Tigecycline Dosing Strategies in Critically Ill Liver-Impaired Patients
Abstract
:1. Introduction
2. Results
2.1. Study Participants
2.2. Pharmacometric Data Analysis
2.2.1. Base Model
2.2.2. Covariate Analysis
2.3. Monte Carlo Simulations
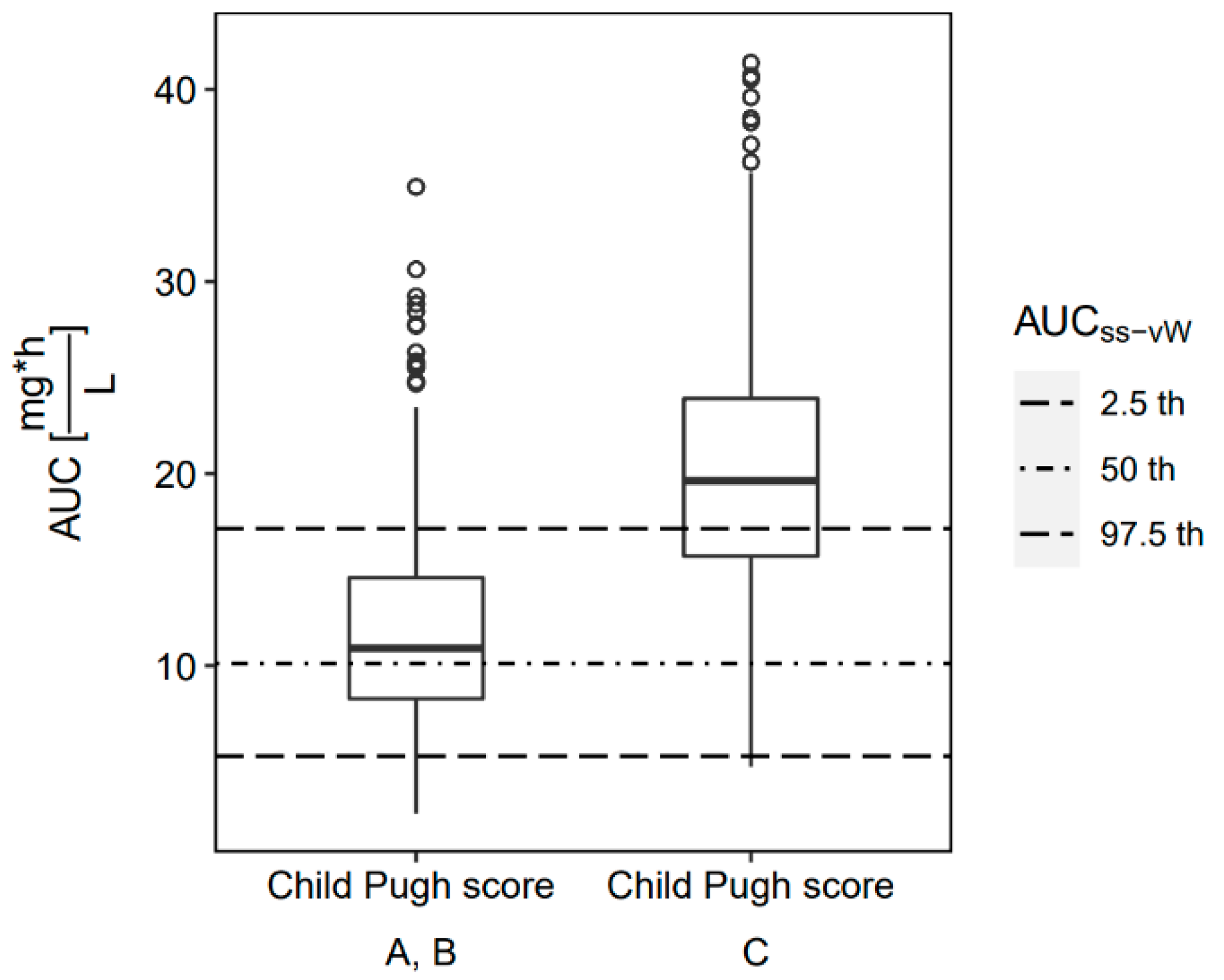
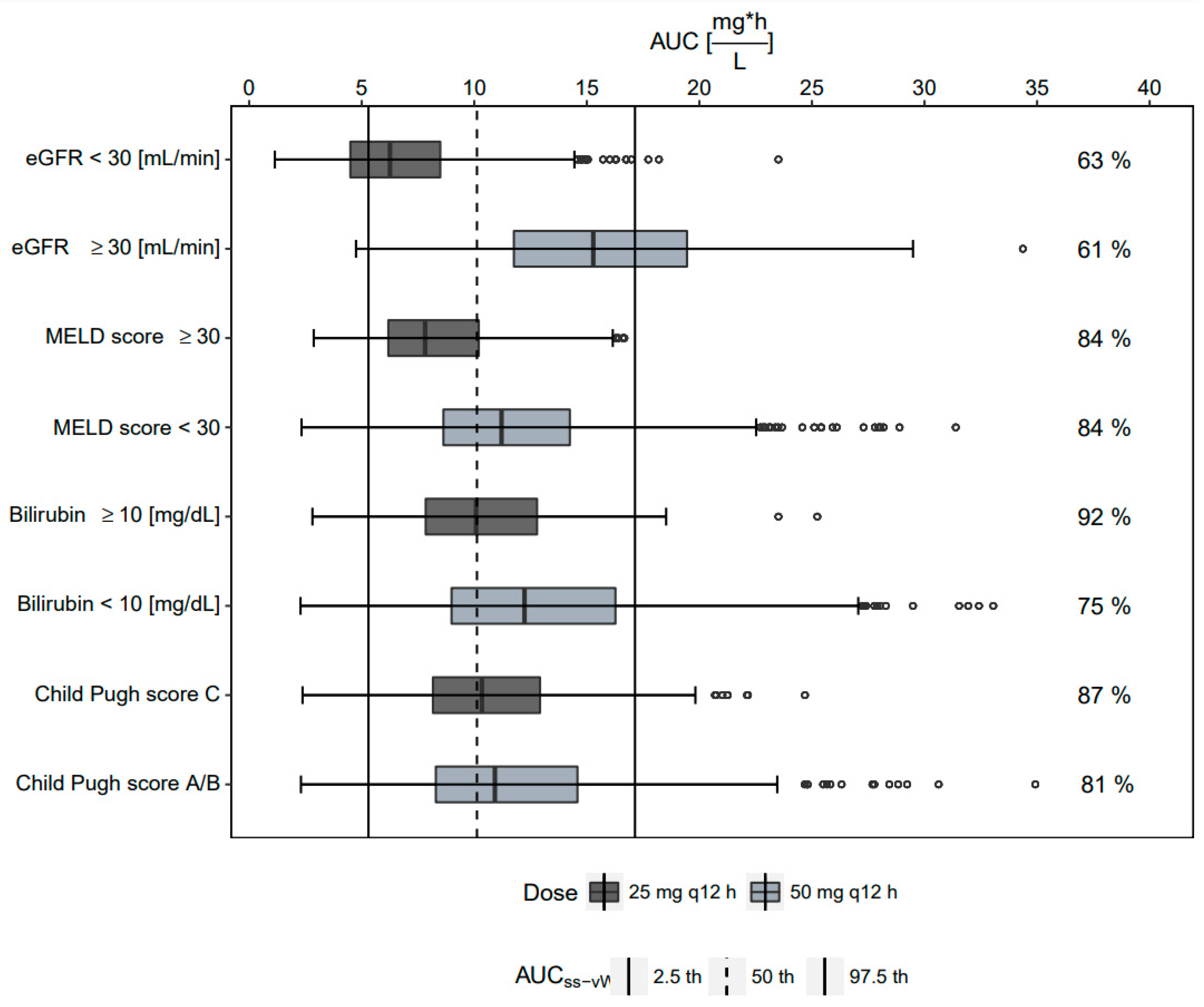
2.4. Probability of Target Attainment
2.5. Study Outcome
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Pharmacometrics Analysis
4.2.1. Base Model
4.2.2. Covariate Analysis
4.2.3. Final Model Evaluation
4.2.4. Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boucher, H.W.; Wennersten, C.B.; Eliopoulos, G.M. In Vitro Activities of the Glycylcycline GAR-936 against Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2000, 44, 2225–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfizer Tygacil® Full Prescribing Information. Available online: https://www.pfizermedicalinformation.com/en-us/tygacil/dosage-admin (accessed on 21 March 2022).
- Meagher, A.K.; Passarell, J.A.; Cirincione, B.B.; Van Wart, S.A.; Liolios, K.; Babinchak, T.; Ellis-Grosse, E.J.; Ambrose, P.G. Exposure-Response Analyses of Tigecycline Efficacy in Patients with Complicated Skin and Skin-Structure Infections. Antimicrob. Agents Chemother. 2007, 51, 1939–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passarell, J.A.; Meagher, A.K.; Liolios, K.; Cirincione, B.B.; Van Wart, S.A.; Babinchak, T.; Ellis-Grosse, E.J.; Ambrose, P.G. Exposure-Response Analyses of Tigecycline Efficacy in Patients with Complicated Intra-Abdominal Infections. Antimicrob. Agents Chemother. 2008, 52, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGovern, P.C.; Wible, M.; El-Tahtawy, A.; Biswas, P.; Meyer, R.D. All-cause mortality imbalance in the tigecycline phase 3 and 4 clinical trials. Int. J. Antimicrob. Agents 2013, 41, 463–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascale, G.; Montini, L.; Pennisi, M.; Bernini, V.; Maviglia, R.; Bello, G.; Spanu, T.; Tumbarello, M.; Antonelli, M. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit. Care 2014, 18, R90. [Google Scholar] [CrossRef] [Green Version]
- De Pascale, G.; Lisi, L.; Ciotti, G.M.P.; Vallecoccia, M.S.; Cutuli, S.L.; Cascarano, L.; Gelormini, C.; Bello, G.; Montini, L.; Carelli, S.; et al. Pharmacokinetics of high-dose tigecycline in critically ill patients with severe infections. Ann. Intensive Care 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Pan, L.; Guo, J.; French, N.; Villanueva, E.V.; Tefsen, B. Effectiveness and Safety of High Dose Tigecycline for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 1049–1064. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, R. Clinical study on the safety and efficacy of high-dose tigecycline in the elderly patients with multidrug-resistant bacterial infections. Medicine 2020, 99, e19466. [Google Scholar] [CrossRef]
- Ni, W.; Li, G.; Zhao, J.; Cui, J.; Wang, R.; Gao, Z.; Liu, Y. Use of Monte Carlo simulation to evaluate the efficacy of tigecycline and minocycline for the treatment of pneumonia due to carbapenemase-producing Klebsiella pneumoniae. Infect. Dis. 2018, 50, 507–513. [Google Scholar] [CrossRef]
- Li, M.X.; Li, N.; Zhu, L.Q.; Liu, W. Optimization of tigecycline dosage regimen for different infections in the patients with hepatic or renal impairment. J. Chemother. 2020, 32, 420–428. [Google Scholar] [CrossRef]
- Fernández, J.; Gustot, T. Management of bacterial infections in cirrhosis. J. Hepatol. 2012, 56, S1–S12. [Google Scholar] [CrossRef]
- Broeker, A.; Wicha, S.G.; Dorn, C.; Kratzer, A.; Schleibinger, M.; Kees, F.; Heininger, A.; Kees, M.G.; Häberle, H. Tigecycline in critically ill patients on continuous renal replacement therapy: A population pharmacokinetic study. Crit. Care 2018, 22, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubino, C.M.; Bhavnani, S.M.; Forrest, A.; Dukart, G.; Dartois, N.; Cooper, A.; Korth-Bradley, J.; Ambrose, P.G. Pharmacokinetics-Pharmacodynamics of Tigecycline in Patients with Community-Acquired Pneumonia. Antimicrob. Agents Chemother. 2012, 56, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsuk-De Moor, A.; Rypulak, E.; Potręć, B.; Piwowarczyk, P.; Borys, M.; Sysiak, J.; Onichimowski, D.; Raszewski, G.; Czuczwar, M.; Wiczling, P. Population Pharmacokinetics of High-Dose Tigecycline in Patients with Sepsis or Septic Shock. Antimicrob. Agents Chemother. 2018, 62, e02273-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, J.; Ramirez, P.; Villarreal, E.; Gordon, M.; Sánchez, M.Á.; Martín, M.; Castellanos-Ortega, Á. Effect of pharmacokinetic/pharmacodynamic ratio on tigecycline clinical response and toxicity in critically ill patients with multidrug-resistant Gram-negative infections. SAGE Open Med. 2020, 8, 2050312120958897. [Google Scholar] [CrossRef]
- Van Wart, S.A.; Cirincione, B.B.; Ludwig, E.A.; Meagher, A.K.; Korth-Bradley, J.M.; Owen, J.S. Population Pharmacokinetics of Tigecycline in Healthy Volunteers. J. Clin. Pharmacol. 2007, 47, 727–737. [Google Scholar] [CrossRef]
- Van Wart, S.A.; Owen, J.S.; Ludwig, E.A.; Meagher, A.K.; Korth-Bradley, J.M.; Cirincione, B.B. Population Pharmacokinetics of Tigecycline in Patients with Complicated Intra-Abdominal or Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2006, 50, 3701–3707. [Google Scholar] [CrossRef] [Green Version]
- Lam, Y.W.F.; Banerji, S.; Hatfield, C.; Talbert, R.L. Principles of Drug Administration in Renal Insufficiency. Clin. Pharmacokinet. 1997, 32, 30–57. [Google Scholar] [CrossRef]
- Korth-Bradley, J.M.; Baird-Bellaire, S.J.; Patat, A.A.; Troy, S.M.; Böhmer, G.M.; Gleiter, C.H.; Buecheler, R.; Morgan, M.Y. Pharmacokinetics and Safety of a Single Intravenous Dose of the Antibiotic Tigecycline in Patients With Cirrhosis. J. Clin. Pharmacol. 2011, 51, 93–101. [Google Scholar] [CrossRef]
- Meagher, A.K.; Ambrose, P.G.; Grasela, T.H.; Ellis-Grosse, E.J. The Pharmacokinetic and Pharmacodynamic Profile of Tigecycline. Clin. Infect. Dis. 2005, 41, S333–S340. [Google Scholar] [CrossRef] [Green Version]
- Bastida, C.; Hernández-Tejero, M.; Aziz, F.; Espinosa, C.; Sanz, M.; Brunet, M.; López, E.; Fernández, J.; Soy, D. Tigecycline population pharmacokinetics in patients with decompensated cirrhosis and severe infections. J. Antimicrob. Chemother. 2020, 75, 3619–3624. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Valla, D. Assessment of the prognosis of cirrhosis: Child–Pugh versus MELD. J. Hepatol. 2005, 42, S100–S107. [Google Scholar] [CrossRef] [PubMed]
- Korth-Bradley, J.M.; Troy, S.M.; Matschke, K.; Muralidharan, G.; Fruncillo, R.J.; Speth, J.L.; Raible, D.G. Tigecycline Pharmacokinetics in Subjects With Various Degrees of Renal Function. J. Clin. Pharmacol. 2012, 52, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Spray, J.W.; Willett, K.; Chase, D.; Sindelar, R.; Connelly, S. Dosage adjustment for hepatic dysfunction based on Child-Pugh scores. Am. J. Health Syst. Pharm. 2007, 64, 690, 692–693. [Google Scholar] [CrossRef]
- Yang, T.; Mei, H.; Wang, J.; Cai, Y. Therapeutic Drug Monitoring of Tigecycline in 67 Infected Patients and a Population Pharmacokinetics/Microbiological Evaluation of A. baumannii Study. Front. Microbiol. 2021, 12, 678165. [Google Scholar] [CrossRef]
- Ramirez, J.; Dartois, N.; Gandjini, H.; Yan, J.L.; Korth-Bradley, J.; McGovern, P.C. Randomized Phase 2 Trial To Evaluate the Clinical Efficacy of Two High-Dosage Tigecycline Regimens versus Imipenem-Cilastatin for Treatment of Hospital-Acquired Pneumonia. Antimicrob. Agents Chemother. 2013, 57, 1756–1762. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wang, T.; Sun, J.; Chen, S.; Cai, J.; Zhang, W.; Dong, H.; Hu, S.; Zhang, D.; Wang, X.; et al. Optimal tigecycline dosage regimen is urgently needed: Results from a pharmacokinetic/pharmacodynamic analysis of tigecycline by Monte Carlo simulation. Int. J. Infect. Dis. 2014, 18, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Kispal, B.; Walker, S.A.N. Monte Carlo simulation evaluation of tigecycline dosing for bacteria with raised minimum inhibitory concentrations in non-critically ill adults. Eur. J. Clin. Pharmacol. 2021, 77, 197–205. [Google Scholar] [CrossRef]
- Montravers, P.; Dupont, H.; Bedos, J.-P.; Bret, P. Tigecycline use in critically ill patients: A multicentre prospective observational study in the intensive care setting. Intensive Care Med. 2014, 40, 988–997. [Google Scholar] [CrossRef]
- Ribbing, J.; Niclas Jonsson, E. Power, Selection Bias and Predictive Performance of the Population Pharmacokinetic Covariate Model. J. Pharmacokinet. Pharmacodyn. 2004, 31, 109–134. [Google Scholar] [CrossRef]
- Anonymous. LiverTox: Clinical and Research Information on Drug Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases; Tigecycline; Bethesda, MD, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547888/ (accessed on 21 March 2022).
- Alraish, R.; Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Wuensch, T.; Kaffarnik, M. Pharmacokinetics of tigecycline in critically ill patients with liver failure defined by maximal liver function capacity test (LiMAx). Ann. Intensive Care 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Alraish, R.; Wuensch, T.; Kaffarnik, M. Linezolid in liver failure: Exploring the value of the maximal liver function capacity (LiMAx) test in a pharmacokinetic pilot study. Int. J. Antimicrob. Agents 2017, 50, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Bednarsch, J.; Valle, E.; Lock, J.F.; Malinowski, M.; Schulz, A.; Seehofer, D.; Jung, T.; Stockmann, M. Reliable assessment of liver function using LiMAx. J. Surg. Res. 2015, 193, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Lindbom, L.; Pihlgren, P.; Jonsson, N. PsN-Toolkit—A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Broeker, A.; Wicha, S.G. Assessing parameter uncertainty in small-n pharmacometric analyses: Value of the log-likelihood profiling-based sampling importance resampling (LLP-SIR) technique. J. Pharmacokinet. Pharmacodyn. 2020, 47, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Characteristics | Total (n = 39) |
|---|---|
| Male (n) | 13 (32.5%) |
| Female (n) | 27 (67.5%) |
| Age (years) | 62 [34, 85] |
| Weight (kg) | 80.0 [44.5, 119] |
| Clinical laboratory parameters | |
| ALT (U/L) | 33.5 [7.00, 928] |
| AST (U/L) | 55.0 [13.0, 1300] |
| Total bilirubin (mg/dL) | 2.64 [0.190, 18.6] |
| De-Ritis ratio | 1.54 [0.167, 4.00] |
| ɣ-Glutamyltransferase (U/L) | 120 [23.0, 1670] |
| INR | 1.44 [0.970, 2.69] |
| LiMAx test [µg/h/kg] | 170 [18.0, 596] |
| MELD score | 18 [9.00, 37.0] |
| Serum creatinine [mg/dL] | 1.09 [0.330, 3.31] |
| eGFR (CKD-EPI) | 68.8 [17.2–149.8] |
| Thrombocytes (g/L) | 148 [15.0, 777] |
| Child–Pugh score A (n) | 21 |
| Child–Pugh score B (n) | 15 |
| Child–Pugh score C (n) | 3 |
| Underlying diseases | |
| Acute liver impairment | 22 |
| Chronic liver disease | 17 |
| Klatskin tumor (type I, IIa, IIb, IV) | 7 |
| Liver abscess | 3 |
| Cholangiocarcinoma | 2 |
| Complicated cholecystitis | 1 |
| Liver cirrhosis | 2 |
| Hypoperfusion of the liver | 1 |
| Cholangiogenic sepsis | 1 |
| Ascites: none (n) | 7 |
| Ascites: Grade 1(n) | 16 |
| Ascites: Grade 2 (n) | 16 |
| Microbiological isolates | |
| Enterococcus avium (n) | 1 |
| Enterococcus faecalis (n) | 4 |
| Enterococcus faecium (n) | 10 |
| Escherichia coli (n) | 4 |
| Klebsiella pneumoniae (n) | 1 |
| MRSA (n) | 2 |
| Staphylococcus epidermidis (n) | 6 |
| VRE (n) | 12 |
| OFV | Implementation of Covariate Relationship | Model | dOFV | IIV | p-Value | |
|---|---|---|---|---|---|---|
| Base model | −914.3 | Two-compartment model with proportional error model | CL: 48.2% Vc: 85% | |||
| Forward inclusion | −928.6 | linear | bilirubintot/CL | −14.3 | CL: 40.9% | <0.001 |
| −947.9 | power | bilirubintot/CL | −33.6 | CL: 36.5% | <0.001 | |
| −931.7 | exponential | bilirubintot/CL | −17.4 | CL: 39.2% | <0.001 | |
| −950.4 | linear | eGFR/CL | −36.1 | CL: 47.3% | <0.001 | |
| −923.4 | power | eGFR/CL | −9.0 | CL: 43% | 0.003 | |
| −942.1 | exponential | eGFR/CL | −28.5 | CL: 48.4% | <0.001 | |
| −930.8 | linear | LiMAx test/CL | −16.5 | CL: 59.1% | <0.001 | |
| −926.5 | power | LiMAx test/CL | −12.3 | CL: 41.7% | <0.001 | |
| −918.1 | exponential | LiMAx test/CL | −3.81 | CL: 54.6% | 0.051 | |
| −926.0 | categorical | Child–Pugh/CL | −11.8 | CL: 41.6% | <0.001 | |
| −918.2 | linear | MELD/CL | −3.94 | CL: 39% | 0.047 | |
| −919.7 | power | MELD/CL | −5.45 | CL: 37.9% | 0.019 | |
| −918.1 | exponential | MELD/CL | −3.83 | CL: 38.7% | 0.050 | |
| −924.1 | linear | WT/Vc | −9.88 | Vc: 68.6% | 0.002 | |
| −920.9 | power | WT/Vc | −6.71 | Vc: 73.6% | 0.009 | |
| −917.9 | exponential | WT/Vc | −3.60 | Vc: 77.7% | 0.058 | |
| −921.4 | linear | age/Vc | −7.08 | Vc: 75.5% | 0.008 | |
| −916.9 | power | age/Vc | −2.60 | Vc: 85% | 0.107 | |
| −920.8 | exponential | age/Vc | −6.51 | Vc: 77.7% | 0.011 | |
| −918.1 | categorical | sex/Vc | −3.9 | Vc: 85.9% | 0.048 | |
| Full model A | −936.0 | Child–Pugh/CL (categorical) WT/Vc (linear) | −21.7 | CL: 41.6% Vc: 70.0% | ||
| Full model B | −929.5 | MELD/CL (power) WT/Vc (linear) | −15.3 | CL: 37.9% Vc: 69.1% | ||
| Full model C | −974.4 | eGFR (linear), bilirubintot (power), on CL WT (linear) on Vc | −60.1 | CL: 37.5% Vc: 70.9% | ||
| Backward elimination | linear | eGFR/CL | 16.9 | <0.001 | ||
| power | bilirubintot/CL | 13.5 | <0.001 | |||
| linear | WT/Vc | 10.1 | 0.0014 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amann, L.F.; Alraish, R.; Broeker, A.; Kaffarnik, M.; Wicha, S.G. Tigecycline Dosing Strategies in Critically Ill Liver-Impaired Patients. Antibiotics 2022, 11, 479. https://doi.org/10.3390/antibiotics11040479
Amann LF, Alraish R, Broeker A, Kaffarnik M, Wicha SG. Tigecycline Dosing Strategies in Critically Ill Liver-Impaired Patients. Antibiotics. 2022; 11(4):479. https://doi.org/10.3390/antibiotics11040479
Chicago/Turabian StyleAmann, Lisa F., Rawan Alraish, Astrid Broeker, Magnus Kaffarnik, and Sebastian G. Wicha. 2022. "Tigecycline Dosing Strategies in Critically Ill Liver-Impaired Patients" Antibiotics 11, no. 4: 479. https://doi.org/10.3390/antibiotics11040479






