel, E.R.; Diamond, D.J.; et al. Effective cancer vaccine platform based on attenuated Salmonella and a type III secretion system. Cancer Res. 2014, 74, 6260–6270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhle, V.; Hensel, M. Cellular microbiology of intracellular Salmonella enterica: Functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cell. Mol. Life Sci. CMLS 2004, 61, 2812–2826. [Google Scholar] [CrossRef] [PubMed]
- Knuff, K.; Finlay, B.B. What the SIF Is Happening-The Role of Intracellular Salmonella-Induced Filaments. Front. Cell. Infect. Microbiol. 2017, 7, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiwick, J.; Salcedo, S.P.; Boucrot, E.; Gilliland, S.M.; Henry, T.; Petermann, N.; Waterman, S.R.; Gorvel, J.P.; Holden, D.W.; Meresse, S. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect. Immun. 2006, 74, 6965–6972. [Google Scholar] [CrossRef] [Green Version]
- Almalki, A.J.; Ibrahim, T.S.; Taher, E.S.; Mohamed, M.F.A.; Youns, M.; Hegazy, W.A.H.; Al-Mahmoudy, A.M.M. Synthesis, Antimicrobial, Anti-Virulence and Anticancer Evaluation of New 5(4H)-Oxazolone-Based Sulfonamides. Molecules 2022, 27, 671. [Google Scholar] [CrossRef]
- Youns, M.; Askoura, M.; Abbas, H.A.; Attia, G.H.; Khayyat, A.N.; Goda, R.M.; Almalki, A.J.; Khafagy, E.S.; Hegazy, W.A.H. Celastrol Modulates Multiple Signaling Pathways to Inhibit Proliferation of Pancreatic Cancer via DDIT3 and ATF3 Up-Regulation and RRM2 and MCM4 Down-Regulation. Onco. Targets Ther. 2021, 14, 3849–3860. [Google Scholar] [CrossRef]
- Askoura, M.; Youns, M.; Halim Hegazy, W.A. Investigating the influence of iron on Campylobacter jejuni transcriptome in response to acid stress. Microb. Pathog. 2020, 138, 103777. [Google Scholar] [CrossRef]

| Target Gene | Primer Sequence: 5′-3′ | Gene Significance | Reference |
|---|---|---|---|
| gyrB | F: GTGATCAGCGTCGCCACT R: GCGCGGTGATCAGCGTC | Housekeeping | [20] |
| ssrB | F: CGCAGGTGCTAATGGCTATG R: TTTGCAATGCCGCTAACAGA | SPI2-expression regulation | [20] |
| ssaE | F: CCGCAGCAATATCAGCAAAA R: AAGTGCGCTGTTATGGTAACGA | SPI2-intracellular replication | [20] |
| ssaJ | F: TGTCGAGCAGTCGCAGTTTATTA R: TGCCTATGCGGATAACCGTTA | SPI2-intracellular replication | [20] |
| sseF | F: TCAGGAATCGCTATTTCTATG R: GTCAGGCTAACGGAGGTAA | SPI2-intracellular replication | [20] |
| sseJ | F: AATAAATCACATCCCAAGC R: ACTCAGTCCAGGTAAATCC | SPI2-intracellular replication | [20] |
| sseI | F: GATACCCCCCCTGAAATGAGTT R: GTGACAAATCGTCCAGATGCA | SPI2-intracellular replication | [20] |
| sifA | F: TACCACCACCGCATACCCA R: ACGAGGAACGCCTGAAACG | Salmonella-inducing filaments (SPI2) | [20] |
| SifB | F: TGATACTCAGCCTGCCCAC R: GCTCAGGGAACAAGCAAC | Salmonella-inducing filaments (SPI2) | [20] |
| sscA | F: GGCTCGCTGCGTATGTTGTT R: GCCGGCGAATTCTTTTACCT | SPI2 chaperon intracellular replication | [20] |
| qseC | F: GGTACCAAATTGACGCAACGTCTCAG R: GAATTCGCCCAACTTACTACGGCCTC | Sensor to adrenergic hormones | [30] |
| qseE | F: GGTACCAGCGACACGTTGAAGCGC R: GAATTCGCGTGTTTGTCAGATGCAGG | Sensor to adrenergic hormones | [30] |
| sdiA | F: AAT ATC GCT TCG TAC CAC R: GTA GGT AAA CGA GGA GCA G | Adhesion | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegazy, W.A.H.; Salem, I.M.; Alotaibi, H.F.; Khafagy, E.-S.; Ibrahim, D. Terazosin Interferes with Quorum Sensing and Type Three Secretion System and Diminishes the Bacterial Espionage to Mitigate the Salmonella Typhimurium Pathogenesis. Antibiotics 2022, 11, 465. https://doi.org/10.3390/antibiotics11040465
Hegazy WAH, Salem IM, Alotaibi HF, Khafagy E-S, Ibrahim D. Terazosin Interferes with Quorum Sensing and Type Three Secretion System and Diminishes the Bacterial Espionage to Mitigate the Salmonella Typhimurium Pathogenesis. Antibiotics. 2022; 11(4):465. https://doi.org/10.3390/antibiotics11040465
Chicago/Turabian StyleHegazy, Wael A. H., Ibrahim M. Salem, Hadil Faris Alotaibi, El-Sayed Khafagy, and Doaa Ibrahim. 2022. "Terazosin Interferes with Quorum Sensing and Type Three Secretion System and Diminishes the Bacterial Espionage to Mitigate the Salmonella Typhimurium Pathogenesis" Antibiotics 11, no. 4: 465. https://doi.org/10.3390/antibiotics11040465
APA StyleHegazy, W. A. H., Salem, I. M., Alotaibi, H. F., Khafagy, E. -S., & Ibrahim, D. (2022). Terazosin Interferes with Quorum Sensing and Type Three Secretion System and Diminishes the Bacterial Espionage to Mitigate the Salmonella Typhimurium Pathogenesis. Antibiotics, 11(4), 465. https://doi.org/10.3390/antibiotics11040465







