Availability, Prices and Affordability of Antibiotics Stocked by Informal Providers in Rural India: A Cross-Sectional Survey
Abstract
:1. Introduction
2. Results
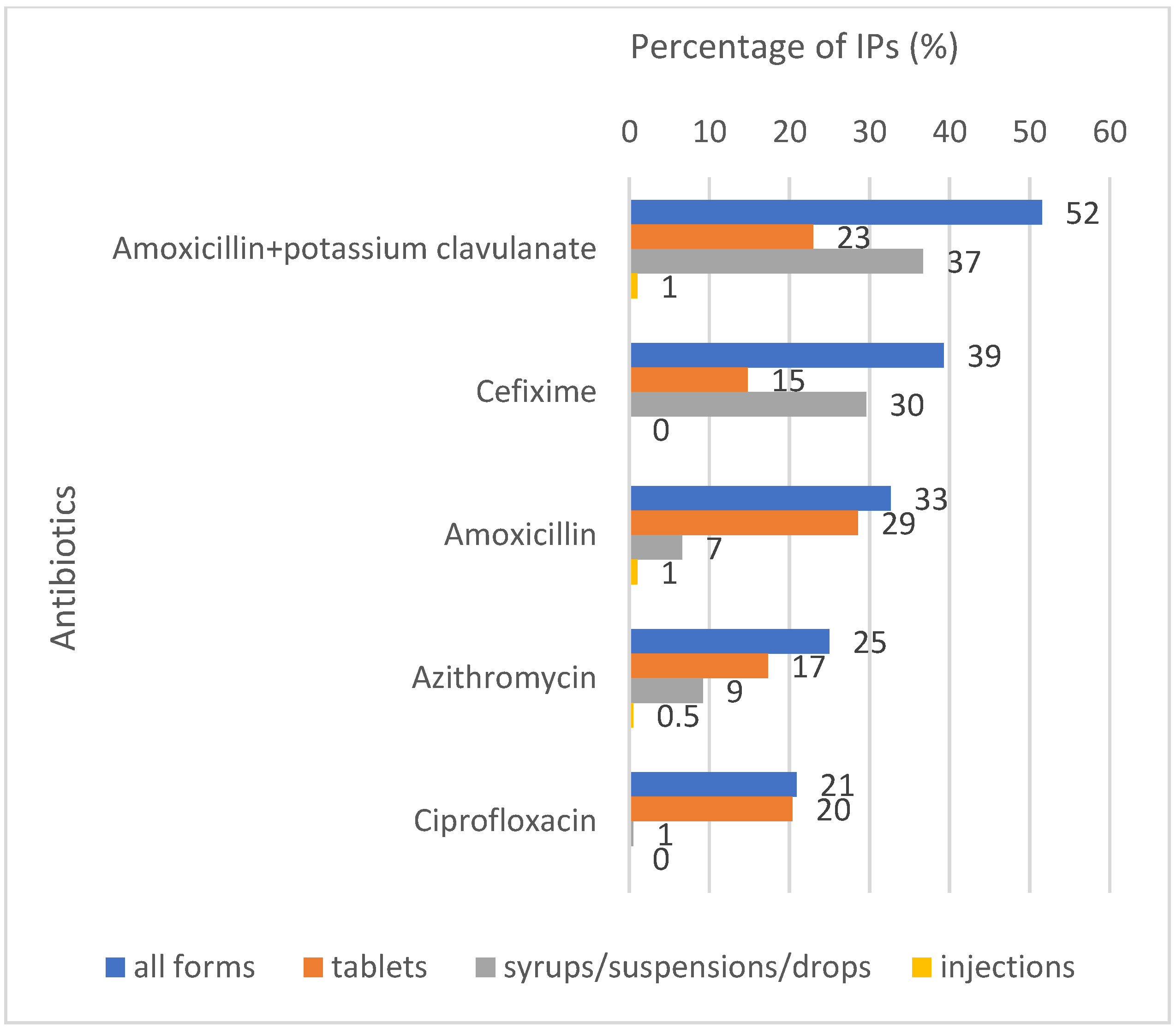
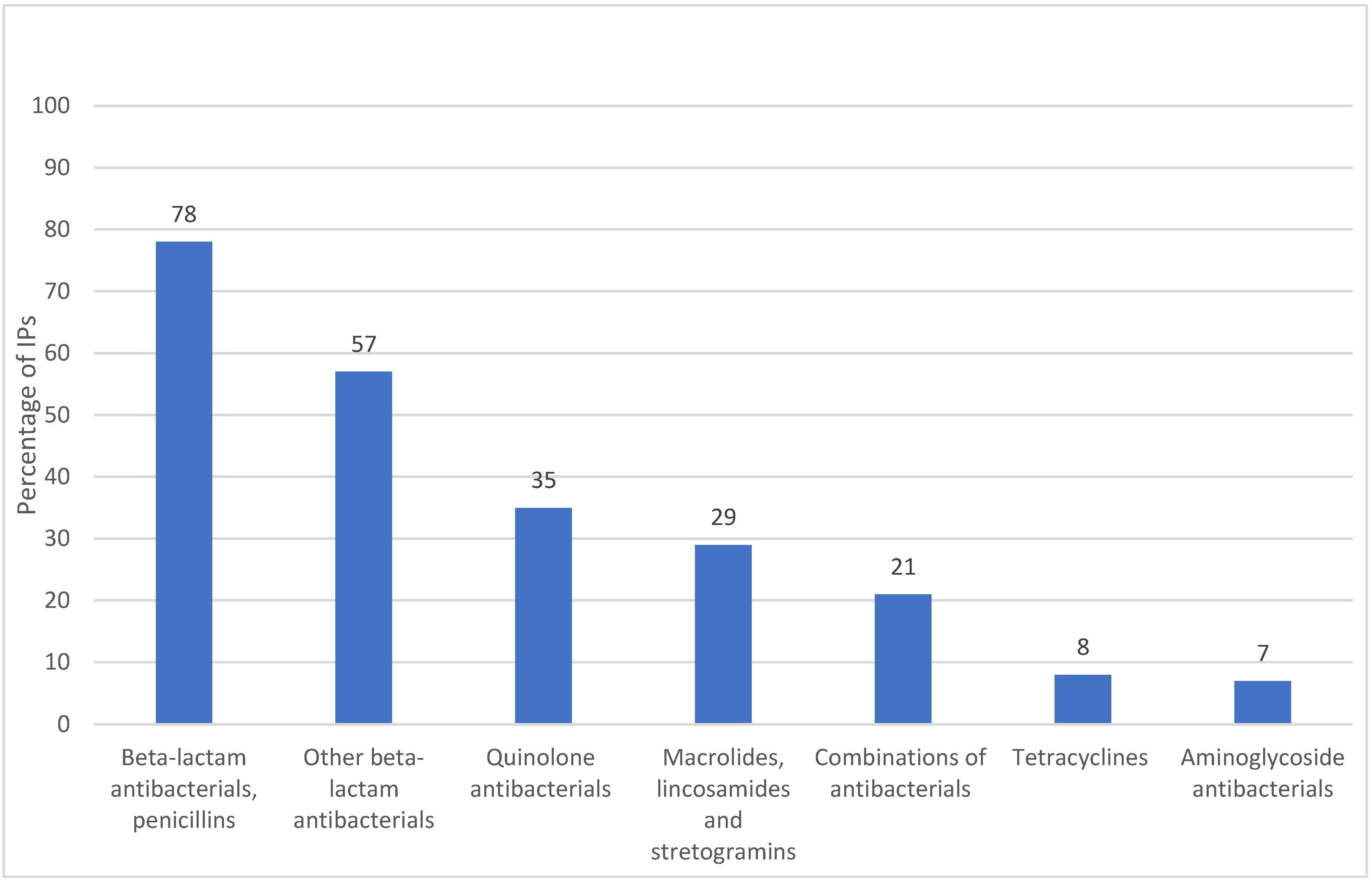
2.1. Antibiotic Availability and Sales Volumes

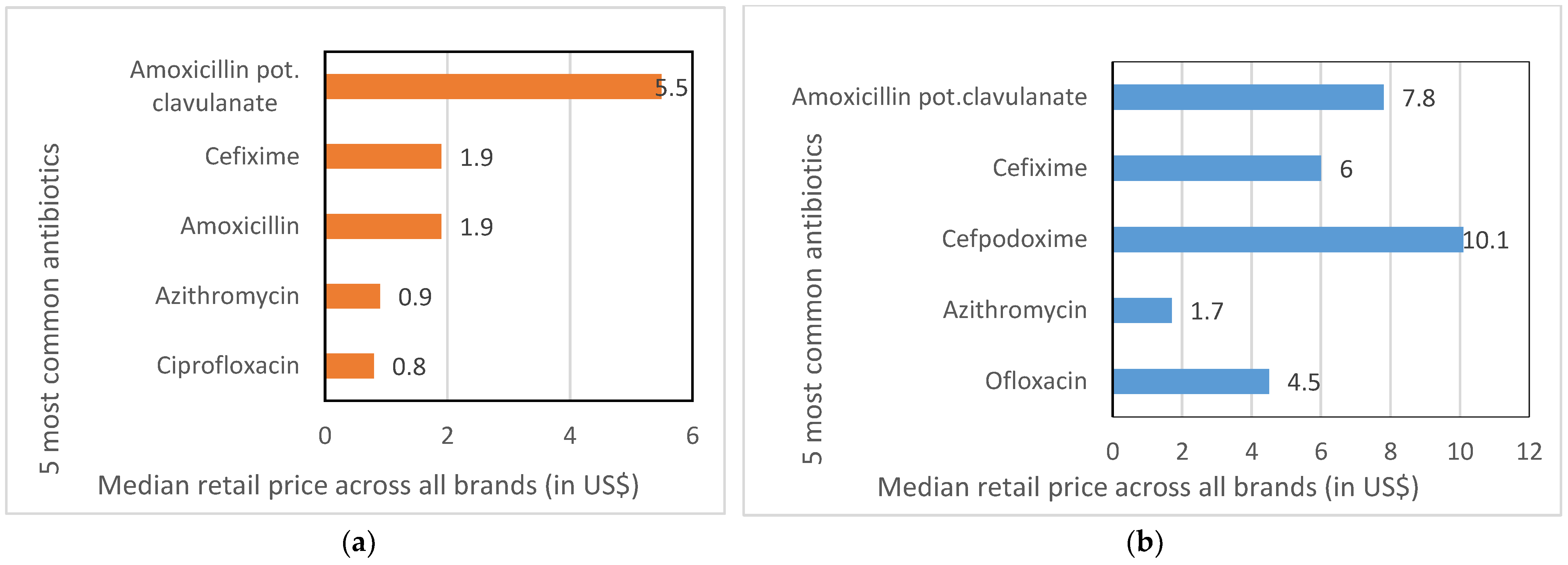
2.2. Brands, Prices and Markups
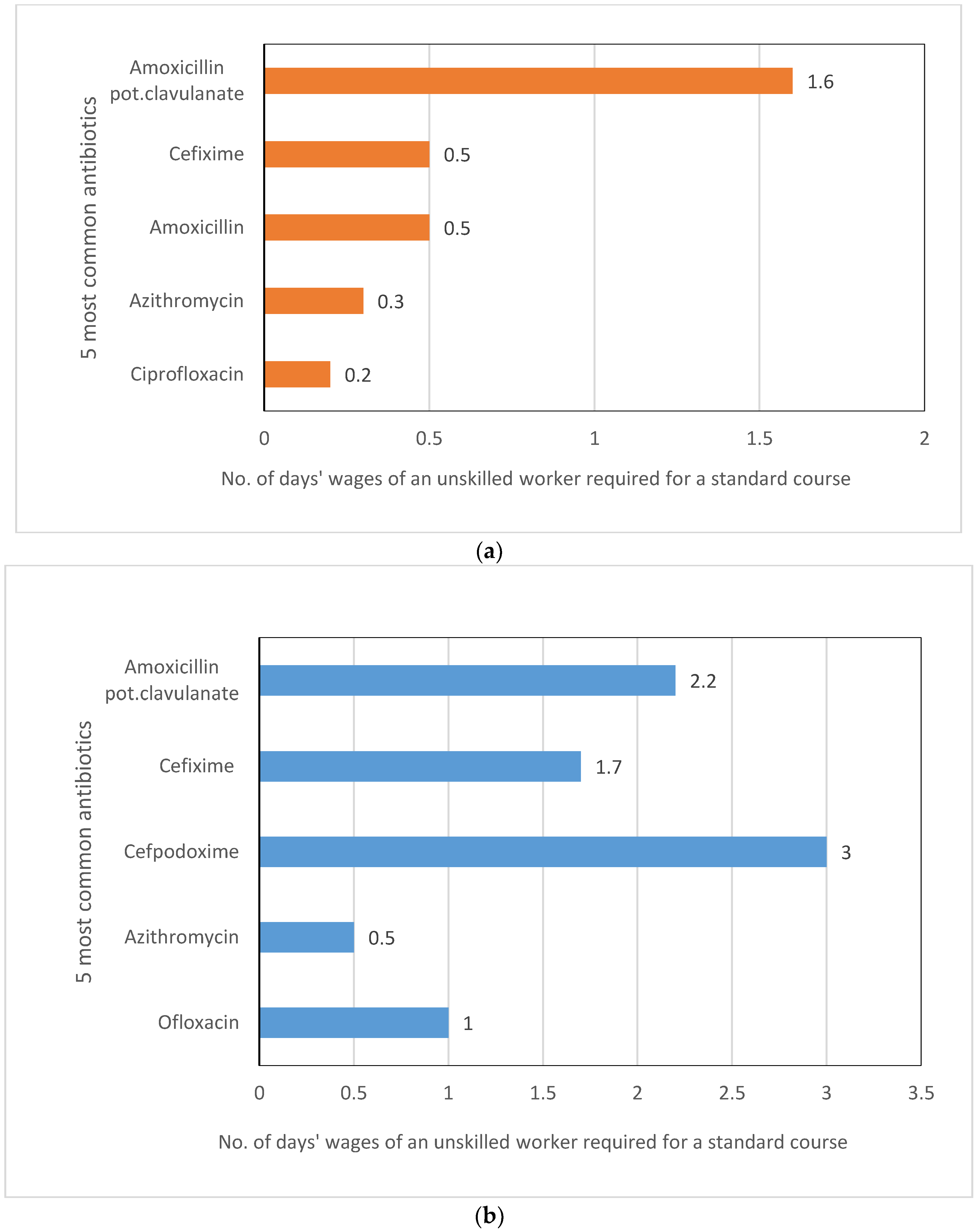
2.3. Affordability
3. Discussion
4. Materials and Methods
4.1. Study Setting
4.2. Sampling and Data Collection
4.3. Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- O’Neill, J. The Review On AMR: Tackling Drug-Resistant Infections Globally; UK Government: London, UK, 2016.
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Klein, E.Y.; Tseng, K.K.; Pant, S.; Laxminarayan, R. Tracking global trends in the effectiveness of antibiotic therapy using the Drug Resistance Index. BMJ Glob. Health 2019, 4, e001315. [Google Scholar] [CrossRef] [PubMed]
- WHO. GLASS Country, Territory and Area Profiles, 2018. 2020. Available online: https://apps.who.int/gho/tableau-public/tpc-frame.jsp?id=2012 (accessed on 29 March 2022).
- Balachandra, S.S.; Sawant, P.S.; Huilgol, P.G.; Vithya, T.; Kumar, G.; Prasad, R. Antimicrobial resistance (AMR) at the community level: An urban and rural case study from Karnataka. J. Fam. Med. Prim. Care 2021, 10, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, P.; Chandel, D.S.; Hansen, N.I.; Sharma, N.; Kandefer, S.; Parida, S.; Satpathy, R.; Pradhan, L.; Mohapatra, A.; Mohapatra, S.S.; et al. Neonatal sepsis in rural India: Timing, microbiology and antibiotic resistance in a population-based prospective study in the community setting. J. Perinatol. 2017, 37, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.H.; Moore, L.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.; Piddock, L. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2015, 387, 168–175. [Google Scholar] [CrossRef]
- WHO. The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. Geneva: World Health Organization. 2019. (WHO/EMP/IAU/2019.11). Available online: https://www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/ (accessed on 20 July 2020).
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Batista, A.; Rodrigues, D.A.; Figueiras, A.; Zapata-Cachafeiro, M.; Roque, F.; Herdeiro, M. Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review. Antibiotics 2020, 9, 786. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Do, N.T.T.; Nguyen, C.T.K.; Tran, T.K.; Ho, P.D.; Nguyen, H.H.; Vu, H.T.L.; Wertheim, H.F.L.; Van Doorn, H.R.; Lewycka, S. Community-level consumption of antibiotics according to the AWaRe (Access, Watch, Reserve) classification in rural Vietnam. JAC-Antimicrob. Resist. 2020, 2, dlaa048. [Google Scholar] [CrossRef]
- Do, N.T.T.; Vu, H.T.L.; Nguyen, C.T.K.; Punpuing, S.; Khan, W.A.; Gyapong, M.; Asante, K.P.; Munguambe, K.; Gómez-Olivé, F.X.; John-Langba, J.; et al. Community-based antibiotic access and use in six low-income and middle-income countries: A mixed-method approach. Lancet Glob. Health 2021, 9, e610–e619. [Google Scholar] [CrossRef]
- Miller, R.; Goodman, C. Do chain pharmacies perform better than independent pharmacies? Evidence from a standardised patient study of the management of childhood diarrhoea and suspected tuberculosis in urban India. BMJ Glob. Health 2017, 2, e000457. [Google Scholar] [CrossRef] [PubMed]
- Sabde, Y.D.; Diwan, V.; Saraf, V.S.; Mahadik, V.K.; Diwan, V.K.; De Costa, A. Mapping private pharmacies and their characteristics in Ujjain district, Central India. BMC Health Serv. Res. 2011, 11, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, R.; Sharland, M.; Hsia, Y.; Magrini, N.; Moja, L.; Siyam, A.; Tayler, E. Measuring antibiotic availability and use in 20 low- and middle-income countries. Bull. World Health Organ. 2020, 98, 177–187C. [Google Scholar] [CrossRef] [PubMed]
- Schäfermann, S.; Neci, R.; Ndze, E.N.; Nyaah, F.; Pondo, V.B.; Heide, L. Availability, prices and affordability of selected antibiotics and medicines against non-communicable diseases in western Cameroon and northeast DR Congo. PLoS ONE 2020, 15, e0227515. [Google Scholar] [CrossRef] [PubMed]
- Nahar, P.; Kannuri, N.K.; Mikkilineni, S.; Murthy, G.; Phillimore, P. At the margins of biomedicine: The ambiguous position of ‘Registered Medical Practitioners’ in rural Indian healthcare. Sociol. Health Illn. 2016, 39, 614–628. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, S.S.; Iqbal, M.; Hanifi SM, A.; Wahed, T.; Bhuiya, A. Are ‘Village Doctors’ in Bangladesh a curse or a blessing? BMC Int. Health Hum. Rights 2010, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Sudhinaraset, M.; Ingram, M.; Lofthouse, H.K.; Montagu, D. What is the Role of Informal Healthcare Providers in Developing Countries? A Systematic Review. PLoS ONE 2013, 8, e54978. [Google Scholar] [CrossRef]
- Mackintosh, M.; Channon, A.; Karan, A.; Selvaraj, S.; Cavagnero, E.; Zhao, H. What is the private sector? Understanding private provision in the health systems of low-income and middle-income countries. Lancet 2016, 388, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Liow, E.; Kassam, R.; Sekiwunga, R. How unlicensed drug vendors in rural Uganda perceive their role in the management of childhood malaria. Acta Trop. 2016, 164, 455–462. [Google Scholar] [CrossRef]
- Wulandari, L.; Wiseman, V. Engaging the private sector to improve antimicrobial use in the community (Editorial). Public Health Prev. Med. Arch. 2018, 6, 79–81. [Google Scholar] [CrossRef]
- Suy, S.; Rego, S.; Bory, S.; Chhorn, S.; Phou, S.; Prien, C.; Heng, S.; Wu, S.; Legido-Quigley, H.; Hanefeld, J.; et al. Invisible medicine sellers and their use of antibiotics: A qualitative study in Cambodia. BMJ Glob. Health 2019, 4, e001787. [Google Scholar] [CrossRef] [PubMed]
- Shahrawat, R.; Rao, K.; Bhatnagar, A. Composition and distribution of the health workforce in India: Estimates based on data from the National Sample Survey. WHO South-East Asia J. Public Health 2016, 5, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, J.; Holla, A.; Mohpal, A.; Muralidharan, K. Quality and Accountability in Healthcare Delivery Audit Evidence from Primary Care Providers in India; Policy Research Working Paper 7334; World Bank: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Khare, S.; Pathak, A.; Purohit, M.R.; Sharma, M.; Marrone, G.; Tamhankar, A.J.; Lundborg, C.S.; Diwan, V. Determinants and pathways of healthcare-seeking behaviours in under-5 children for common childhood illnesses and antibiotic prescribing: A cohort study in rural India. BMJ Open 2021, 11, e052435. [Google Scholar] [CrossRef] [PubMed]
- Gautham, M.; Shyamprasad, K.M.; Singh, R.; Zachariah, A.; Bloom, G. Informal rural healthcare providers in North and South India. Health Policy Plan. 2014, 29, i20–i29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautham, M.; Spicer, N.; Chatterjee, S.; Goodman, C. What are the challenges for antibiotic stewardship at the community level? An analysis of the drivers of antibiotic provision by informal healthcare providers in rural India. Soc. Sci. Med. 2021, 275, 113813. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.; MacGregor, H.N. Knowledge, legitimacy and economic practice in informal markets for medicine: A critical review of research. Soc. Sci. Med. 2010, 71, 1593–1600. [Google Scholar] [CrossRef]
- Chandra, S.; Bhattacharya, S. Unqualified Medical Practitioners: Their Illegal but Indispensable Role in Primary Healthcare. Econ. Political Wkly. 2019, 54, 36–44. [Google Scholar]
- Nair, M.; Tripathi, S.; Mazumdar, S.; Mahajan, R.; Harshana, A.; Pereira, A.; Jimenez, C.; Halder, D.; Burza, S. Knowledge, attitudes, and practices related to antibiotic use in Paschim Bardhaman District: A survey of healthcare providers in West Bengal, India. PLoS ONE 2019, 14, e0217818. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Chowdhury, A.; Hussam, R.; Banerjee, A.V. The impact of training informal health care providers in India: A randomized controlled trial. Science 2016, 354, aaf7384. [Google Scholar] [CrossRef]
- Nahar, P.; Unicomb, L.; Lucas, P.; Uddin, M.R.; Islam, M.A.; Alam Nizame, F.; Khisa, N.; Akter, S.M.S.; Rousham, E.K. What contributes to inappropriate antibiotic dispensing among qualified and unqualified healthcare providers in Bangladesh? A qualitative study. BMC Health Serv. Res. 2020, 20, 656. [Google Scholar] [CrossRef] [PubMed]
- WHO. Measuring Medicine Prices, Availability, Affordability and Price Components, 2nd ed.; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Holloway, K.A.; Batmanabane, G.; Puri, M.; Tisocki, K. Antibiotic use in South East Asia and policies to promote appropriate use: Reports from country situational analyses. BMJ 2017, 358, j2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Khare, S.; Purohit, M.; Sharma, M.; Tamhankar, A.J.; Lundborg, C.S.; Diwan, V.; Pathak, A. Antibiotic Prescribing by Informal Healthcare Providers for Common Illnesses: A Repeated Cross-Sectional Study in Rural India. Antibiotics 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. ATC Structure and Principles. 2020, WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2021. Norwegin Institute for Public Health, Oslo, Norway. Available online: https://www.whocc.no/atc/structure_and_principles/ (accessed on 29 March 2022).
- GOI. The Indian Drugs and Cosmetics Act, 1940 (as amended upto 31st December, 2016); Ministry of Health and Family Welfare: New Delhi, India, 2016.
- GOI. List of Drugs Prohibited for Manufacture and Sale through Gazette Notifications under Section 26a of Drugs & Cosmetics Act 1940 by the Ministry of Health and Family Welfare. 2018. Available online: https://cdsco.gov.in/opencms/opencms/en/consumer/List-Of-Banned-Drugs/ (accessed on 20 December 2020).
- WHO. WHO Model Formulary; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- GoWB. Minimum Rates of Wages in the Employment of Agriculture in West Bengal. No. 1374/Stat/2RW/16/94/LCS/JLC; Office of the Labour Commissioner, Government of West Bengal: Kolkata, India, 2016.
- Conteh, L.; Hanson, K. Methods for studying private sector supply of public health products in developing countries: A conceptual framework and review. Soc. Sci. Med. 2003, 57, 1147–1161. [Google Scholar] [CrossRef]
- O’Connell, K.A.; Poyer, S.; Solomon, T.; Munroe, E.; Patouillard, E.; Njogu, J.; Evance, I.; Hanson, K.; Shewchuk, T.; Goodman, C. Methods for implementing a medicine outlet survey: Lessons from the anti-malarial market. Malar. J. 2013, 12, 52. Available online: http://www.malariajournal.com/content/12/1/52 (accessed on 29 March 2022). [CrossRef] [Green Version]
- Gandra, S.; Kotwani, A. Need to improve availability of “access” group antibiotics and reduce the use of “watch” group antibiotics in India for optimum use of antibiotics to contain antimicrobial resistance. J. Pharm. Policy Pr. 2019, 12, 20. [Google Scholar] [CrossRef]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2020, 21, 107–115. [Google Scholar] [CrossRef]
- GOI. Drugs Prices Control Order, 2013; Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India: New Delhi, India, 2013.
- GOI. ORDER: Price Revision as per Annual Wholesale Index, 1st April 2017; National Pharmaceutical Pricing Authority, Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India: New Delhi, India, 2017.
- Babar, Z.U.D.; Ibrahim, M.I.M.; Singh, H.; Bukahri, N.I.; Creese, A. Evaluating Drug Prices, Availability, Affordability, and Price Components: Implications for Access to Drugs in Malaysia. PLOS Med. 2007, 4, e82. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Ramachandran, S.S. Fixed dose drug combinations: Issues and challenges in India. Indian J. Pharmacol. 2016, 48, 347–349. [Google Scholar] [CrossRef]
- Phadnis, A. Supreme Court Restrains Govt from Banning 15 Combination Drugs, in Business Standard. 2018. Available online: https://www.business-standard.com/article/companies/supreme-court-restrains-govt-from-banning-15-fixed-dose-drugs-118090701000_1.html (accessed on 3 January 2021).
- Abbott. Why Branded Generics Matter. Available online: https://www.abbott.com/corpnewsroom/strategy-and-strength/why-branded-generic-matter.html (accessed on 7 February 2022).
- IBEF. Indian Pharmaceuticals. March 2021. Available online: https://www.ibef.org/download/Pharmaceuticals-March-2021.pdf (accessed on 7 February 2022).
- Atal, S.; Atal, S.; Deshmankar, B.; Nawaz, S.A. Cost analysis of commonly used drugs under price control in India: Assessing the effect of drug price control order on brand price variation. Int. J. Pharm. Pharm. Sci. 2016, 8, 2016. [Google Scholar]
- Reeler, A.V. Anthropological perspectives on injections: A review. Bull. World Health Organ. 2000, 78, 135–143. [Google Scholar] [PubMed]
- Gautham, M.; Alarcon, P.; Bhattacharyya, S.; Bloom, G.; Chowdhury, A.; Ebata, A.; Goodman, C.; Hashiguchi, L.; Mateus, A.L.P.; Samanta, I.; et al. A Multi-Stakeholder Approach Towards Operationalising Antibiotic Stewardship in India’s Pluralistic Rural Health System. Available online: https://gtr.ukri.org/projects?ref=MR%2FS013598%2F1#/tabOverview (accessed on 29 March 2022).
- ICMR. Treatment Guidelines for Antimicrobial Use in Common Syndromes, 2nd ed.; Indian Council of Medical Research: New Delhi, India, 2019.
- GoWB. State Statistical Handbook 2014; Bureau of Applied Economics & Statistics, Department of Statistics & Programme Implementation, Government of West Bengal: Kolkata, India, 2014.
- GOWB. District Human Development Report: Birbhum; Development & Planning Department, Government of West Bengal: Kolkata, India, 2009.
- GOWB. District Human Development Report: South 24 Parganas; Development and Planning Department, Government of West Bengal: Kolkata, India, 2009.

| Characteristics | % of IPs (n = 196) |
|---|---|
| Gender | |
| Male | 98% |
| Age | |
| <35 years | 23.5% |
| 36–45 years | 31.5% |
| 46–55 years | 22.5% |
| >55 years | 22.5% |
| School education | |
| Up to Class 10 | 34% |
| Up to Class 12 | 31% |
| Graduate or postgraduate | 35% |
| Years of practice | |
| <10 years | 32% |
| 10.1–20 years | 32% |
| >20 years | 36% |
| Any allied health certification | 73% |
| Worked as a compounder to formal doctors | 83% |
| Operate out of a small clinic | 100% |
| Days per week the clinic is open | |
| 7 days | 75% |
| 6 days | 21% |
| 5 days or less | 4% |
| Mode of using antibiotics | |
| Dispense | 95% |
| Prescribe | 86% |
| Health services | |
| Outpatient care for common illnesses (fevers, diarrhea and cold/cough) | 97% |
| Home visits (on call) | 92% |
| Inpatient care | 15% |
| Diabetes | 66% |
| Hypertension | 91% |
| Dental care | 91% |
| Eye care | 86% |
| Wound suturing | 90% |
| Small surgeries (e.g., draining an abscess) | 78% |
| Piles | 5% |
| Delivery care | 26% |
| Abortions | 19% |
| Animal healthcare (mainly cattle and poultry) | 40% |
| Tablets | Syrups/Suspensions/Drops | Injections | |||
|---|---|---|---|---|---|
| Antibiotic | % of IPs Stocking | Antibiotic | % of IPs Stocking | Antibiotic | % of IPs Stocking |
| Amoxicillin | 29% | Amoxicillin potassium clavulanate | 37% | Amikacin | 6% |
| Amoxicillin potassium clavulanate | 23% | Cefixime | 30% | Cefotaxime | 4% |
| Ciprofloxacin | 20% | Cefpodoxime | 13% | Ceftriaxone | 4% |
| Azithromycin | 17% | Azithromycin | 9% | Ampicillin | 1% |
| Cefixime | 15% | Ofloxacin | 8% | Gentamicin | 1% |
| Antibiotic (Number of IPs Stocking) | DDDs Available, Median (IQR) | DDDs Dispensed Last 7 Days, Median (IQR) | No. of Patients Dispensed Antibiotic Last 7 Days, Median (IQR) | DDDs Dispensed Per Patient, Median (IQR) |
|---|---|---|---|---|
| TABLETS | ||||
| Amoxicillin (n = 56) | 10 (6, 15) | 10 (7, 20) | 7 (3, 11) | 2 (1, 3) |
| Amoxicillin potassium clavulanate (n = 45) | 10 (6, 12) | 7 (5, 20) | 4 (2, 7) | 2 (2, 3) |
| Ciprofloxacin (n = 38) | 15 (10, 19) | 15 (8, 25) | 5 (3, 10) | 3 (2, 4) |
| Azithromycin (n = 36) | 25 (10, 40) | 20 (15, 50) | 4 (3, 7) | 5 (5, 7) |
| Cefixime (n = 30) | 15 (10, 20) | 10 (8, 25) | 4 (2, 7) | 4 (2, 5) |
| SYRUPS/SUSPENSIONS/DROPS | ||||
| Amoxicillin potassium clavulanate (n = 72) | 2 (1, 4) | 4 (2, 6) | 4 (1, 7) | 1 (1, 1) |
| Cefixime (n = 59) | 2 (1, 3) | 3 (1, 8) | 4 (2, 10) | 1 (0.50, 1) |
| Cefpodoxime (n = 26) | 1.5 (1.50, 3) | 2 (1.5, 7.50) | 3 (2, 7) | 0.75 (0.75, 0.75) |
| Azithromycin (n = 18) | 4 (2, 6) | 6 (2, 10) | 3 (1, 5) | 2 (2, 2) |
| Ofloxacin (n = 17) | 1.50 (0.75, 3) | 2 (1.50, 4.50) | 2 (1, 4) | 0.75 (0.75, 0.75) |
| INJECTIONS | ||||
| Amikacin (n = 13) | 1.5 (0.75, 3) | 2 (0.50, 4.50) | 4 (2, 6) | 0.50 (0.25, 0.75) |
| Cefotaxime (n = 9) | 0.19 (0.08, 0. 25) | 0.31 (0.13, 0.63) | 7 (4, 10) | 0.06 (0.01, 0.08) |
| Ceftriaxone (n = 8) | 1.25 (0.70, 1.60) | 1.46 (0.22, 5.50) | 3 (1, 11) | 0.46 (0.19, 0.50) |
| Ampicillin (n = 2) | 2.29 (0.83, 3.75) | 2.94 (1.38, 4.50) | 17 (5, 30) | 0.21 (0.15, 0.30) |
| Gentamicin (n = 2) | 1.91 (0.50, 3.33) | 0.75 (0.17, 1.33) | 4 (4, 5) | 0.18 (0.03, 0.33) |
| Antibiotic (Number of Products Available in Different Clinics) | Brand Name, Manufacturer | Median Retail Price in INR/USD for Standard Course * |
|---|---|---|
| TABLETS | ||
| Amoxicillin potassium clavulanate | Total brands: 20 | |
| Lowest priced brand (n = 1) | Medimox Plus, Alkem | 89/1.36 |
| Most stocked brand (n = 11) | Clavam 625, Alkem | 357/5.49 |
| Highest priced brand (n = 1) | Augmentin 100, Medreich | 564/8.67 |
| Price ratio: highest:lowest | 6.2 | |
| Cefixime | Total brands: 19 | |
| Lowest priced brand (n = 1) | Sefbactum, Akums | 76/1.16 |
| Most stocked brand (n = 6) | Taxim-O, Alkem | 125/1.92 |
| Highest priced brand (n = 1) | Noxcef-O, Embark | 252/3.87 |
| Price ratio: highest:lowest | 3.3 | |
| Amoxicillin | Total brands: 11 | |
| Lowest priced brand (n = 1) | Moxileb, Leben | 109/1.67 |
| Most stocked brand (n = 17) | Wymox, Pfizer | 125/1.92 |
| Highest priced brand (n = 1) | Moxilium, Biochem | 244/3.75 |
| Price ratio: highest:lowest | 2.2 | |
| Azithromycin | Total brands: 22 | |
| Lowest priced brand (n = 1) | Zedcin-500, Medmark | 21/0.32 |
| Most stocked brand (n = 6) | Azithral, Alembic | 66/1.00 |
| Highest priced brand (n = 2) | Trulimax, Pfizer | 71/1.09 |
| Price ratio: highest:lowest | 3.6 | |
| Ciprofloxacin | Total brands: 14 | |
| Lowest priced brand (n = 1) | CPGLO-500, Arose Pharma | 17/ 0.26 |
| Most stocked brand (n = 11) | Cifran, Sun Pharma | 48/0.73 |
| Highest priced brand (n = 2) | Cifran, Ranbaxy | 108/1.66 |
| Price ratio: highest:lowest | 5.6 | |
| SYRUPS/SUSPENSIONS/DROPS | ||
| Amoxicillin potassium clavulanate | Total Brands: 28 | |
| Lowest priced brand (n = 1) | Clavactum, Park Pharma | 372/5.72 |
| Most stocked brand (n = 25) | Clavam, Alkem | 507/7.80 |
| Highest priced brand (n = 1) | Safemox-CL, Magma Allianz | 622/9.56 |
| Price ratio: highest:lowest | 1.7 | |
| Cefixime | Total Brands: 19 | |
| Lowest priced brand (n = 2) | Zofix, Alembic | 203/3.12 |
| Most stocked brand (n = 30) | Taxim-O, Alkem | 392/6.03 |
| Highest priced brand (n = 1) | Cefix, Cipla | 849/13.06 |
| Price ratio: highest:lowest | 4.2 | |
| Cefpodoxime | Total Brands: 17 | |
| Lowest priced brand (n = 1) | Opox-CV 50, Hetero | 140/2.15 |
| Most stocked brand (n = 7) | Monocef-O, Aristo | 653/10.04 |
| Highest priced brand (n = 1) | Cefpo-CV 50, Finecure | 887/13.64 |
| Price ratio: highest:lowest | 6.3 | |
| Azithromycin | Total Brands: 7 | |
| Lowest priced brand (n = 1) | Azid, Indi Pharma | 65/1.00 |
| Most stocked brand (n = 4) | Azithral, Alembic | 99/1.52 |
| Highest priced brand (n = 1) | Zithrocin, Biochem | 200/3.07 |
| Price ratio: highest:lowest | 3.1 | |
| Ofloxacin | Total Brands: 10 | |
| Lowest priced brand (n = 4) | OFM, Lekar Pharma | 117/1.80 |
| Most stocked brand (n = 7) | O2, Medley | 420/6.46 |
| Highest priced brand (n = 1) | Powergyl, Cipla | 569/8.75 |
| Price ratio: highest:lowest | 4.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautham, M.; Miller, R.; Rego, S.; Goodman, C. Availability, Prices and Affordability of Antibiotics Stocked by Informal Providers in Rural India: A Cross-Sectional Survey. Antibiotics 2022, 11, 523. https://doi.org/10.3390/antibiotics11040523
Gautham M, Miller R, Rego S, Goodman C. Availability, Prices and Affordability of Antibiotics Stocked by Informal Providers in Rural India: A Cross-Sectional Survey. Antibiotics. 2022; 11(4):523. https://doi.org/10.3390/antibiotics11040523
Chicago/Turabian StyleGautham, Meenakshi, Rosalind Miller, Sonia Rego, and Catherine Goodman. 2022. "Availability, Prices and Affordability of Antibiotics Stocked by Informal Providers in Rural India: A Cross-Sectional Survey" Antibiotics 11, no. 4: 523. https://doi.org/10.3390/antibiotics11040523
APA StyleGautham, M., Miller, R., Rego, S., & Goodman, C. (2022). Availability, Prices and Affordability of Antibiotics Stocked by Informal Providers in Rural India: A Cross-Sectional Survey. Antibiotics, 11(4), 523. https://doi.org/10.3390/antibiotics11040523






