Secondary Metabolite Variation and Bioactivities of Two Marine Aspergillus Strains in Static Co-Culture Investigated by Molecular Network Analysis and Multiple Database Mining Based on LC-PDA-MS/MS
Abstract
:1. Introduction
2. Results
2.1. Morphological Comparison
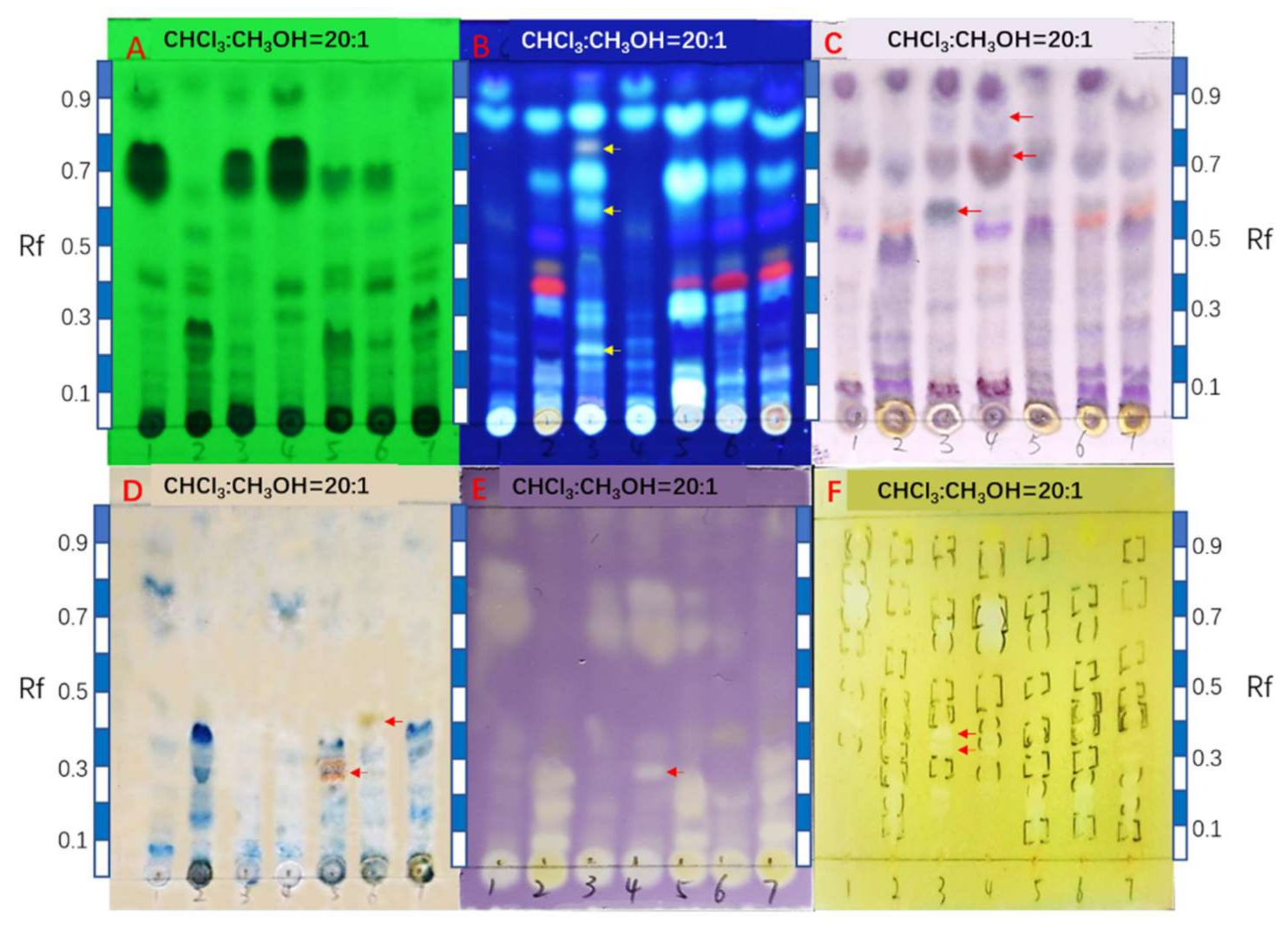
2.2. Comparison of HPTLC Fingerprints
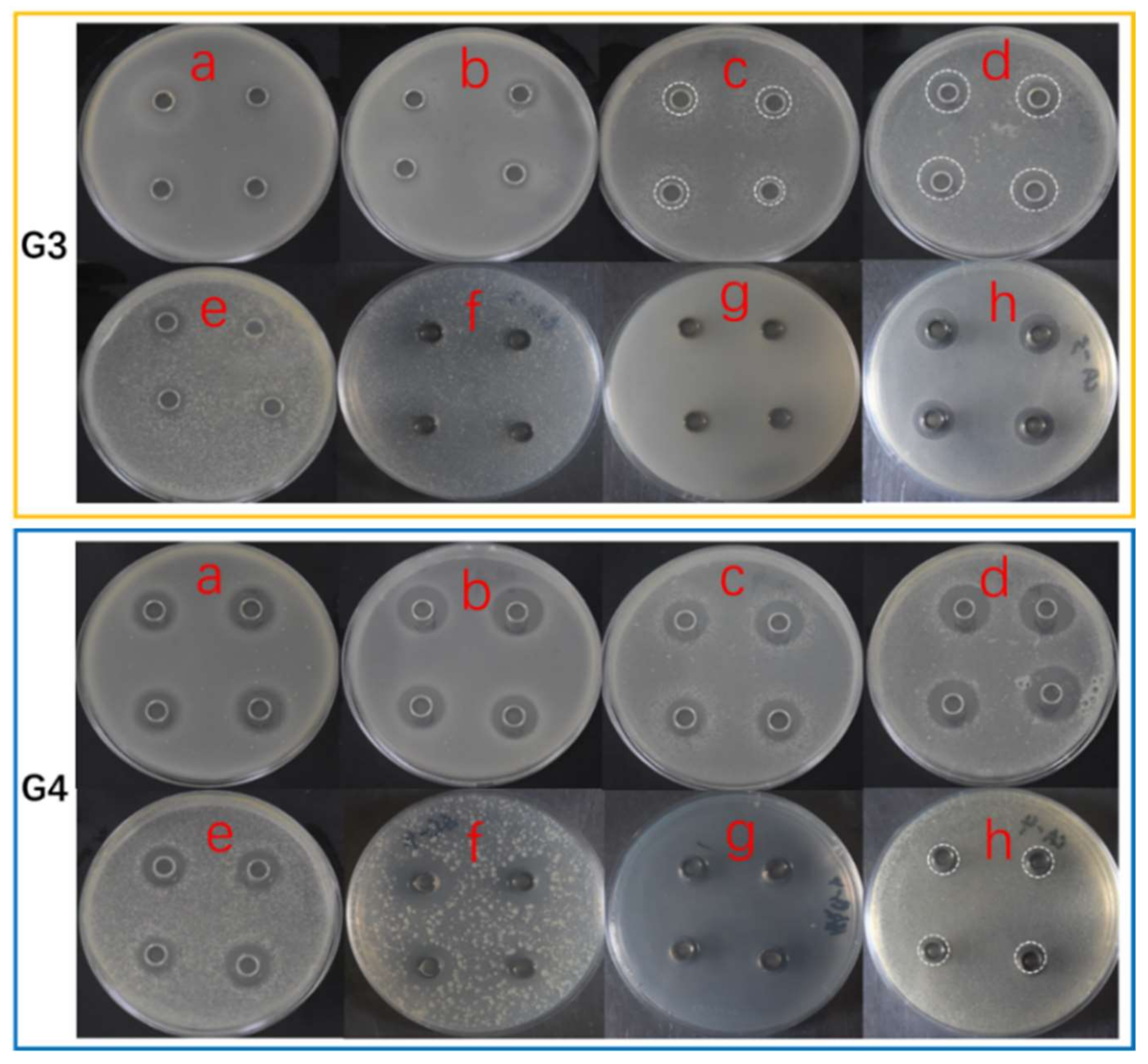
2.3. Antimicrobial Activity
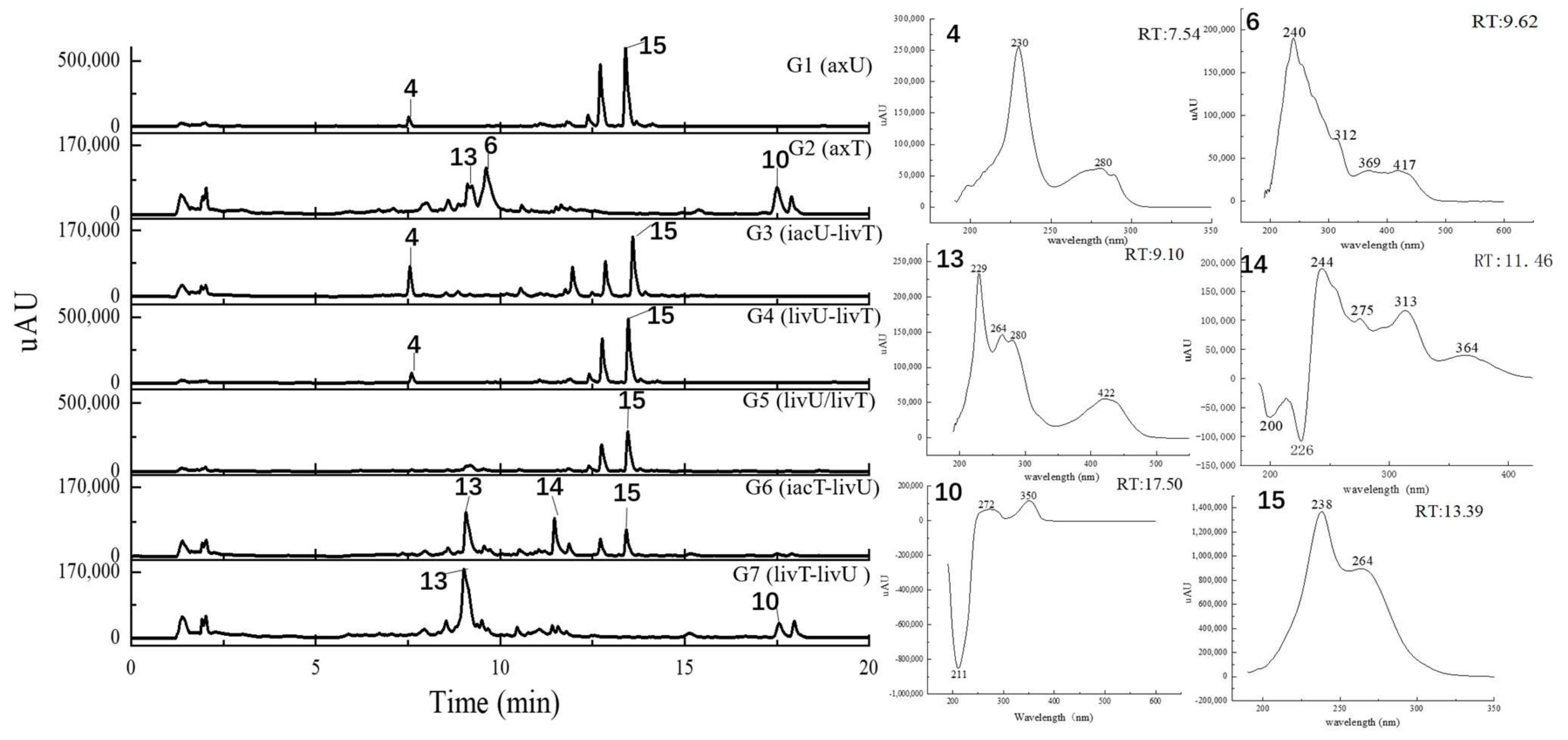
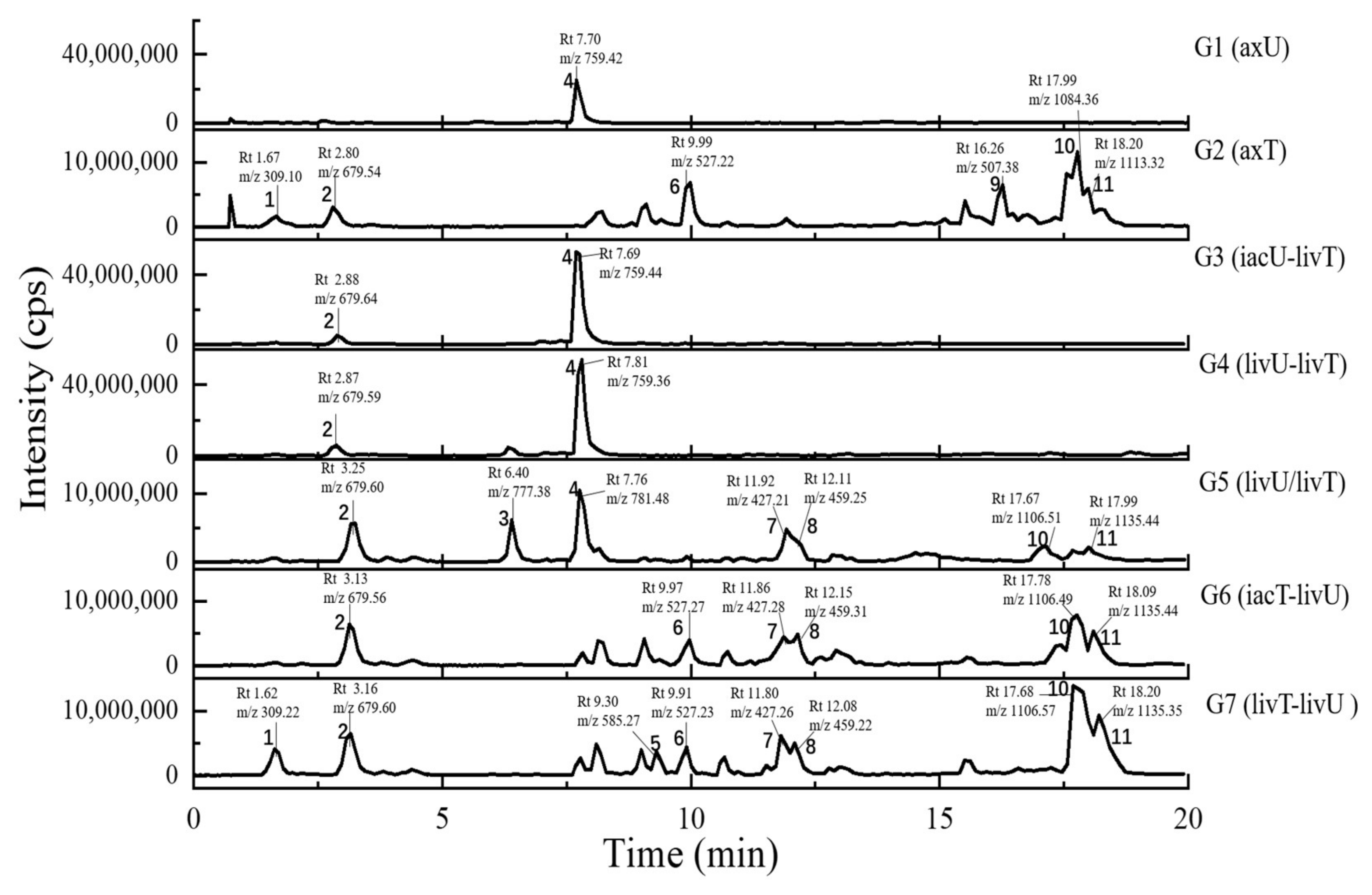
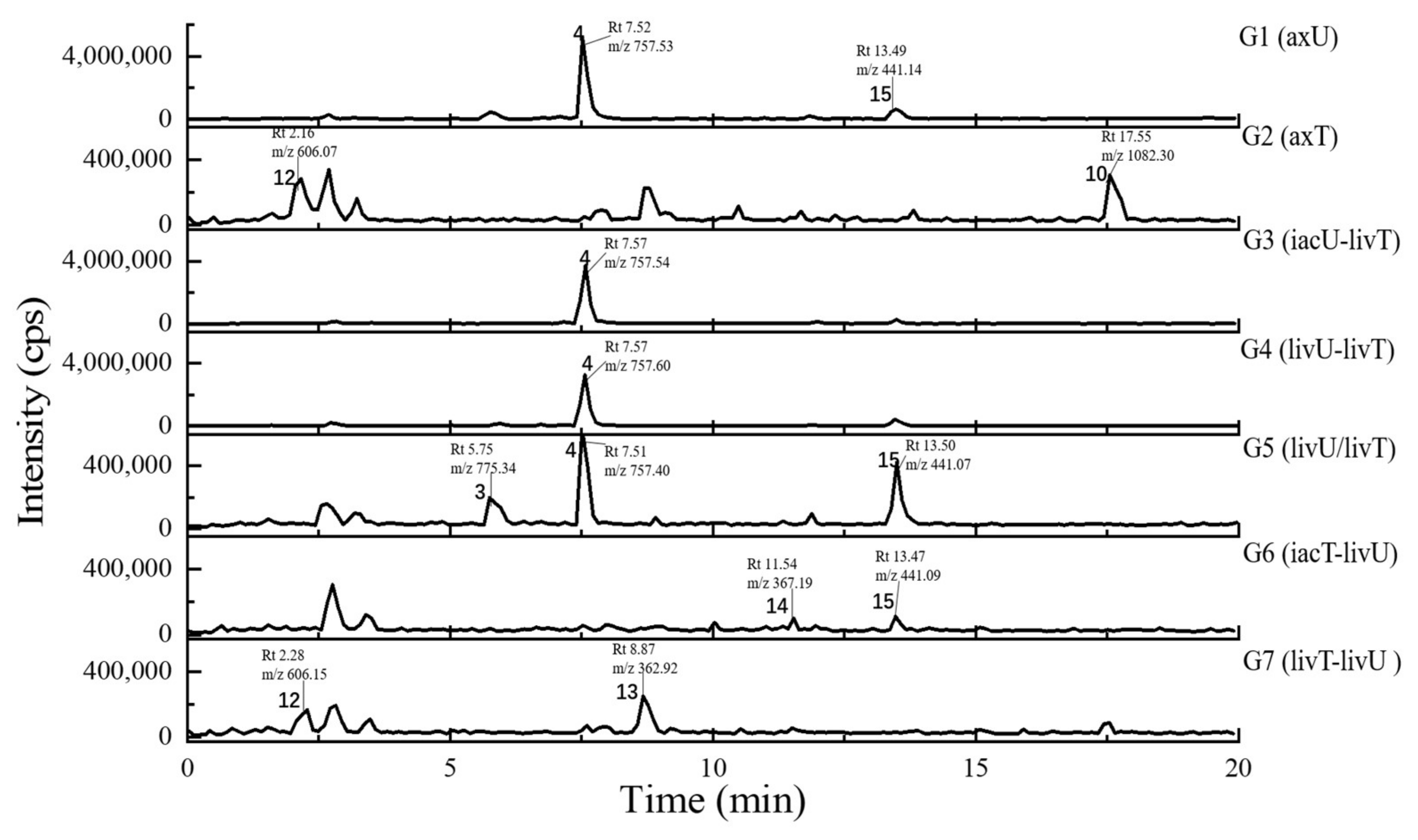
2.4. Metabolits Profile Comparison by LC-PDA-MS/MS and Multiple Database Mining
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Co-cultivation and Extraction of Strains
- G1:
- A. unguis cultivated separately for 4 weeks (axenic A. unguis, abbreviated as axU);
- G2:
- A. terreus cultured separately for 4 weeks (axenic A. terreus, abbreviated as axT);
- G3:
- Inactivated A. unguis + live A. terreus (abbreviated as iacU-livT. In detail, A. unguis was inoculated first, cultured for one week and then iactivated by autoclaving; Afterwards, A. terreus was inoculated into the same flask and cultured for the next three weeks);
- G4:
- Live A. unguis + live A. terreus (abbreviated as livU-livT; Similar to G3, but the first inoculated A. unguis was not autoclaved);
- G5:
- Live A. unguis/Live A. terreus (abbreviated as livU/livT; The two strains were simultaneously inoculated into the same flask and cultivated for 4 weeks);
- G6:
- Inactivated A. terreus + live A. unguis (abbreviated as iacT/livU; Similar to G3, but the inoculation order was opposite);
- G7:
- Live A. terreus + A. unguis (abbreviated as livT-livU; Similar to G4, but the inoculation order was opposite).
4.2.2. Thin Layer Chromatography (TLC) Analysis and Bioautography
4.2.3. Antimicrobial Assay
4.2.4. LC-MS/MS Analysis
4.2.5. GNPS Molecular Network Analysis
4.2.6. Multiple Natural Product Databases Mining
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, A.M.S.; Guerrero, A.J.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2016–2017: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2021, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Marmann, A.; Aly, A.H.; Lin, W.H.; Wang, B.G.; Proksch, P. Co-cultivation—A powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar. Drugs. 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.B.; Henrikson, J.C.; Hoover, A.R.; Lee, A.E.; Cichewicz, R.H. Epigenetic remodeling of the fungal secondary metabolome. Org. Biomol. Chem. 2008, 6, 1895–1897. [Google Scholar] [CrossRef]
- Peng, X.Y.; Wu, J.T.; Shao, C.L.; Li, Z.Y.; Chen, M.; Wang, C.Y. Co-culture: Stimulate the metabolic potential and explore the molecular diversity of natural products from microorganisms. Mar. Life Sci. Technol. 2020, 3, 363–374. [Google Scholar] [CrossRef]
- Rateb, M.E.; Hallyburton, I.; Houssen, W.E.; Bull, A.T.; Goodfellow, M.; Santhanam, R. Induction of diverse secondary metabolites in Aspergillus fumigatus by microbial co-culture. RSC Adv. 2013, 3, 14444–14450. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.X.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Oppong-Danquah, E.; Parrot, D.; Blumel, M.; Labes, A.; Tasdemir, D. Molecular networking-based metabolome and bioactivity analyses of marine-adapted fungi co-cultivated with phytopathogens. Front. Microbiol. 2018, 9, 2072–2108. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M. γ-Butyrolactones from Aspergillus species: Structures, biosynthesis, and biological activities. Nat. Prod. Commun. 2017, 12, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.W.; Zhang, Y.; Niu, X.T.; Mohyuddin, S.G.; Wen, J.; Bao, M.L.; Yu, T.Y.; Wu, L.Y.; Hu, C.Y.; Yong, Y.H.; et al. Coral-derived endophytic fungal product, butyrolactone-I, alleviates LPS induced intestinal epithelial cell inflammatory response through TLR4/NF-κB and MAPK signaling pathways: An in vitro and in vivo studies. Front. Nutr. 2021, 8, 748118. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, Y.; Yao, Y.B.; Lei, X.L.; Qian, Z.J. Butyrolactone-I from coral-derived fungus Aspergillus terreus attenuates neuro-inflammatory response via suppression of NF-κB pathway in BV-2 cells. Mar. Drugs 2018, 16, 202. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.; Jang, J.; Sim, J. A review of the pharmacological activities and recent synthetic advances of γ-butyrolactones. Int. J. Mol. Sci. 2021, 22, 2769. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Ross, S.A. Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor. Z. Nat. 2016, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, H.M.; Zhang, X.Y.; He, X.; Wang, W.D.; Gang, C.; Wang, H.F.; Pei, Y.H. A new butyrolactone from Aspergillus sp. Chem. Nat. Compd. 2018, 54, 1035–1037. [Google Scholar] [CrossRef]
- Ma, X.X.; Liu, Y.Y.; Nie, Y.Y.; Li, Y.M.; Wang, Y.; Xue, X.Y.; Hong, P.Z.; Zhang, Y. LC-MS/MS based molecular network analysis of the effects of chemical regulation on the secondary metabolites and biological activities of a marine-derived fungal strain Aspergillus terreus C23-3. Biotechnol. Bull. Shengwu Jishu Tongbao 2021, 37, 27–41. [Google Scholar] [CrossRef]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A highiy potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholestesterol-lowering agent. Biochemistry 1980, 77, 3957–3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.C.; Bao, H.Y.; Liu, Y.Y.; Nie, Y.Y.; Yang, J.M.; Hong, P.Z.; Zhang, Y. Depsidone derivatives and a cyclopeptide produced by marine Fungus Aspergillus unguis under chemical induction and by its plasma induced mutant. Molecules 2018, 23, 2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Mu, J.; Feng, Y.; Wen, L.X.; Han, J. Four chlorinated depsidones from a seaweed-derived strain of Aspergillus unguis and their new biological activities. Nat. Prod. Res. 2014, 28, 503–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.C.; Nei, Y.Y.; Yang, Z.Y.; Liu, Y.Y.; Song, C.; Hong, P.Z. Application of Aspergillusidone G in the Preparation of Neuroprotective Drugs. Patent Application CN11060473A, 24 December 2019. [Google Scholar]
- Zhang, Y.; Yang, W.C.; Nei, Y.Y.; Yang, Z.Y.; Song, C.; Hong, P.Z. Application of Depsidone Compounds in the Preparation of Neuroprotective Drugs. Patent Application CN110559290A, 13 December 2019. [Google Scholar]
- Sureram, S.; Kesornpun, C.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Directed biosynthesis through biohalogenation of secondary metabolites of the marine-derived fungus Aspergillus unguis. RSC Adv. 2013, 3, 1781–1788. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [CrossRef]
- Sureram, S.; Wiyakrutta, S.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Depsidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Med. 2012, 78, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, K.; Saepua, S.; Bunbamrung, N.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Diphenyl ethers and depsidones from the endophytic fungus Aspergillus unguis BCC54176. Tetrahedron 2022, 105, 132612. [Google Scholar] [CrossRef]
- Saetang, P.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J.; Hadsadee, S.; Jungsuttiwong, S. Antibacterial and antifungal polyketides from the fungus Aspergillus unguis PSU-MF16. J. Nat. Prod. 2021, 84, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Gyetvai, A.; Emri, T.; Takacs, K.; Dergez, T.; Fekete, A.; Pesti, M.; Pocsi, I.; Lenkey, B. Lovastatin possesses a fungistatic effect against Candida albicans, but does not trigger apoptosis in this opportunistic human pathogen. FEMS. Yeast Res. 2006, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Kontoyiannis, D.P.; Wan, Z.; Li, R.; Liu, W. Antifungal activity of statins against Aspergillus species. Med. Mycol. 2007, 45, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Nong, X.; Zhe, R.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorg. Med. Chem. Lett. 2017, 27, 787–791. [Google Scholar] [CrossRef]
- Johnson, L.J.; Koulman, A.; Christensen, M.; Lane, G.A.; Fraser, K.; Forester, N.; Johnson, R.D.; Bryan, G.T.; Rasmussen, S. An extracellular siderophore is required to maintain the mutualistic interaction of Epichloe festucae with Lolium perenne. PLoS Pathog. 2013, 9, 332–351. [Google Scholar] [CrossRef] [Green Version]
- Bunbamrung, N.; Intaraudom, C.; Dramae, A.; Komwijit, S.; Laorob, T.; Khamsaeng, S.; Pittayakhajonwut, P. Antimicrobial, antimalarial and anticholinesterase substances from the marine-derived fungus Aspergillus terreus BCC51799. Tetrahedron 2020, 76, 131496. [Google Scholar] [CrossRef]
- Yoshimi, A.; Yamaguchi, S.; Fujioka, T.; Kawai, K.; Gomi, K.; Machida, M.; Abe, K. Heterologous production of a novel cyclic peptide compound, KK-1, in Aspergillus oryzae. Front. Microbiol. 2018, 9, 690–702. [Google Scholar] [CrossRef]
- Nong, X.H.; Wang, Y.F.; Zhang, X.Y.; Zhou, M.P.; Xu, X.Y.; Qi, S.H. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs. 2014, 12, 6113–6124. [Google Scholar] [CrossRef]
- Nie, Y.Y. Screening and Secondary Metabolite Research of Seaweeds and Marine Fungi with Anti-Alzheimer’s Disease Related Activity. Master’s Thesis, Guangdong Ocean University, Guangdong, China, 3 December 2017. [Google Scholar]
- Fukuda, T.; Kurihara, Y.; Kanamoto, A.; Tomoda, H. Terretonin G, a new sesterterpenoid antibiotic from marine-derived Aspergillus sp. OPMF00272. J. Antibiot. 2014, 67, 593–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wei, X.Y.; Kim, E.L.; Lin, X.P.; Yang, X.W.; Zhou, X.F.; Yang, B.; Jung, J.H.; Liu, Y.H. New glucosidated pyrazinoquinazoline indole alkaloids from fungus Aspergillus fumigatus derived of a jellyfish. Tetrahedron 2015, 71, 271–275. [Google Scholar] [CrossRef]
- Gombodorj, S.; Yang, M.H.; Shang, Z.C.; Liu, R.H.; Li, T.X.; Yin, G.P.; Kong, L.Y. New phenalenone derivatives from Pinellia ternata tubers derived Aspergillus sp. Fitoterapia 2017, 120, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Katsuhiro, S.A.K.; Hiroshi, Y.; Shinji, F.; Takaaki, N.; Taizo, N. NF 00659A1, A2, A3, B1 and B2, novel antitumor antibiotics produced by Aspergillus sp. NF 00659. J. Antibiot. 1997, 4, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.M.; Wang, B.G. Nigerasperones A~C, new monomeric and dimeric naphtho-γ-pyrones from a marine alga-derived endophytic fungus Aspergillus niger EN-13. J. Antibiot. 2007, 60, 204–210. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaaban, K.A.; Abdel-Aziz, M.S. Seven naphtho-γ-pyrones from the marine derived fungus Alternaria alternata: Structure elucidation and biological properties. Org. Med. Chem. Lett. 2012, 2, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Priestap, H.A. New naphththopyrones from Aspergillus fonsecaeus. Tetrahedron 1984, 40, 3617–3624. [Google Scholar] [CrossRef]
- Liao, G.F.; Wu, P.; Xue, J.H.; Liu, L.; Li, H.X.; Wei, X.Y. Asperimides A-D, anti-inflammatory aromatic butenolides from a tropical endophytic fungus Aspergillus terreus. Fitoterapia 2018, 131, 50–54. [Google Scholar] [CrossRef]
- Steyn, P.S. Austamide, a new toxic metabolite from Aspergillus ustus. Tetrahedron Lett. 1971, 12, 3331–3334. [Google Scholar] [CrossRef]
- Malmstrøm, J. Unguisins A and B: New cyclic peptides from the marine-derived fungus Emericella unguis. J. Nat. Prod. 1999, 62, 787–789. [Google Scholar] [CrossRef]
- Shiono, Y.; Miyazaki, N.; Murayama, T.; Koseki, T.; Harizon; Katia, D.G.; Supratman, U.; Nakata, J.; Kakihara, Y.; Saeki, M.; et al. GSK-3β inhibitory activities of novel dichroloresorcinol derivatives from Cosmospora vilior isolated from a mangrove plant. Phytochem. Lett. 2016, 18, 122–127. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Satpradit, S.; Klaiklay, S.; Phongpaichit, S.; Borwornwiriyapan, K.; Sakayaroj, J. Polyketide anthraquinone, diphenyl ether, and xanthone derivatives from the soil fungus Penicillium sp. PSU-RSPG99. Tetrahedron 2014, 70, 5148–5152. [Google Scholar] [CrossRef]
- Phainuphong, P.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J.; Kanjanasirirat, P.; Borwornpinyo, S.; Akrimajirachoote, N.; Yimnual, C.; Muanprasat, C. Depsides and depsidones from the soil-derived fungus Aspergillus unguis PSU-RSPG204. Tetrahedron 2018, 74, 5691–5699. [Google Scholar] [CrossRef]
- Elsbaey, M.; Sallam, A.; El-Metwally, M.; Nagata, M.; Tanaka, C.; Shimizu, K.; Miyamoto, T. Melanogenesis inhibitors from the endophytic fungus Aspergillus amstelodami. Chem. Biodivers. 2019, 16, e1900237. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Sweany, R.R.; Mack, B.M.; Moore, G.G.; Gilbert, M.K.; Cary, J.W.; Lebar, M.D.; Rajasekaran, K.; Daman, K.E., Jr. Genetic responses and aflatoxin inhibition during co-culture of aflatoxigenic and non-aflatoxigenic Aspergillus flavus. Toxins 2021, 13, 794. [Google Scholar] [CrossRef]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 734–750. [Google Scholar] [CrossRef]
- Boruta, T.; Scigaczewska, A.; Bizukojc, M. “Microbial Wars” in a stirred tank bioreactor: Investigating the co-cultures of Streptomyces rimosus and Aspergillus terreus, filamentous microorganisms equipped with a rich arsenal of secondary metabolites. Front. Bioeng. Biotechnol. 2021, 9, 713639. [Google Scholar] [CrossRef]
- Liu, C.; Kakeya, H. Cryptic chemical communication: Secondary metabolic responses revealed by microbial co-culture. Chem. Asian J. 2020, 15, 327–337. [Google Scholar] [CrossRef]
- Fischer, J.; Muller, S.Y.; Netzker, T.; Jager, N.; Gacek-Mattews, A.; Pezzini, F.; Schoeler, H.; Reichelt, M.; Gershenzon, J.; Krespach, M.K.; et al. Chromatin mapping identifies BasR, a key regulator of bacteria-triggered production of fungal secondary metabolites. eLife. 2018, 7, 69–104. [Google Scholar] [CrossRef]
- Glauser, G.; Gindro, K.; Fringeli, J.; Joffrey, D.J.P.; Rudaz, S.; Wolfender, J.L. Differential analysis of mycoalexins in confrontation zones of grapevine fungal pathogens by ultrahigh pressure liquid chromatography/time-of-flight mass spectrometry and capillary nuclear magnetic resonance. J. Agric. Food Chem. 2009, 57, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Awakawa, T.; Wakimoto, T.; Abe, I. Induced biosyntheses of a novel butyrophenone and two aromatic polyketides in the plant pathogen Stagonospora nodorum. Nat. Prod. Bioprospect. 2013, 3, 141–144. [Google Scholar] [CrossRef]
- Ariawan, A.D.; Webb, J.E.A.; Howe, E.N.W.; Gale, P.A.; Thordarson, P.; Hunter, L. Cyclic peptide unguisin A is an anion receptor with high affinity for phosphate and pyrophosphate. Org. Biomol. Chem. 2017, 15, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.R.; Wang, J.T. Competition between prorocentrum donghaiense and heterotrophic bacteria for phosphate. Acta Hydrobiol. Sin. Shuisheng Shengwu Xuebao 2010, 36, 663–668. [Google Scholar] [CrossRef]
- Chiu, C.H.; Paszkowski, U. Mechanisms and impact of symbiotic phosphate acquisition. Cold Spring Harbor Perspect. Biol. 2019, 11, 603–636. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B.Q.; Cheng, C.P.; Liang, J.R.; Yang, Q.L.; Gao, Y.H. Interspecies competition between Woloszynskia sp. and Alexandrium tamarense based on phosphate concentration and initial cell density. J. Xiamen Univ. Xiamen Daxue Xuebao 2008, 47, 163–172. [Google Scholar]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E produced by Streptomyces griseus stimulates growth and development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef]
- Macreadie, I.G.; Johnson, G.; Schlosser, T.; Macreadie, P.I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 2006, 262, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Tavakkoli, A.; Johnston, T.P.; Sahebkar, A. Antifungal effects of statins. J. Pharmacol. Ther. 2020, 208, 83–123. [Google Scholar] [CrossRef]
- Boruta, T.; Milczarek, I.; Bizukojc, M. Evaluating the outcomes of submerged co-cultivation: Production of lovastatin and other secondary metabolites by Aspergillus terreus in fungal co-cultures. Appl. Microbiol. Biotechnol. 2019, 103, 5593–5605. [Google Scholar] [CrossRef] [Green Version]
- Boruta, T.; Marczyk, A.; Rychta, K.; Przydacz, K.; Bizukojc, M. Confrontation between Penicillium rubens and Aspergillus terreus: Investigating the production of fungal secondary metabolites in submerged co-cultures. J. Biosci. Bioeng. 2020, 130, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, H.; Lan, J.; Zaman, K.H.A.; Cao, S. A review: Halogenated compounds from marine fungi. Molecules 2021, 26, 458. [Google Scholar] [CrossRef] [PubMed]
- Dean, F.M.; Robertson, A.; Roberts, J.C.; Rapeb, K.B. Nidulin and ‘ustin’: Two chlorine-containing metabolic products of Aspergillus nidulans. Nature 1953, 172, 344. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Nakajima, S.; Satoh, Y.; Yamazaki, M.; Kawai, K. Studies on fungal products. XVIII. Isolation and structures of a new fungal depsidone related to nidulin and a new phthalide from Emericella unguis. Chem. Pharm. Bull. 1988, 36, 1970–1975. [Google Scholar] [CrossRef] [Green Version]
- Isaka, M.; Yangchum, A.; Supothina, S.; Veeranondha, S.; Komwijit, S.; Phongpaichit, S. Semisynthesis and antibacterial activities of nidulin derivatives. J. Antibiot. 2019, 72, 181–184. [Google Scholar] [CrossRef]
- Vala, A.K.; Dave, B.P.; Dube, H.C. Chemical characterization and quantification of siderophores produced by marine and terrestrial Aspergilli. Can. J. Microbiol. 2006, 52, 603–607. [Google Scholar] [CrossRef]
- Renshaw, J.C.; Robson, G.D.; Trinci, A.P.J.; Wiebe, M.G.; Livens, F.R.; Collison, D.; Taylor, R.J. Fungal siderophores: Structures, functions and applications. Mycol. Res. 2002, 106, 1123–1142. [Google Scholar] [CrossRef]
- Nie, Y.Y.; Yang, W.C.; Liu, Y.Y.; Yang, J.M.; Lei, X.L.; Gerwick, W.H.; Zhang, Y. Acetylcholinesterase inhibitors and antioxidants mining from marine fungi: Bioassays, bioactivity coupled LC–MS/MS analyses and molecular networking. Mar. Life Sci. Technol. 2020, 2, 386–397. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K. Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J. Ethnopharmacol. 2014, 151, 158–175. [Google Scholar] [CrossRef]


 : axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster for statins including the sodiated ion of peak 7 (lovastatin). (B) is an enlarged cluster containing a possible fusarine-like siderophore. (C) is an enlarged cluster containing heme B. (D) is an enlarged cluster containing protonated ion of peak 7. (E) is an enlarged cluster containing peak 6 (territrem B). (F) conclude two nodes annotated as a diketopiperazine and a simvastatin fragment. (G) is a node annotated as a cyclopeptide.
: axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster for statins including the sodiated ion of peak 7 (lovastatin). (B) is an enlarged cluster containing a possible fusarine-like siderophore. (C) is an enlarged cluster containing heme B. (D) is an enlarged cluster containing protonated ion of peak 7. (E) is an enlarged cluster containing peak 6 (territrem B). (F) conclude two nodes annotated as a diketopiperazine and a simvastatin fragment. (G) is a node annotated as a cyclopeptide.
 : axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster for statins including the sodiated ion of peak 7 (lovastatin). (B) is an enlarged cluster containing a possible fusarine-like siderophore. (C) is an enlarged cluster containing heme B. (D) is an enlarged cluster containing protonated ion of peak 7. (E) is an enlarged cluster containing peak 6 (territrem B). (F) conclude two nodes annotated as a diketopiperazine and a simvastatin fragment. (G) is a node annotated as a cyclopeptide.
: axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster for statins including the sodiated ion of peak 7 (lovastatin). (B) is an enlarged cluster containing a possible fusarine-like siderophore. (C) is an enlarged cluster containing heme B. (D) is an enlarged cluster containing protonated ion of peak 7. (E) is an enlarged cluster containing peak 6 (territrem B). (F) conclude two nodes annotated as a diketopiperazine and a simvastatin fragment. (G) is a node annotated as a cyclopeptide.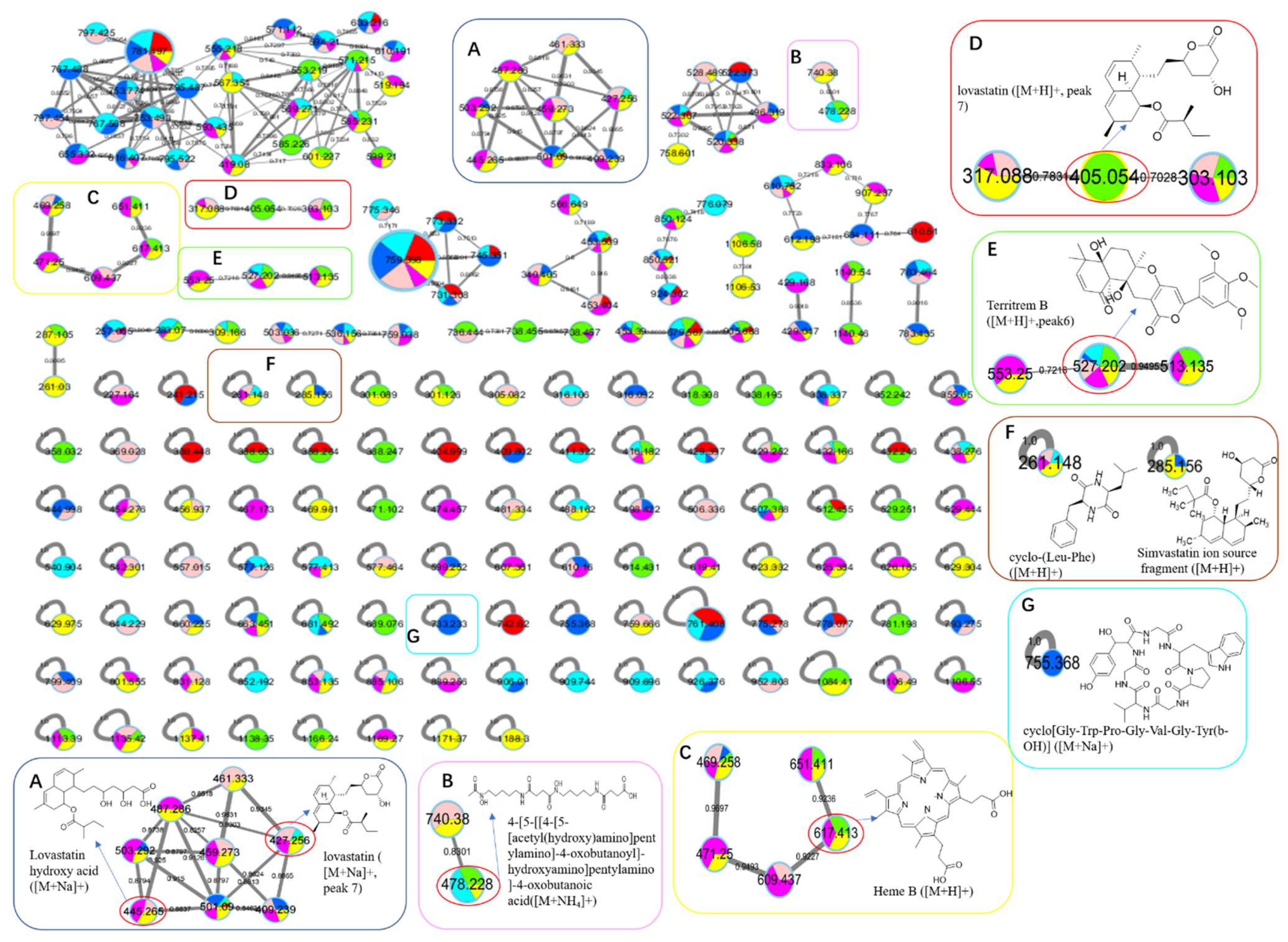
 : axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster containing peak 15 (nidulin). (B) is an enlarged cluster containing nornidulin.
: axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster containing peak 15 (nidulin). (B) is an enlarged cluster containing nornidulin.
 : axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster containing peak 15 (nidulin). (B) is an enlarged cluster containing nornidulin.
: axU (G1), axT (G2), iacU-livT (G3), livU-livT (G4), livU/livT (G5), iacT-livU (G6), and livT-livU (G7), respectively. (A) is an enlarged cluster containing peak 15 (nidulin). (B) is an enlarged cluster containing nornidulin.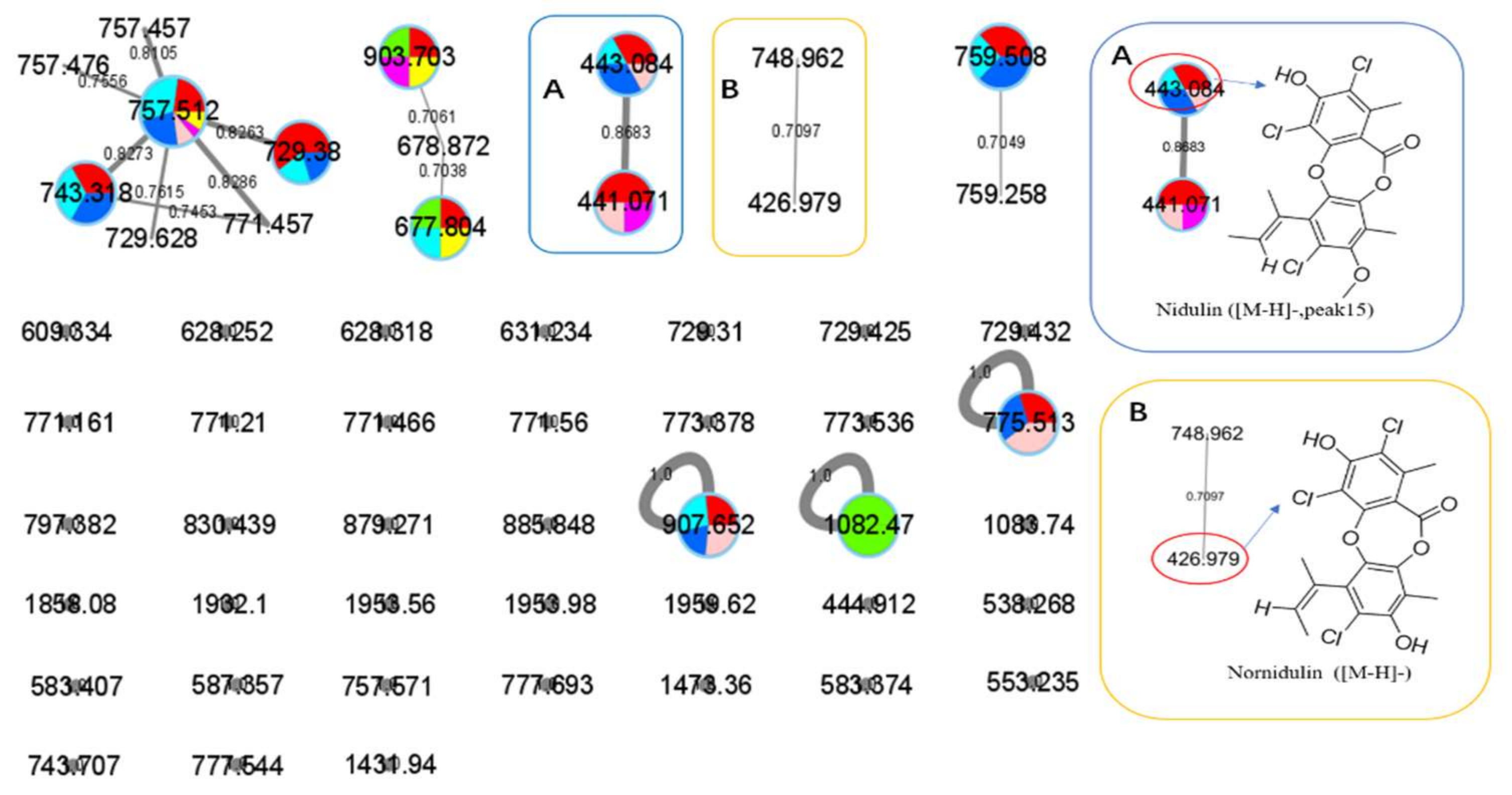

| Sample Number | Total Sample Amount (Yield: mg/flask) | Diameters of Inhibition Zones Against Indicator Microbes (mm) # | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MRSA | Bacillus subtilis | Pseudomonus aeruginosa | Vibro parahemolyticus | Vibro alginolyticus | Shewanella putrefaciens | Yersinia pseudotuberculosis | Candida albicans | ||
| axU (G1) | 345 ± 40 | 15.7 ± 0.6 | 13.2 ± 0.5 | 14.0 ± 0.9 | 18.6 ± 0.9 | 17.8 ± 0.8 | 17.1 ± 1.2 | - | 13 ± 0.5 |
| axT (G2) | 624 ± 10 | - | - | - | 7.3 ± 0.4 | 8.1 ± 0.6 | 9.3 ± 0.5 | - | - |
| iacU-livT (G3) | 560 ± 20 | - | 7.5 ± 0.8 | 8.9 ± 0.9 | 11.8 ± 0.4 | 7.3 ± 0.9 | 7.1 ± 0.5 | - | 14.2 ± 0.7 |
| livU-livT (G4) | 309 ± 30 | 13.0 ± 0.4 | 15.7 ± 0.5 | 15.6 ± 1.0 | 17.8 ± 0.6 | 16.2 ± 0.6 | 17.4 ± 0.5 | - | 11.7 ± 0.5 |
| livU/livT (G5) | 420 ± 80 | 10.2 ± 0.6 | 14.3 ± 0.7 | 14.2 ± 0.7 | - | 14.3 ± 0.5 | 18 ± 0.8 | - | 13.0 ± 0.8 |
| iacT-livU (G6) | 440 ± 20 | - | 7.0 ± 0.4 | - | - | 9.6 ± 0.6 | 10.4 ± 0.8 | - | 12.3 ± 0.5 |
| livT-livU (G7) | 638 ± 40 | 8.1 ± 0.3 | - | - | 8. 1 ± 0.9 | - | - | - | 9.2 ± 0.7 |
| Ampicillin | - | 14.1 ± 0.4 | 17.5 ± 0.2 | 19.7 ± 0.5 | 24.1 ± 0.9 | 18.6 ± 0.4 | 16.1 ± 0.6 | 11.3 ± 0.2 | - |
| Ketoconazole | - | - | - | - | - | - | - | - | 16.3 ± 0.8 |
| Feature Peak Number | G3(iacU-livT) | G4(livU-livT) | G5(livU/livT) | G6(iacT-livU) | G7(livT-livU) | |
|---|---|---|---|---|---|---|
| G2(axT) | 1 | ↓0.14 | ↓<0.01 | ↓0.02 | ↓0.13 | ↑1.58 |
| 2 | ↑1.59 | ↑1.96 | ↑2.11 | ↑2.24 | ↑2.46 | |
| 5 | ↓0.23 | ↓0.02 | ↓0.08 | ↑1.49 | ↑3.49 | |
| 6 | ↓0.12 | ↓<0.01 | ↓0.11 | ↓0.7 | ↓0.72 | |
| 7 | ↓0.21 | ↓0.08 | ↑3.57 | ↑3.15 | ↑4.42 | |
| 8 | ↓0.52 | ↓0.19 | ↑8.41 | ↑7.31 | ↑10.47 | |
| 9 | ↓0.02 | ↓0.06 | ↓<0.01 | ↓0.08 | ↓0.05 | |
| 10 | ↓<0.01 | ↓<0.01 | ↓0.09 | ↓0.64 | ↑1.36 | |
| 11 | ↓0.02 | ↓0.04 | ↓0.52 | ↑1.33 | ↑3.03 | |
| 12 | ↓0.2 | ↓0.06 | ↓0.08 | ↓0.02 | ↓0.57 | |
| 13 | ↓0.03 | ↓0.02 | ↓0.14 | ↓0.27 | ↓0.91 | |
| G1(axU) | 2 | ↑4.57 | ↑5.66 | ↑6.07 | ↑6.45 | ↑7.09 |
| 3 | ↓0.05 | ↑3.46 | ↑3.08 | ↓<0.01 | ↓0.01 | |
| 4 | ↑3.65 | ↑3.48 | ↓0.78 | ↓0.09 | ↓0.14 | |
| 15 | ↓0.23 | ↓0.56 | ↓0.5 | ↓0.14 | ↓0 | |
| 14 | ↓0 | ↓0 | ↑1 | ↑13.39 | ↑1.89 |
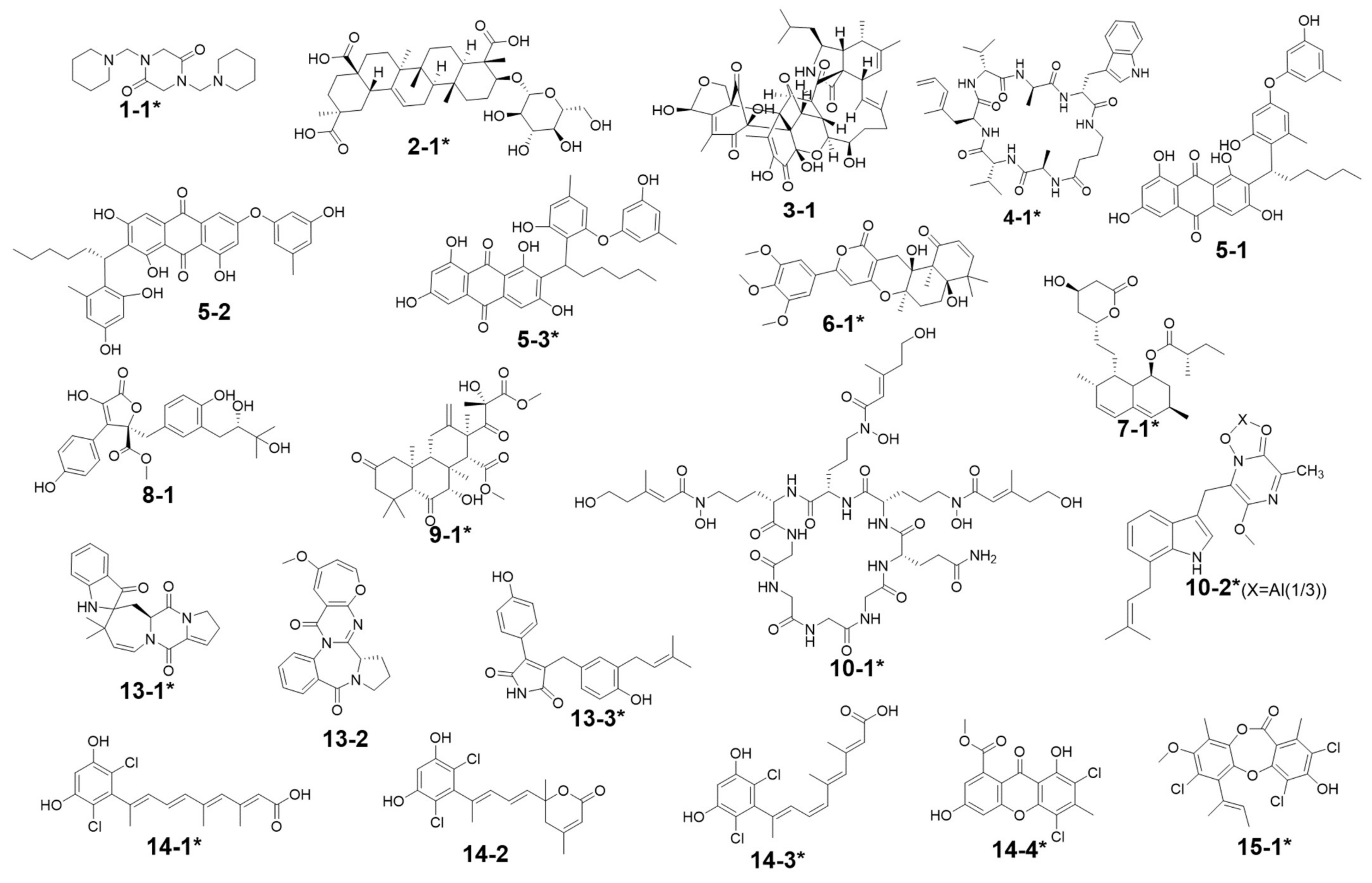
| Peak Number | Presence in Sample | m/z Value Measured | Retention Time (min) | UV Maximum Measured (nm) | Compound Hits in Library | Molecular Weight in Libraries | Libraries & IDs | MS2 Similarity (Cosine) | Molecular Formula | UV Maximum Absportive Peaks in Libraries/Literature (nm) | Bioresource | DOI | Biological Activity | Structures Code of the Compound Hits |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | G2, G7 | 309.10 [M + H]+ (presumed) | 1.67 | 232 | 1,4-Bis(piperidin-1-ylmethyl)piperazine-2,5-dione | 308.33 | Dictionary of Natural Products | N/A | C16H28N4O2 | N/A (isolate amide) | A. terreus | N/A | N/A | 1-1 * |
| 2 | G1–G7 | 679.59 [M + H]+ 677.88 [M − H]− | 2.87 | 231 | 3β-(β-D-glucopyranosyloxy)olean-12-ene-23,28,30-trioic acid | 678.81 | NMRDATA, 1331571; Natural Product Atlas, NPA026397; Pubchem, 146682840 | N/A | C36H54O12 | N/A (isolate double bonds) | A. amstelodami | 10.1002/cbdv.201900237 | anti-melanogenic and anti-allergic activity | 2-1 * |
| 3 | G1, G4, G5 | 777.38 [M + H]+ 775.34 [M − H]− | 6.40 | 231 | Aspergilasine B | 775.84 | NMRDATA, 999923; Dictionary of Natural Products | N/A | C42H49NO13 | 202, 240 | A. flavipes QCS12 | 10.1021/acs.orglett.7b02146 | no inhibitory activities againest seven cancer cell lines up to a concentration of 40μM. | 3-1 |
| 4 | G1, G3–G7 | 759.36 [M + H]+ 757.60 [M − H]− | 7.81 | 230, 280 | Unguisin A | 758.92 | NMRDATA, 29553 | N/A | C40H54N8O7 | 290, 281, 274, 219 | A. unguis | 10.1021/np980539z;10.1039/C7OB00316A | moderately inhibited Staphylococcus aureus;as an anion receptor with high affinity for phosphate and pyrophosphate | 4-1 * |
| 5 | G2, G7 | 585.27 [M + H]+ 583.24 [M − H]− | 9.30 | 229, 264, 280, 422 | Aspergilol A | 584.62 | NMRDATA, 895659; Pubchem, 132915662; Natural Product Atlas, NPA009011; Dictionary of Natural Products | N/A | C34H32O9 | 196, 293, 452 | A. versicolor | 10.1016/j.tet.2015.10.038 | possessing antioxidant activities | 5-1 |
| Aspergilol B | 584.61 | Dictionary of Natural Products | N/A | C34H32O9 | 194, 293, 462 | Aspergillus | 10.1016/j.tet.2015.10.038 | possessing antioxidant activities | 5-2 | |||||
| Aspergilol G | 584.61 | Dictionary of Natural Products | N/A | C34H32O9 | 206, 265, 295, 458 | Aspergillus | 10.1016/J.BMCL.2017.01.032 | N/A | 5-3 * | |||||
| 6 | G2, G6, G7 | 527.22 [M + H]+ | 9.99 | 240, 312, 369, 417 | Territrem B | 526.57 | GNPS, CCMSLIB00005436075 | 0.70 | C29H34O9 | 195, 220, 236, 330, 284 | A. terreus | 10.3390/md12126113 | strong inhibitory activity against acetylcholinesterase, potent antifouling activity | 6-1 * |
| 526.57 | Dictionary of Natural Products | N/A | ||||||||||||
| 7 | G2, G5–G7 | 427.26 [M + Na]+ | 11.80 | 243, 275, 313, 364 | Lovastatin | 404.54 | GNPS, CCMSLIB00000852214 | 0.76 | C24H36O5 | 231, 238, 247 | A. terreus | 10.1080/10826068.2020.1805624 | the competitive inhibitors of the enzyme hydroxy-methyl-glutaryl coenzyme A (HMG-CoA) reductase | 7-1 * |
| 404.54 | Dictionary of Natural Products | N/A | ||||||||||||
| 8 | G5–G7 | 459.31 [M + H]+ (presumed) | 12.15 | 246, 285 | Unannotated statin | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Aspernolide D | 458.46 | NMRDATA, 152713; Pubchem, 46930025; Natural Product Atlas, NPA003511 | N/A | C24H26O9 | 290 | A. terreus RCBC1002 | 10.1248/cpb.58.1221 | Inactive against all bacterial strains | 8-1 | |||||
| 9 | G2, G7 | 507.38 [M + H]+ (presumed) | 16.26 | N/A (no obvious absorption) | Terretonin G | 506.58 | NMRDATA, 809567; Dictionary of Natural Products | N/A | C27H38O9 | End absorption | Aspergillus sp. OPMF00272 | 10.1038/ja.2014.46 | Moderate antimicrobial activity against Gram-positive bacteria | 9-1 * |
| 10 | G2, G5–G7 | 1106.49 [M + Na]+ 1082.38 [M − H]− | 17.78 | 272, 350 | Epichloenin A | 1083.15 | Dictionary of Natural Products | N/A | C46H74N12O18 | N/A (containing a,b-unsaturated amides) | Epichloe¨ festucae | 10.1371/journal.ppat.1003332 | as an important molecular/cellular signal for controlling fungal growth and hence the symbiotic interaction. | 10-1 * |
| Astalluminoxide | 1084.22 | Natural Product Atlas, NPA032177 | N/A | C60H66AlN9O9 | 201, 222, 272, 349 | A. terreus BCC51799 | 10.1016/j.tet.2020.131496 | moderate to weak cytotoxicity against both cancerous and non-cancerous cells. | 10-2 * | |||||
| 11 | G2, G5–G7 | 1135.35 [M + Na]+ | 18.20 | 273, 346 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 12 | G2, G7 | 606.07 [M − H]− (presumed) | 2.16 | 231 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 13 | G2, G7 | 362.92 [M − H]− (presumed) | 8.87 | 226, 264, 278, 420 | Austamide | 363.41 | Dictionary of Natural Products | N/A | C21H21N3O3 | 234, 256, 282, 392 | A.ustus | 10.1016/s0040-4039(01)97170-9 | toxic to ducklings | 13-1 * |
| Circumdatin B | 363.37 | Dictionary of Natural Products | N/A | C20H17N3O4 | 284, 358 | A. ochraceus | 10.1021/jo981536u | Inactive in the assay against NCI’s 60 cancer cell line panel | 13-2 | |||||
| Asperimide A | 363.41 | Natural Product Atlas, NPA028229 | N/A | C22H21NO4 | 229, 278, 360 | A.terreus | 10.1016/j.fitote.2018.10.011 | not found exhibited cytotoxicity | 13-3 * | |||||
| 14 | G6, G7 | 367.19 [M − H]− (presumed) (367.19:369.11:371.19 = 9:6:1, in intensity) revealing to be dichlorinated compound | 11.54 | 244, 275, 313, 361 | Cosmochlorin A | 369.24 | Dictionary of Natural Products; Natural Product Atlas, NPA030107 | N/A | C18H18Cl2O4 | 323 | Cosmospora vilior IM2-155 | 10.1016/j.phytol.2016.09.007 | moderate antimicrobial activity against gram-positive bacteria and fungi; partially restored the growth inhibition caused by hyperactivated Ca2+-signaling in mutant yeast and showed GSK-3b inhibition | 14-1 * |
| Cosmochlorin B | 369.24 | Dictionary of Natural Products; Natural Product Atlas, NPA030108 | N/A | C18H18Cl2O4 | 230, 290 | Cosmospora vilior IM2-155 | 10.1016/j.phytol.2016.09.007 | Inactive against microbes; similar restoring the growth inhibition activity to cosmochlorin A, promoting osteoclast formation | 14-2 * | |||||
| Cosmochlorin C | 369.24 | Dictionary of Natural Products; Natural Product Atlas, NPA030109 | N/A | C18H18Cl2O4 | 323 | Cosmospora vilior IM2-155 | 10.1016/j.phytol.2016.09.007 | Similar antimicrobial activity to cosmochlorin A | 14-3 * | |||||
| Penicillixanthone | 369.15 | Dictionary of Natural Products; Natural Product Atlas, NPA008373 | N/A | C16H10Cl2O6 | 230, 294, 369 | Penicillium sp. PSU-RSPG99 | 10.1016/j.tet.2014.05.105 | No antimycobacterial and cytotoxic activities | 14-4 * | |||||
| 15 | G1, G3–G6 | 441.07 [M − H]− (441.07:443.02:444.99:446.12 = 27:27:9:1, in intensity) | 13.50 | 237, 264 | Nidulin | 443.70 | GNPS, CCMSLIB00005436077 | 0.70 | C20H17Cl3O5 | 267 | A. unguis | 10.1055/s-0031-1298228 10.1080/14786419.2013.879305 | aromatase inhibitory and antimicrobial and DNA damaging activities | 15-1 * |
| Pubchem, 6450195; Dictionary of Natural Products | N/A |
| Injection Volume (μL) | Elution Conditions | Flow Rate (mL/min) | |
|---|---|---|---|
| Time (min) | Proportion | ||
| 25 | 0.00–1.00 | 30% ACN-H2O | 0.6 |
| 1.00–10.00 | 30–99% ACN-H2O | ||
| 10.00–16.00 | 99% ACN-H2O | ||
| 16.00–16.20 | 99–30% ACN-H2O | ||
| 16.20–20.00 | 30% ACN-H2O | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Glukhov, E.; He, Y.; Liu, Y.; Zhou, L.; Ma, X.; Hu, X.; Hong, P.; Gerwick, W.H.; Zhang, Y. Secondary Metabolite Variation and Bioactivities of Two Marine Aspergillus Strains in Static Co-Culture Investigated by Molecular Network Analysis and Multiple Database Mining Based on LC-PDA-MS/MS. Antibiotics 2022, 11, 513. https://doi.org/10.3390/antibiotics11040513
Wang Y, Glukhov E, He Y, Liu Y, Zhou L, Ma X, Hu X, Hong P, Gerwick WH, Zhang Y. Secondary Metabolite Variation and Bioactivities of Two Marine Aspergillus Strains in Static Co-Culture Investigated by Molecular Network Analysis and Multiple Database Mining Based on LC-PDA-MS/MS. Antibiotics. 2022; 11(4):513. https://doi.org/10.3390/antibiotics11040513
Chicago/Turabian StyleWang, Yuan, Evgenia Glukhov, Yifan He, Yayue Liu, Longjian Zhou, Xiaoxiang Ma, Xueqiong Hu, Pengzhi Hong, William H. Gerwick, and Yi Zhang. 2022. "Secondary Metabolite Variation and Bioactivities of Two Marine Aspergillus Strains in Static Co-Culture Investigated by Molecular Network Analysis and Multiple Database Mining Based on LC-PDA-MS/MS" Antibiotics 11, no. 4: 513. https://doi.org/10.3390/antibiotics11040513
APA StyleWang, Y., Glukhov, E., He, Y., Liu, Y., Zhou, L., Ma, X., Hu, X., Hong, P., Gerwick, W. H., & Zhang, Y. (2022). Secondary Metabolite Variation and Bioactivities of Two Marine Aspergillus Strains in Static Co-Culture Investigated by Molecular Network Analysis and Multiple Database Mining Based on LC-PDA-MS/MS. Antibiotics, 11(4), 513. https://doi.org/10.3390/antibiotics11040513







