Phytocompounds as an Alternative Antimicrobial Approach in Aquaculture
Abstract
1. Introduction
2. Phytocompounds
2.1. Flavonoids
2.2. Alkaloids
2.3. Phenolic Acids
2.4. Terpenoids
2.5. Saponins
3. Extraction Methods of Phytochemicals from Plants
3.1. Extraction Process
3.1.1. Conventional Extraction Method
Soxhlet Method
Maceration
Percolation
Decoction
3.1.2. Advanced Extraction Method
Microwave-Assisted Extraction (MAE)
3.2. Importance of Extraction of Phytocompounds
| Method | Solvent | Temperature | Duration | Compound(s) Extracted | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Maceration | Water, aqueous and non-aqueous solvents | 28–30 °C | 3–4 days | Phenolics, flavonoids and alkaloids | Cheap, with no special tools required and less energy processes. | Time-consuming and high solvent usage. | [119] |
| Percolation | Water, aqueous and non-aqueous solvents | 25–40 °C | 24 h | Phenolics and flavonoids | Shorter time than maceration and is possible to extract thermolabile constituents. | Needs skill and takes longer time than Soxhlet extraction. | [120] |
| Soxhlet extraction | Organic solvents | <60 °C | 16–20 h | Andrographolide and deoxyandrographolide | Able to extract large sample materials, less skill required and solvent savings. | High risk of thermal destruction of compounds and time-consuming. | [121] |
| Decoction | Water | 70 °C | 0.5–1 h | Catechoo-tannins, anthraquinones, phenolics and alkaloids | Suitable for heat-stable compounds and less skill required. | Unsuitable for heat-sensitive compounds. | [122] |
| Microwave-assisted extraction | Water, aqueous and non-aqueous solvents | 70–80 °C | 3–5 min | Phenolics, alkaloids and carotenoids | Less organic solvents are needed, high extraction rate and no airborne contamination. | Limited amount of sample that can be extracted. | [123] |
4. Phytocompounds as Alternatives to Antimicrobial Approach in Aquaculture
4.1. Antimicrobial Activities in Aquaculture
4.1.1. Antibacterial Activity
4.1.2. Antiparasitic Activity
4.1.3. Antiviral Activity
4.1.4. Antifungal Activity
5. Challenges and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- FAO. Fisheries and Aquaculture Software. FishStatJ: Software for Fishery and Aquaculture Statistical Time Series; FAO Fisheries Division: Rome, Italy, 2019. [Google Scholar]
- The Insight Partners. Aquaculture Market Forecast to 2028—COVID-19 Impact and Global Analysis by Culture Environment (Freshwater, Marine Water, and Brackish Water) and Product Type (Fish, Aquatic Plants, Crustaceans, Mollusca, and Others). GlobeNewswire. 2022. Available online: https://www.globenewswire.com/news-release/2022/02/10/2382597/0/en/Aquaculture-Market-Size-Worth-357-903-27-Mn-Globally-by-2028-at-4-3-CAGR-Exclusive-Report-by-The-Insight-Partners.html (accessed on 15 February 2022).
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018; pp. 40–41. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture. Sustainability in Action; FAO: Rome, Italy, 2020; pp. 4–6. [Google Scholar]
- Bombardelli, R.A.; Mewes, J.K.; Buzzi, A.H.; de Oliveira Pedreira, A.C.; Syperreck, M.A.; Dalmaso, A.C.S.; Chagas, T.V.; Chiella, R.J.; Meurer, F. Diets containing crude glycerin modify the ovary histology, cause reproductive harm on Nile tilapia females and impair the offspring quality. Aquaculture 2021, 533, 736098. [Google Scholar] [CrossRef]
- Schrag, S.J.; Wiener, P. Emerging infectious disease: What are the relative roles of ecology and evolution? Trends Ecol. Evol. 1995, 10, 319–324. [Google Scholar] [CrossRef]
- Patz, J.A.; Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef]
- Dobson, A.; Foufopoulos, J. Emerging infectious pathogens of wildlife. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2001, 356, 1001–1012. [Google Scholar] [CrossRef]
- Murray, A.G.; Peeler, E.J. A framework for understanding the potential for emerging diseases in aquaculture. Prev. Vet. Med. 2005, 67, 223–235. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Pulkkinen, K.; Pulkkinen, K.; Suomalainen, L.R.; Read, A.F.; Ebert, D.; Rintamäki, P.; Valtonen, E.T. Intensive fish farming and the evolution of pathogen virulence: The case of columnaris disease in Finland. Proc. R. Soc. B Biol. Sci. 2010, 277, 593–600. [Google Scholar] [CrossRef]
- Rodger, H.D. Fish disease causing economic impact in global aquaculture. In Fish Vaccines; Springer: Basel, Switzerland, 2016; pp. 1–34. [Google Scholar]
- Stentiford, G.D.; Sritunyalucksana, K.; Flegel, T.W.; Williams, B.A.; Withyachumnarnkul, B.; Itsathitphaisarn, O.; Bass, D. New paradigms to help solve the global aquaculture disease crisis. PLoS Pathog. 2017, 13, e1006160. [Google Scholar] [CrossRef]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Dadar, M.; Doan, H.V.; Harikrishnan, R. Feed additives impacts on shellfish microbiota, health, and development. In Microbial Communities in Aquaculture Ecosystems; Springer: Cham, Switzerland, 2019; pp. 143–163. [Google Scholar]
- Hoque, M.S.; Jacxsens, L.; De Meulenaer, B.; Alam, A.N. Quantitative risk assessment for formalin treatment in fish preservation: Food safety concern in local market of Bangladesh. Procedia Food Sci. 2016, 6, 151–158. [Google Scholar] [CrossRef]
- Pham, D.K.; Chu, J.; Do, N.T.; Brose, F.; Degand, G.; Delahaut, P.; De Pauw, E.; Douny, C.; Van Nguyen, K.; Vu, T.D.; et al. Monitoring antibiotic use and residue in freshwater aquaculture for domestic use in Vietnam. EcoHealth 2015, 12, 480–489. [Google Scholar] [CrossRef] [PubMed]
- De La Pena, A.M.; Espinosa-Mansilla, A. Analysis of antibiotics in fish samples. Anal. Bioanal. Chem. 2009, 395, 987–1008. [Google Scholar]
- Won, S.Y.; Lee, C.H.; Chang, H.S.; Kim, S.O.; Lee, S.H.; Kim, D.S. Monitoring of 14 sulfonamide antibiotic residues in marine products using HPLC-PDA and LC-MS/MS. Food Control 2011, 22, 1101–1107. [Google Scholar] [CrossRef]
- He, X.; Wang, Z.; Nie, X.; Yang, Y.; Pan, D.; Leung, A.O.; Cheng, Z.; Yang, Y.; Li, K.; Chen, K. Residues of fluoroquinolones in marine aquaculture environment of the Pearl River Delta, South China. Environ. Geochem. Health 2012, 34, 323–335. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013. [Google Scholar]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Buschmann, A.H.; Dölz, H.J. Aquaculture as yet anoHassther environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 2016, 16, e127–e133. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.; Gilbert, M.; Van Boeckel, T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12, 5384. [Google Scholar] [CrossRef]
- Kragesteen, T.J.; Simonsen, K.; Visser, A.W.; Andersen, K.H. Identifying salmon lice transmission characteristics between Faroese salmon farms. Aquac. Environ. Interact. 2018, 10, 49–60. [Google Scholar] [CrossRef]
- Cantrell, D.L.; Groner, M.L.; Ben-Horin, T.; Grant, J.; Revie, C.W. Modeling pathogen dispersal in marine fish and shellfish. Trends Parasitol. 2020, 36, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Dang, K.D.H.; Rohrbach, E.; Breider, F.; Rossi, P. Seasonal and spatial variability of antibiotic resistance genes and Class I integrons in the rivers of the Mekong delta, Vietnam. bioRxiv 2021, 8–21. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Liu, C.; Xue, Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Doan, H.V.; Soltani, E.; Ingelbrecht, J.; Soltani, M. Medicinal herbs and plants: Potential treatment of monogenean infections in fish. Rev. Fish. Sci. Aquac. 2020, 28, 260–282. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Markham, K.R.; Andersen, O.M. Flavonoids: Chemistry, Biochemistry, and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Tuli, H.S. Current Aspects of Flavonoids: Their Role in Cancer Treatment; Springer: Singapore, 2019. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Yamagata, K. Metabolic Syndrome: Preventive Effects of Dietary Flavonoids. Stud. Nat. Prod. Chem. 2019, 60, 1–4. [Google Scholar]
- Sinsinwar, S.; Vadivel, V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 8279–8297. [Google Scholar] [CrossRef]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The combination of catechin and epicatechin gallate from Fructus crataegi potentiates β-lactam antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) in vitro and in vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.A.; Rahuman, A.A.; Bagavan, A.; Geetha, K.; Kamaraj, C.; Elango, G. Evaluation of medicinal plant extracts and isolated compound epicatechin from Ricinus communis against Paramphistomum cervi. Parasitol. Res. 2012, 111, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Mansoor, A.A.; Gross, A.; Ashfaq, M.K.; Jacob, M.; Khan, S.I.; Hamann, M.T. Methicillin-resistant Staphylococcus aureus (MRSA)-active metabolites from Platanus occidentalis (American sycamore). J. Nat. Prod. 2009, 72, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Potential antihyperglycaemic effect of myricetin derivatives from Syzygium malaccense. J. Funct. Foods 2016, 22, 325–336. [Google Scholar] [CrossRef]
- Boulaaba, M.; Snoussi, M.; Saada, M.; Mkadmini, K.; Smaoui, A.; Abdelly, C.; Ksouri, R. Antimicrobial activities and phytochemical analysis of Tamarix gallica extracts. Ind. Crops Prod. 2015, 76, 1114–1122. [Google Scholar] [CrossRef]
- Shafaghat, A.; Farzaneh, P.; Shafaghatlonbar, M. Luteolin derivatives and antimicrobial activity of Achillea tenuifolia Lam. methanol extract. Ind. Crops Prod. 2014, 62, 533–536. [Google Scholar] [CrossRef]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K.; Tukappa, A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 175851. [Google Scholar] [CrossRef]
- Fallah, D.F.; Arasteh, A. Investigation of antioxidant and Inhibitory properties of soybean seeds extract (Glycine max) on the acetylcholinesterase and the production of Amyloid Nano- biofibrils. Exp. Anim. Biol. 2021, 10, 11–22. [Google Scholar]
- Moovendhan, M.; Ramasubburayan, R.; Vairamani, S.; Shanmugam, A.; Palavesam, A.; Immanuel, G. Antibiotic susceptibility of Genistein and Alkaloids from Rhizophora apiculata. Biocatal. Agric. Biotechnol. 2014, 3, 323–327. [Google Scholar] [CrossRef]
- Husni, E.F.I.; Afriyandi, D. Standardization study of simplicia and extract of calamondin (citrus microcarpa bunge) peel, quantification of hesperidin and antibacterial assay. Pharmacogn. J. 2020, 12, 777–783. [Google Scholar] [CrossRef]
- Prakash, S.; Elavarasan, N.; Subashini, K.; Kanaga, S.; Dhandapani, R.; Sivanandam, M.; Kumaradhas, P.; Thirunavukkarasu, C.; Sujatha, V. Isolation of hesperetin-A flavonoid from Cordia sebestena flower extract through antioxidant assay guided method and its antibacterial, anticancer effect on cervical cancer via in vitro and in silico molecular docking studies. J. Mol. Struct. 2020, 1207, 127751. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Vorsa, N.; Marderosian, A.D.; Foo, L.Y. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N. Engl. J. Med. 1998, 339, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Encyclopedia Britannica. The Editors of Encyclopaedia. Alkaloid. 2020. Available online: https://www.britannica.com/science/alkaloid (accessed on 22 December 2021).
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 6. [Google Scholar]
- Buckingham, J.; Baggaley, K.H.; Roberts, A.D.; Szabo, L.F. (Eds.) Dictionary of Alkaloids with CD-ROM; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Venkateshwarlu, R.; Gopal, Y.V.; Raju, A.B.; Prasad, K.B. Antitumor activity of Alangium salvifolium against Dalton’s ascitic Lymphoma. Med. Chem. Drug Discov. 2012, 3, 122–133. [Google Scholar]
- Jiang, H.Y.; Wang, C.F.; Fan, L.; Yang, K.; Feng, J.B.; Geng, Z.F.; Xu, J.; Deng, Z.W.; Du, S.S.; Yin, H.B. Cytotoxic constituents from the stems of Clausena lansium (Lour.) Skeels. Molecules 2013, 18, 10768–10775. [Google Scholar] [CrossRef]
- Altameme, H.J.; Hameed, I.H.; Kareem, M.A. Analysis of alkaloid phytochemical compounds in the ethanolic extract of Datura stramonium and evaluation of antimicrobial activity. Afr. J. Biotechnol. 2015, 14, 1668–1674. [Google Scholar]
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and antimicrobial activity of alkaloid extracts from seeds of different genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Dicko, M.H.; Gruppen, H.; Traoré, A.S.; van Berkel, W.J.; Voragen, A.G. Evaluation of the effect of germination on phenolic compounds and antioxidant activities in sorghum varieties. J. Agric. Food Chem. 2005, 53, 2581–2588. [Google Scholar] [CrossRef]
- Lisete-Torres, P.; Losada-Barreiro, S.; Albuquerque, H.; Sanchez-Paz, V.; Paiva-Martins, F.; Bravo-Diaz, C. Distribution of hydroxytyrosol and hydroxytyrosol acetate in olive oil emulsions and their antioxidant efficiency. J. Agric. Food Chem. 2012, 60, 7318–7325. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Boulekbache-Makhlouf, L.S.S.; Madani, K. Total phenolic content, antioxidant and antibacterial activities of fruits of Eucalyptus globulus cultivated in Algeria. Ind. Crops Prod. 2013, 41, 85–89. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, Q.H.; Xiong, X.G.; Wang, Y.; Zhang, Z.H.; Sun, M.J.; Lu, X.; Wu, D. Anti-inflammatory effects of p-coumaric acid, a natural compound of Oldenlandia diffusa, on arthritis model rats. Evid. -Based Complementary Altern. Med. 2018, 2018, 5198594. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.-Y.; Chou, T.-Z.; Liang, C.-H. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from Origanum vulgare. Food Chem. 2010, 123, 254–262. [Google Scholar] [CrossRef]
- Mateo, A.N.; van den Berg, R.; Havenaar, R.; Bast, A.; RMM Haenen, G. Ferulic acid from aleurone determines the antioxidant potency of wheat grain (Triticum aestivum L.). J. Agric. Food Chem. 2008, 56, 5589–5594. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.-Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef]
- Berthelot, K.; Estevez, Y.; Deffieux, A.; Peruch, F. Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis. Biochimie 2012, 94, 1621–1634. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. The Cosmeceutical Properties of Compounds Derived from Marine Algae. Mar. Macro-Microalgae Overv. 2018, 198. [Google Scholar]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Heiling, S.; Schuman, M.C.; Schoettner, M.; Mukerjee, P.; Berger, B.; Schneider, B.; Jassbi, A.R.; Baldwin, I.T. Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell 2010, 22, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Serrano, M.A.; Vallejo, M.; Efferth, T.; Alvarez, M.; Marin, J.J. Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med. 2006, 72, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Sinha, P.; Yadav, K.S.; Shukla, A.; Saxena, A.; Bawankule, D.U.; Tandon, S.; Khan, F.; Chanotiya, C.S.; Yadav, N.P. Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components linalool and linalyl acetate. J. Ethnopharmacol. 2020, 261, 113127. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.; Yahaya, M.; Abubakar, H.; Sanusi, A.; Adamu, H.W.; Alebiosu, C.O. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, 1–5. [Google Scholar]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef]
- El Aziz, M.M.A.; Ashour, A.S.; Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 282–288. [Google Scholar]
- Netala, V.R.; Ghosh, S.B.; Bobbu, P.; Anitha, D.; Tartte, V. Triterpenoid saponins: A review on biosynthesis, applications and mechanism of their action. Int. J. Pharm. Pharm. Sci. 2015, 7, 24–28. [Google Scholar]
- Liu, W.; Deng, S.; Zhou, D.; Huang, Y.; Li, C.; Hao, L.; Zhang, G.; Su, S.; Xu, X.; Yang, R.; et al. 3, 4-Seco-dammarane triterpenoid saponins with anti-inflammatory activity isolated from the leaves of Cyclocarya paliurus. J. Agric. Food Chem. 2020, 68, 2041–2053. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, L.; Xu, Y.; Guo, Y.; Wei, L.; Li, X.; Song, W. Antidiabetic effect of Momordica charantia saponins in rats induced by high-fat diet combined with STZ. Electron. J. Biotechnol. 2020, 43, 41–47. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, R.; Zhang, W.; Yao, G.L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crops Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- Kozińska, N.; Tokarska, K.; Chudy, M.; Wojciechowski, K. Cytotoxicity of Quillaja saponaria Saponins towards Lung Cells Is Higher for Cholesterol-Rich Cells. Biophysica 2021, 1, 126–136. [Google Scholar] [CrossRef]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- Lee, I.-A.; Park, Y.J.; Joh, E.H.; Kim, D.H. Soyasaponin Ab ameliorates colitis by inhibiting the binding of lipopolysaccharide (LPS) to Toll-like receptor (TLR) 4 on macrophages. J. Agric. Food Chem. 2011, 59, 13165–13172. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Z.; Zhou, B.; Fu, S.; Hong, T.; Li, P.; Liu, J. A new ocotillol-type ginsenoside from stems and leaves of Panax quinquefolium L. and its anti-oxidative effect on hydrogen peroxide exposed A549 cells. Nat. Prod. Res. 2020, 34, 2474–2481. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, 140–148. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Z.; Li, Y.; Qian, Z. Overview of the quality standard research of traditional Chinese medicine. Front. Med. 2011, 5, 195–202. [Google Scholar] [CrossRef]
- Barbero, G.F. Extraction and Analysis of Natural Product in Plant. Agronomy 2021, 11, 415. [Google Scholar] [CrossRef]
- De Castro, M.L.; Garcıa-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging technologies for the extraction of marine phenolics: Opportunities and challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Seidel, V. Initial and bulk extraction of natural products isolation. Nat. Prod. Isol. 2012, 864, 27–41. [Google Scholar]
- De Castro, MD Luque, and Feliciano Priego-Capote. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Rasul, M.G. Conventional Extraction Methods Use in Medicinal Plants, their Advantages and Disadvantages. Int. J. Basic Sci. App. Comput. 2018, 2, 10–14. [Google Scholar]
- Handa, S.S. An overview of extraction techniques for medicinal and aromatic plants. Extr. Technol. Med. Aromat. Plants 2008, 1, 21–40. [Google Scholar]
- Zhang, Q.-W.; Lin, L.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- United Nations Industrial Development Organization; Handa, S.S.; Khanuja, S.P.S.; Longo, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants; United Nations Industrial Development Organization and the International Centre for Science and High Technology: Trieste, Italiy, 2008; pp. 200–266. [Google Scholar]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem. 2017, 6, 32–36. [Google Scholar]
- Alonso-Salces, R.M.; Korta, E.; Barranco, A.; Berrueta, L.A.; Gallo, B.; Vicente, F. Determination of polyphenolic profiles of Basque cider apple varieties using accelerated solvent extraction. J. Agric. Food Chem. 2001, 49, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Guntero, V.A.; Mancini, P.M.E.; Kneeteman, M.N. Introducing Organic Chemistry Students to the Extraction of Natural Products Found in Vegetal Species. World J. Chem. Educ. 2017, 5, 142–147. [Google Scholar] [CrossRef]
- Cristianini, M.; Sánchez, J.S.G. Extraction of bioactive compounds from purple corn using emerging technologies: A review. J. Food Sci. 2020, 85, 862–869. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Chemat, F.; Cravotto, G. (Eds.) Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 4. [Google Scholar]
- Tambun, R.; Alexander, V.; Ginting, Y. Performance comparison of maceration method, soxhletation method, and microwave-assisted extraction in extracting active compounds from soursop leaves (Annona muricata): A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1122, 012095. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.A.; Barreiro, M.F.; Rodrigues, A.; Curran, T.P.; Barros, L.; Ferreira, I. Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crops Prod. 2017, 95, 404–415. [Google Scholar] [CrossRef]
- Stéphane, F.F.Y.; Jules, B.K.J.; Batiha, G.E.S.; Ali, I.; Bruno, L.N. Extraction of Bioactive Compounds from Medicinal Plants and Herbs; IntechOpen: London, UK, 2021. [Google Scholar]
- Nn, A. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar]
- Ebada, S.S.; Edrada, R.A.; Lin, W.; Proksch, P. Methods for Isolation, Purification and Structural Elucidation of Bioactive Secondary Metabolites from Marine Invertebrates. Nat. Protoc. 2008, 3, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Chigayo, K.; Mojapelo, P.E.L.; Mnyakeni-Moleele, S.; Misihairabgwi, J.M. Phytochemical and antioxidant properties of different solvent extracts of Kirkia wilmsii tubers. Asian Pac. J. Trop. Biomed. 2016, 6, 1037–1043. [Google Scholar] [CrossRef]
- Biscaia, D.; Ferreira, S.R.S. Propolis extracts obtained by low pressure methods and supercritical fluid extraction. J. Supercrit. Fluids 2009, 51, 17–23. [Google Scholar] [CrossRef]
- Abdullahi, M.I.; Musa, A.M.; Haruna, A.K.; Sule, I.M.; Abdullahi, M.S.; Abdulmalik, M.; Akinwande, Y.; Abimiku, A.G.; Iliya, I. Anti-microbial Flavonoid Diglycoside from the leaves of Ochna schweinfurthiana F Hoffm (Ochnaceae). Niger. J. Pharm. Sci. 2011, 10, 1–7. [Google Scholar]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F.; Ghaziaskar, S.H. Optimization of phenolic and flavonoid content and antioxidants capacity of pressurized liquid extraction from Dracocephalum kotschyi via circumscribed central composite. J. Supercrit. Fluids 2016, 107, 307–314. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Hasan, M.; Singh, H. Effects of solvent properties on the Soxhlet extraction of diterpenoid lactones from Andrographis paniculata leaves. Sci. Asia 2009, 35, 306–309. [Google Scholar] [CrossRef]
- Zintchem, R.; Fankem, G.O.; Fokunang, C.; Mkounga, P.; Tembe, E.A.; Kamgang, R.; Gatsing, D.; Dimo, T. In vitro evaluation of the antimicrobial properties of Mallotus oppositifolium decoction leaf extracts and fractions. Int. J. Biol. Chem. Sci. 2015, 9, 815–824. [Google Scholar] [CrossRef][Green Version]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M. Optimization of saponins, phenolics, and antioxidants extracted from fenugreek seeds using microwave-assisted extraction and response surface methodology as an optimizing tool. Comptes Rendus Chim. 2019, 22, 714–727. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vijayakumar, S.; Gopinath, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. In vivo and in vitro antimicrobial activity of Azadirachta indica (Lin) against Citrobacter freundii isolated from naturally infected Tilapia (Oreochromis mossambicus). Aquaculture 2015, 437, 252–255. [Google Scholar] [CrossRef]
- Schrader, K.K. Plant natural compounds with antibacterial activity towards common pathogens of pond-cultured channel catfish (Ictalurus punctatus). Toxins 2010, 2, 1676–1689. [Google Scholar] [CrossRef]
- Verma, V.K.; Prakash, O.; Kumar, R.S.R.; Rani, K.V.; Sehgal, N. Water hyacinth (Eichhornia crassipes) leaves enhances disease resistance in Channa punctata from Vibrio harveyi infection. J. Basic Appl. Zool. 2021, 82, 6. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, Y.; Wang, Z.; Zhou, A.; Xie, S.; Zhang, Y.; Xiang, Q.; Song, E.; Zou, J. Effects of dietary Gelsemium elegans alkaloids on growth performance, immune responses and disease resistance of Megalobrama amblycephala. Fish Shellfish Immunol. 2019, 91, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bussabong, P.; Rairat, T.; Chuchird, N.; Keetanon, A.; Phansawat, P.; Cherdkeattipol, K.; Pichitkul, P.; Kraitavin, W. Effects of isoquinoline alkaloids from Macleaya cordata on growth performance, survival, immune response, and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei). PLoS ONE 2021, 16, e0251343. [Google Scholar] [CrossRef] [PubMed]
- Schrader, K.K.; Hamann, M.T.; McChesney, J.D.; Rodenburg, D.L.; Ibrahim, M.A. Antibacterial activities of metabolites from Platanus occidentalis (american sycamore) against fish pathogenic bacteria. J. Aquac. Res. Dev. 2015, 6, 364. [Google Scholar] [CrossRef]
- Zheng, W.; Yan, C.-M.; Zhang, Y.-B.; Li, Z.-H.; Li, Z.; Li, X.-Y.; Wang, Z.-W.; Wang, X.; Chen, W.-Q.; Yu, X.-H. Antiparasitic Efficacy of Gracillin and Zingibernsis Newsaponin from Costus Speciosus (Koen Ex. Retz) Sm. against Ichthyophthirius multifiliis. Parasitology 2015, 142, 473–479. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, D.-H.; Klesius, P.H. Evaluation of an Antiparasitic Compound Extracted from Galla Chinensis against Fish Parasite Ichthyophthirius Multifiliis. Vet. Parasitol. 2013, 198, 45–53. [Google Scholar] [CrossRef]
- Wang, G.-X.; Zhou, Z.; Jiang, D.X.; Han, J.; Wang, J.F.; Zhao, L.W.; Li, J. In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet. Parasitol. 2010, 171, 305–313. [Google Scholar] [CrossRef]
- Yao, J.-Y.; Zhou, Z.-M.; Li, X.-L.; Yin, W.-L.; Ru, H.-S.; Pan, X.-Y.; Hao, G.-J.; Xu, Y.; Shen, J.-Y. Antiparasitic Efficacy of Dihydrosanguinarine and Dihydrochelerythrine from Macleaya microcarpa against Ichthyophthirius multifiliis in Richadsin (Squaliobarbus curriculus). Vet. Parasitol. 2011, 183, 8–13. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Liu, Y.-M.; Zhang, Q.-Z.; Fu, Y.-W.; Lin, D.-J. Evaluation of an Antiparasitic Compound Extracted from Polygonum Cuspidatum against Ichthyophthirius Multifiliis in Grass Carp. Vet. Parasitol. 2018, 253, 22–25. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Molla, M.H.R.; Ahammad, F. Compounds identified from marine mangrove plant (Avicennia Alba) as potential antiviral drug candidates against WDSV, an in-silico approach. Mar. Drugs 2021, 19, 253. [Google Scholar] [CrossRef]
- Gopiesh, K.V.; Kannabiran, K.; Sarath Babu, V.; Sahul Hameed, A.S. Inhibition of fish nodavirus by gymnemagenol extracted from Gymnema sylvestre. J. Ocean Univ. China 2011, 10, 402–408. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Babu, M.M.; Punitha, S.M.J.; Viji, V.T.; Citarasu, T. Screening and Characterization of Antiviral Compounds from Psidium Guajava Linn. Root Bark against White Spot Syndrome Virus. Indian J. Nat. Prod. Resour. 2012, 3, 208–214. [Google Scholar]
- Kang, S.Y.; Kang Ji Oh, M.-J. Antiviral activities of flavonoids isolated from the bark of Rhus verniciflua stokes against fish pathogenic viruses In Vitro. J. Microbiol. 2012, 50, 293–300. [Google Scholar] [CrossRef]
- Naeini, A.R.; Nazeri, M.; Shokri, H. Antifungal activity of Zataria multiflora, Pelargonium graveolens and Cuminum cyminum essential oils towards three species of Malassezia isolated from patients with pityriasis versicolor. J. Mycol. Médicale 2011, 21, 87–91. [Google Scholar] [CrossRef]
- Shah, T.K.; Tandel, R.S.; Kumar, A.; Bhat, R.A.H.; Dash, P.; Sarma, D. Chemical composition, antifungal activity and molecular docking of Himalayan thyme leaf extract (Thymus linearis) against fish pathogenic oomycete Saprolegnia parasitica. Aquaculture 2021, 543, 736988. [Google Scholar] [CrossRef]
- Barot, M.; Kumar, N.J.I.; Kumar, R.N. Bioactive compounds and antifungal activity of three different seaweed species Ulva lactuca, Sargassum tenerrimum and Laurencia obtusa collected from Okha coast, Western India. J. Coast. Life Med. 2016, 4, 284–289. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.S. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr. Drug Discov. Technol. 2020, 17, 430–447. [Google Scholar] [CrossRef]
- Murray, T.S.; Kazmierczak, B.I. Pseudomonas aeruginosa exhibits sliding motility in the absence of type IV pili and flagella. J. Bacteriol. 2008, 190, 2700–2708. [Google Scholar] [CrossRef]
- Tkachenko, H.; Buyun, L.; Terech-Majewska, E.; Osadowski, Z. In vitro antimicrobial activity of ethanolic extracts obtained from Ficus spp. leaves against the fish pathogen Aeromonas hydrophila. Fish. Aquat. Life 2016, 24, 219–230. [Google Scholar] [CrossRef]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, T.G.; Jung, M.; Park, K.H.; Chong, Y. In vitro synergism and anti-biofilm activity of quercetin–pivaloxymethyl conjugate against Staphylococcus aureus and Enterococcus Species. Chem. Pharm. Bull. 2018, 66, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-X.; Schrader, K.K.; Mizuno, C.S.; Rimando, A.M. Activity of lycorine analogues against the fish bacterial pathogen Flavobacterium columnare. J. Agric. Food Chem. 2011, 59, 5977–5985. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Wendt, M.; Heo, G.A. Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria. Lab. Anim. Res. 2016, 32, 87–90. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhang, J.; Xie, J.; Yang, L.; Xing, Y.; Li, Z. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2020, 46, 759–770. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Alexpandi, R.; Abirami, G.; Satish, L.; Swasthikka, R.P.; Krishnaveni, N.; Jayakumar, R.; Pandian, S.K.; Ravi, A.V. Tocopherol and phytol possess anti-quorum sensing mediated anti-infective behavior against Vibrio campbellii in aquaculture: An in vitro and in vivo study. Microb. Pathog. 2021, 161, 105221. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Piampiano, E.; Pini, F.; Biondi, N.; Garcia, C.J.; Decorosi, F.; Tomàs-Barberàn, F.A.; Giovannetti, L.; Viti, C. Tetraselmis suecica F&M-M33 phycosphere: Associated bacteria and exo-metabolome characterization. Eur. J. Phycol. 2021, 56, 61–71. [Google Scholar]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Poulin, R.; Morand, S. The diversity of parasites. Q. Rev. Biol. 2000, 75, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R. The many roads to parasitism: A tale of convergence. Adv. Parasitol. 2011, 74, 1–40. [Google Scholar]
- Adams, A.M.; Murrell, K.D.; Cross, J.H. Parasites of fish and risks to public health. Rev. Sci. Tech. 1997, 16, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J. Fish Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Chandra, K.J. Checklist of monogenetic trematodes infesting Bangladesh and Indian freshwater fishes. J. Bangladesh Agric. Univ. 2004, 2, 113–124. [Google Scholar]
- Faruk, M.A.R. Fish parasite: Infectious diseases associated with fish parasite. In Seafood Safety and Quality; CRC Press: Boca Raton, FL, USA, 2018; pp. 154–176. [Google Scholar]
- Louten, J. Poliovirus. In Essential Human Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 257–271. [Google Scholar]
- Lucas, W.; Knipe, D.M. Viral Capsids and Envelopes: Structure and Function; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Sudheer, N.S.; Philip, R.; Singh, I.S.B. In vivo screening of mangrove plants for anti WSSV activity in Penaeus monodon, and evaluation of Ceriops tagal as a potential source of antiviral molecules. Aquaculture 2011, 311, 36–41. [Google Scholar] [CrossRef]
- Kurnianda, V.; Khairunnisa, W.A.; Mauliza, A.; Ulfah, M.; Nurfadillah, I.R.; Kobayashi, M. Polyhydroxy Isocopalane from Indonesian’s Marine Sponge Callyspongia sp. as Anti–White Spot Syndrome Virus from Litopenaeus vannamei. J. Pharmacogn. Nat. Prod. 2017, 3, 2–4. [Google Scholar] [CrossRef]
- Qian, X.; Zhu, F. Hesperetin protects crayfish Procambarus clarkii against white spot syndrome virus infection. Fish Shellfish Immunol. 2019, 93, 116–123. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Q.; Xiao, H.; Li, M.; Huang, Y.; Zhang, Q.; Li, P. The Inhibitory Activities and Antiviral Mechanism of Medicinal Plant Ingredient Quercetin against Grouper Iridovirus Infection. Front. Microbiol. 2020, 11, 2457. [Google Scholar] [CrossRef]
- Khoa, L.V.; Hatai, K.; Aoki, T. Fusarium incarnatum isolated from black tiger shrimp, Penaeus monodon Fabricius, with black gill disease cultured in Vietnam. J. Fish Dis. 2004, 27, 507–515. [Google Scholar] [CrossRef]
- Otta, S.K.; Praveena, P.E.; Raj, R.A.; Saravanan, P.; Priya, M.S.; Amarnath, C.B.; Bhuvaneswari, T.; Panigrahi, A.; Ravichandran, P. Pythium Insidiosum as a New Opportunistic Fungal Pathogen for Pacific White Shrimp, Litopenaeus vannamei; Researchgate: Berlin, Germany, 2018. [Google Scholar]
- Gozlan, R.E.; Marshall, W.; Lilje, O.; Jessop, C.; Gleason, F.H.; Andreou, D. Current ecological understanding of fungal-like pathogens of fish: What lies beneath? Front. Microbiol. 2014, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Canter-Lund, H.; Lund, J. Freshwater algae: Their Microscopic World Explored; Bio Press: Devon, UK, 1995. [Google Scholar]
- James, T.Y.; Letcher, P.M.; Longcore, J.E.; Mozley-Standridge, S.E.; Porter, D.; Powell, M.J.; Griffith, G.W.; Vilgalys, R. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 2006, 98, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Baleta, F.N.; Bolaños, J.M.; Ruma, O.C.; Baleta, A.N.; Cairel, J.D. Phytochemicals screening and antimicrobial properties of Sargassum oligocystum and Sargassum crassifolium Extracts. J. Med. Plants 2017, 5, 382–387. [Google Scholar]
- Kausalya, M.; Rao, G.M.N. Antimicrobial activity of marine algae. J. Algal Biomass Utln. 2015, 6, 78–87. [Google Scholar]
- Huang, X.L.; Liu, R.J.; Whyte, S.; Du, Z.J.; Chen, D.F.; Deng, Y.Q.; Wang, K.Y.; Geng, Y. The in vitro antifungal activity of 30 Chinese herb extracts to Saprolegnia sp. J. Appl. Ichthyol. 2015, 31, 681–686. [Google Scholar] [CrossRef]
- Effiong, B.N.; Sanni, A. Antifungal properties and phytochemical screening of crude extract of Lemna pauciscostata (Helgelm) against fish feed spoilage fungi. Life Sci. J. 2009, 6, 19–22. [Google Scholar]
- Lee, P.-T.; Yamamoto, F.Y.; Low, C.F.; Loh, J.Y.; Chong, C.M. Gut immune system and the implications of oral-administered immunoprophylaxis in finfish aquaculture. Front. Immunol. 2021, 12, 773193. [Google Scholar] [CrossRef]
- Chong, C.M.; Murthy, A.G.; Choy, C.Y.; Lai, K.S. Phytotherapy in aquaculture: Integration of endogenous application with science. J. Environ. Biol. 2020, 41, 1204–1214. [Google Scholar] [CrossRef]
- Zhuo, L.-C.; Yong, A.S.K.; Shapawi, R.; Lin, Y.H. Effects of fermented lemon peel supplementation in diet on growth, immune responses, and intestinal morphology of Asian sea bass, Lates calcarifer. Aquac. Rep. 2021, 21, 100801. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Mohammady, E.Y.; Elashry, M.A.; El-Haroun, E.R.; Davies, S.J. Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 2018, 495, 592–601. [Google Scholar] [CrossRef]
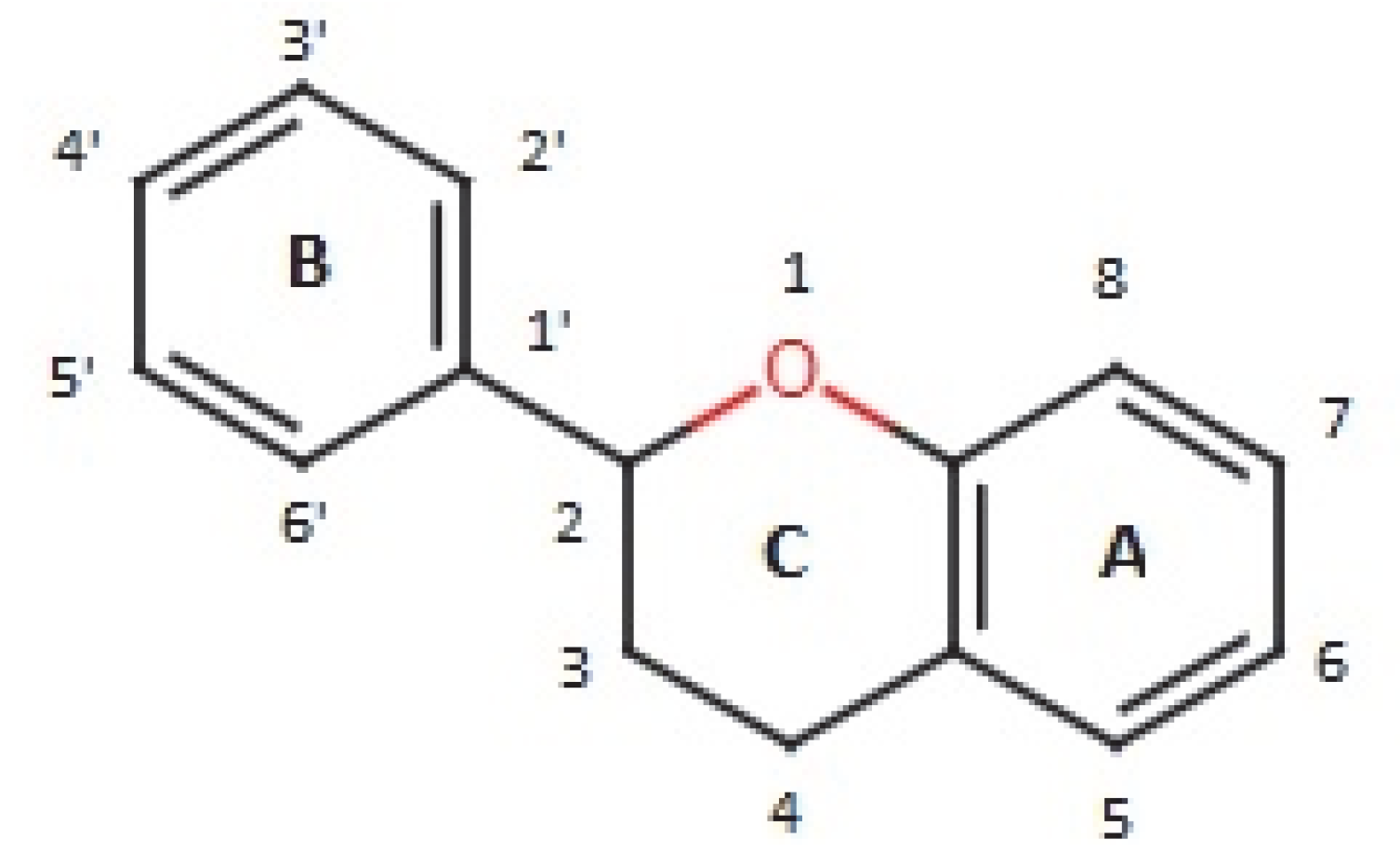
| Class | General Structure | Phytocompound and Its Antimicrobial Properties | Plant Sources |
|---|---|---|---|
| Flavanol | 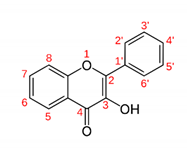 | Catechin: able to inhibit the growth of methicillin-resistant S. aureus ATCC 33591 (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSA, ATCC 25923). | Anacardium occidentale [40] |
| Epicatechin gallate: epicatechin gallate enhanced the antibacterial effect of β-lactam antibiotics against MRSA in vitro and in vivo. | Fructus crataegi [41] | ||
| Epicatechin: high efficacy of phytoisolate compound against the parasitic activity of Paramphistomum cervi. | Ricinus communis [42] | ||
| Flavonol |  | Kaempferol 3-O-α-L-(2″, 3″-di-Z-p-coumaroyl)rhamnoside: showed high efficacy against MRSA (IC50 0.4 mg/L) and Streptococcus iniae LA94-426. | Platanus occidentalis [43] |
| Myricetin 3′-glucoside and myricetin 3-alpha-L-arabinofuranoside: showed strong antiglycemic activity by inhibiting carbohydrate-hydrolyzing enzymes. | Syzygium malaccense [44] | ||
| Quercetin 3-O-glucuronide: significant inhibitory effect of bacterial growth against S. aureus, E. faecalis, E. coli, P. aeruginosa and Salmonella typhi with inhibition zone diameters greater than 13 mm. | Tamarix gallica [45] | ||
| Flavone | 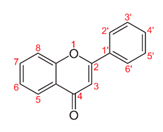 | 5-Hydroxy-3′,4′-dimethoxyflavone-7-O-(rhamnoside) and 5-hydroxy-3′-methoxyflavone-4′-O-(penthenyl-4-one)-7-O-(2″-(rhamnosyl) rhamnoside): able to inhibit the growth of B. subtilis (21.4 mm) and E. faecalis (8.2 mm) compared to tetracycline (22.2 mm and 9.6 mm, respectively). | Achillea tenuifolia [46] |
| Apigenin: apigenin (10 µL) had antibacterial effects that were more significant on S. typhimurium and P. mirabilis when compared with streptomycin as a control (10 µL). | Portulaca oleracea L. [47] | ||
| Isoflavone | 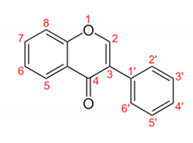 | Genistein: Increased the acetylcholinesterase (AChE) activity and, in contrast, reduced both glutathione and catalase activity. The results may suggest beneficial impacts on cognitive defects related to Alzheimer’s disease. | Glycine max [48] |
| Genistein: methanolic extracts containing genistein displayed antibiotic response against all bacterial strains and maximum zone of inhibition at a low concentration level at 350 µg/mL. | Rhizophora apiculate [49] | ||
| Flavanone |  | Hesperidin: able to inhibit the growth Streptococcus aureus, Escherichia coli, Enterococcus faecalis and Pseudomonas auraginosa at a 15% concenteration with inhibitory diameter range of 7.65 mm ± 0.36 mm to 9.96 mm ± 0.52 mm, and at a concentration of 20% with a diameter range of 9.26 mm ± 0.72 mm to 13.39 mm ± 028 mm. | Citrus microparpa [50] |
| Hesperetin-A: showed a noteworthy cytotoxicity effect (IC50: 2.86 μg/mL) on HeLa cell line, and an in silico molecular docking study portrayed hesperetin as having a good interaction with the E6 protein of HPV16 cervical carcinoma, which is beneficial for cancer treatment. | Cordia sebestena [51] | ||
| Anthocyanidin |  | Pelargonidin: possessed potent scavenging activity for superoxide radicals to attract more neutrophils in plasma. | Punica granatum [52] |
| Proanthocyanidins: exhibits anti-Escherichia coli adhesion activity with P-type fimbriae on the wall of the urinary tract. | Vaccinium sect. Cyanococcus [53] |
| Alkaloids | General Structure | Phytocompound and Bioactive Properties | Plant Origin |
|---|---|---|---|
| Deoxytubulosine | 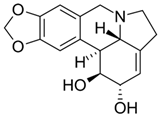 | β-carboline-benzoquinolizidine alkaloid deoxytubulosine: exhibits cytotoxicity and anticancer activity against Dalton’s ascitic lymphoma cells. | Alangium salvifolium [57] |
| Carbazole | 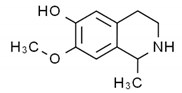 | Methyl carbazole-3-carboxylate: showed the best in vitro cytotoxic activities against Hela, K562, A549, H1299 and SMMC-7721 tumor cell lines. | Clausena lansium [58] |
| Pyridazine | 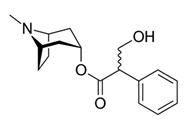 | 2,7-Diphenyl-1,6-dioxopyridazino[4,5:2,3]pyrrolo[4,5-d]pyridazine: showed high potency of antibacterial effects through inhibition zone against Escherichia coli, Pseudomonas eurogenosa, Staphylococcus aureus, Proteus mirabilis and Klebsiella pneumonia. | Datura stramonium [59] |
| Quinolizidine | 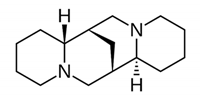 | Lupanine, 13α-hydroxylupanine and albine: alkaloid extracts showed high antimicrobial activity against K. pneumonia and moderate activity against P. aeruginosa clinical isolates. | Lupinus albus [60] |
| Trigonelline | 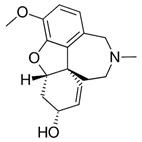 | Trigonelline: at the dose of 1 g/L, it showed (1) an antihistamine effect on guinea pig ileum; (2) an anticholinergic effect on rat colon; (3) a stimulant effect on rat uterus. | Trigonella foenum-graecum [61] |
| Phenolic Acid | General Structure | Phytocompound and Bioactive Properties | Reference |
|---|---|---|---|
| Gallic acid | 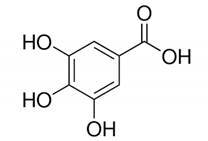 | Gallic acid: showed a high zone of inhibition of 13.67 ± 0.58 mm towards S. Aureus through the disc diffusion method. | Eucalyptus globulus [65] |
| p-Coumaric acid | 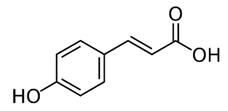 | 4-Hydroxycinnamic acid: exerted anti-inflammatory effects, in a mechanism that included suppression of inflammatory cell infiltration as well as the levels of tumor necrosis factor-α and interleukin 6. | Oldenlandia diffusa [66] |
| Rosmarinic acid |  | Rosmarinic acid methyl ester found in Origanum vulgare that possessed a strong antioxidant effect is much safer and less toxic than either arbutin or l-ascorbic acid in human fibroblast cells. | Origanum vulgare [67] |
| Ferulic acid | 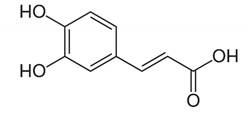 | Trans-4-hydroxy-3-methoxycinnamicacid: inhibited UVB-induced matrix metalloproteinases that contribute to the development of skin cancer via post-translational mechanisms. | Triticum aestivum [68] |
| Terpenoids | Chemical Structure | Phytocompound | Plant Species |
|---|---|---|---|
| Sesquiterpenoids |  | Artemisinin: acts as an inhibitor of the production of Flaviviridae viruses, and its effect is additive to interferon-α and ribavirin. | Artemisia annua [75] |
| Monoterpenoids |  | Linalool: responsible for the antipsoriatic activity of lavender oil as the compound showed more than 50% recovery in psoriasis area severity index scores and recovery level of Th-17 cell cytokines. | Lavandula angustifolia [76] |
| Triterpenoids | 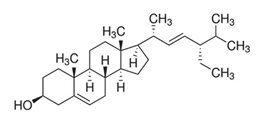 | Stigmasterol: the compound exhibited 29 mm as the zone of inhibition against Staphylococcus aureus. | Neocarya macrophylla [77] |
| Diterpenoids | 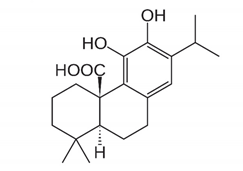 | Carnosic acid and carnosol: exhibited a significant increase in antibacterial activity against Listeria monocytogenes and Staphylococcus aureus strains. | Rosmarinus officinalis [78] |
| Saponins | Chemical Structure | Compound and Bioactive Properties | Plant Origin |
|---|---|---|---|
| Quinoa saponins | 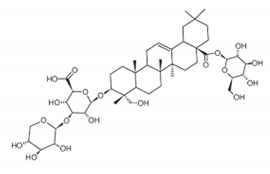 | Compound exerted obvious bacteriostatic and bactericidal effects on Gram-positive bacteria such as Staphylococcus aureus, Staphylococcus epidermidis and Bacillus cereus. | Chenopodium quinoa [86] |
| Soyasaponin |  | Soyasaponin Ab: colon shortening, myeloperoxidase activity, the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) and activation of the transcription factor nuclear factor-kB (NF-kB). | Glycine max [87] |
| Ginsenosides | 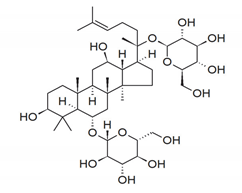 | 12-One-pseudoginsenoside F11: prevented H2O2-stimulated cell damage in A549 cells, which may be strongly related to the antioxidative effects of 12-one-PF11. | Panax quinquefolium [88] |
| Water | Ethanol | Methanol | Chloroform | Dichloromethanol | Ether | n-Hexane |
|---|---|---|---|---|---|---|
| Tannins Anthocyanins Terpenoids Saponins | Tannins Terpenoids Polyphenols Flavonoids Alkaloids | Phenolic acids Alkaloids Carotenoids Flavonoids | Flavonoids Terpenoids | Terpenoids | Alkaloids Terpenoids | Carotenoids |
| Bioactivity | Plant Species | Phytocompound | Application | References |
|---|---|---|---|---|
| Antibacterial | Chelidonium majus |
| Displayed strong toxicity against Edwarsiella ictaluri with a 24 h LC of 7.3 ± 0.8 mg/L and MIC of 2.1 ± 1.7 mg/L. | [125] |
| Eichhornia crassipes |
| Increased bacterial resistance in Channa punctate against Vibrio harveyi infection. | [126] | |
| Gelsemium elegans |
| Significantly increased survival rates in Megalobroma amblycephala after the challenge with Aeromonas hydrophila. | [127] | |
| Macleaya cordata |
| Two concentrations (1 and 1.5 mg/kg of feed) improved the survival rate and resistance to Vibrio parahaemolyticus infection of Litopenaeus vannamei. | [128] | |
| Platanus occidentalis |
| High antibacterial efficacy against MRSA (IC50 0.4 mg/L) and Streptococcus iniae LA94-426. | [129] | |
| Antiparasitic | Costus speciosus |
| Can achieve 100% killing with in vitro treatments of gracillin and zingibernsis newsaponin. The EC50 values were 0.53 and 3.2 mg L−1, respectively. | [130] |
| Antiviral | Galla chinensis |
| Elimination of all Ichthyophthirius multifiliis theronts at the concentrations of 2.5–20 mg/L, and complete interference of reproduction of tomonts at 40 mg/L. A 93.3% rate of survival was achieved in the Ich-infected catfish that were treated with pentagalloylglucose at 20 mg/L, whereas all infected fish were dead in the negative control group. | [131] |
| Macleaya microparpa |
| Potent anthelmintic activity against Dactylogyrus intermedius in Carassius auratus with EC50 values of 0.37, 4.64 and 3.63 mg L−1, respectively. | [132] | |
| Macleaya microparpa |
| The EC50 values of dihydrosanguinarine and dihydrochelerythrine against I. multifiliis were 5.18 and 9.43 mg/L, respectively. | [133] | |
| Polygonum cuspidatum |
| At 96 min, in vitro treatment of emodin at 1 mg/L was able to kill all I. multifiliis. Recovery of the Ich-infected Ctenopharyngodon idella can be achieved by continuously adding emodin for 10 days. | [134] | |
| Avicennia alba |
| Compounds friedlein, phytosterols and 1-triacontanol were determined to be potential drug candidates against WDSV using molecular docking simulation, with docking scores of −8.5 kcal/mol, −8.0 kcal/mol and −7.9 kcal/mol, respectively. | [135] | |
| Gymnema sylvestre |
| At 20 µg/mL of gymnemagenol, it inhibited 50% of cell viability of grouper nervous necrosis virus (GNNV) that showed effectiveness in inhibiting the proliferation of GNNV in infected SIGE cells. | [136] | |
| Polysiphonia morrowii |
| 3-Bromo-4, 5-dihydroxybenzy methyl ether exhibited significant antiviral activities showing selective index values (SI = CC50/EC50) of 20 to 40 against infectious hematopoietic necrosis virus (IHNV) and infectious pancreatic necrosis virus (IPNV). | [137] | |
| Psidium guajava |
| The F1-treated Fennerropenaeus indicus survived significantly against white shrimp syndrome virus (p < 0.05) at 80%, and while survival rates of 20, 30, 40, 35, 35 and 25 were found in the F2 to F7 fraction-treated groups, respectively. Meanwhile, the control group faced a 100% mortality rate. | [138] | |
| Rhus verniciflua |
| Fisetin showed the highest significant anti-infectious activities against hemorrhagic necrosis virus and antiviral hemorrhagic septicemia virus, showing EC50 values of 27.1 and 33.3 µM. Fustin and sulfuretin displayed significant antiviral activities, showing EC50 values of 91.2–197.3 μM against infectious hemorrhagic necrosis virus and viral hemorrhagic septicemia virus. | [139] | |
| Antifungal | Eucalyptus camaldulensis |
| E. camaldolensis at a concentration of 25 ppm and G. herbarium at a concentration of 100 ppm for 60 min daily with three repetitions were the best treatments in Onchorynchus mykiss, represented by the prevention of fungal attack, and the increase in the hatching rate, the eyed egg rate and the final larvae rate. | [140] |
| Geranium herbarium |
| |||
| Thymus linearis |
| More specificity of hexadec-2-en-1-ol towards the V-type ATPase site, and of carvacrol towards TKL protein kinase, of Saprolegnia parasitica responsible for the virulence of pathogens. | [141] | |
| Ulva lactuta |
| Displayed a significant zone of inhibition of antifungal activity against Aspergillus niger (36 mm). | [142] | |
| Zataria multiflora |
| A concentration of 25 ppm for 60 min daily with three repetitions was the best treatment in Onchorynchus mykiss, represented by the prevention of fungal attack, and the increase in the hatching rate, the eyed egg rate and the final larvae rate. | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nik Mohamad Nek Rahimi, N.; Natrah, I.; Loh, J.-Y.; Ervin Ranzil, F.K.; Gina, M.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Phytocompounds as an Alternative Antimicrobial Approach in Aquaculture. Antibiotics 2022, 11, 469. https://doi.org/10.3390/antibiotics11040469
Nik Mohamad Nek Rahimi N, Natrah I, Loh J-Y, Ervin Ranzil FK, Gina M, Lim S-HE, Lai K-S, Chong C-M. Phytocompounds as an Alternative Antimicrobial Approach in Aquaculture. Antibiotics. 2022; 11(4):469. https://doi.org/10.3390/antibiotics11040469
Chicago/Turabian StyleNik Mohamad Nek Rahimi, Naqiuddin, Ikhsan Natrah, Jiun-Yan Loh, Francis Kumar Ervin Ranzil, Madi Gina, Swee-Hua Erin Lim, Kok-Song Lai, and Chou-Min Chong. 2022. "Phytocompounds as an Alternative Antimicrobial Approach in Aquaculture" Antibiotics 11, no. 4: 469. https://doi.org/10.3390/antibiotics11040469
APA StyleNik Mohamad Nek Rahimi, N., Natrah, I., Loh, J.-Y., Ervin Ranzil, F. K., Gina, M., Lim, S.-H. E., Lai, K.-S., & Chong, C.-M. (2022). Phytocompounds as an Alternative Antimicrobial Approach in Aquaculture. Antibiotics, 11(4), 469. https://doi.org/10.3390/antibiotics11040469






