Plasmid-Mediated Transfer of Antibiotic Resistance Genes in Soil
Abstract
:1. Introduction
2. Comparison of Plasmid Extraction and Analysis Methods
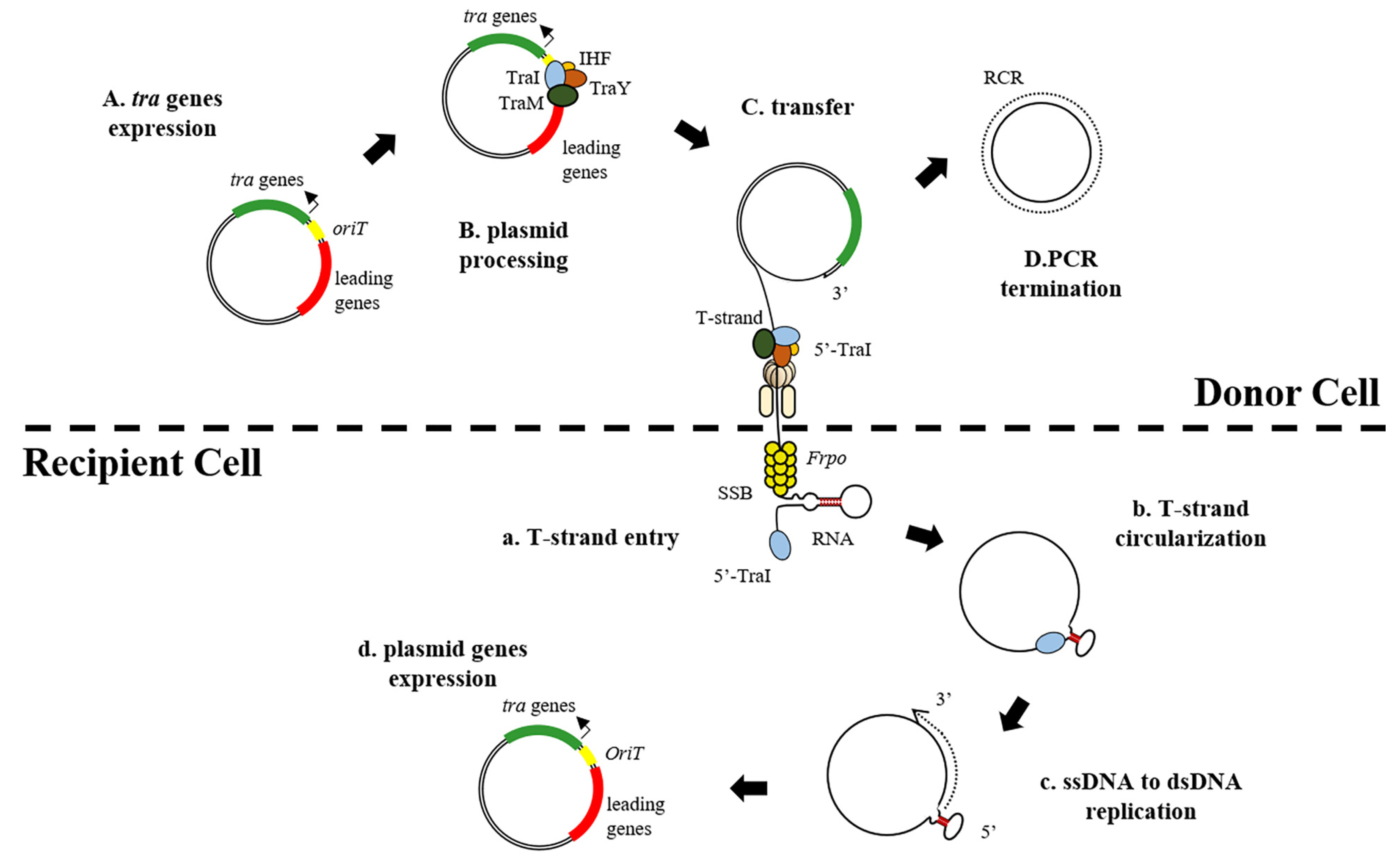
3. Plasmid Transfer Mechanisms
3.1. Within the Donor Cell
3.2. Within the Receptor Cell
4. Plasmid-Mediated Transfer of Antibiotic Resistance Genes
4.1. Presence of ARGs in the Natural Environment
4.2. Prevalence and Spread of ARGs under Antibiotic Selection Pressure
4.2.1. Transfer of ARGs from Severely Contaminated Sites
4.2.2. Human Activities Affect the Transfer of ARGs in the Environment
4.3. Transfer of ARGs under Other Selection Pressures
5. Phage-Mediated Transfer of Antibiotic Resistance Genes
6. One Health Approach of Antibiotics Resistance
7. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Checcucci, A.; Trevisi, P.; Luise, D.; Modesto, M.; Blasioli, S.; Braschi, I.; Mattarelli, P. Exploring the Animal Waste Resistome: The Spread of Antimicrobial Resistance Genes through the Use of Livestock Manure. Front. Microbiol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Baluja, M.; Abouelnaga, M.; Romalde, J.; Su, J.-Q.; Yu, Y.; Gomez-Lopez, M.; Smets, B.; Zhu, Y.-G.; Graham, D.W. Spatial ecology of a wastewater network defines the antibiotic resistance genes in downstream receiving waters. Water Res. 2019, 162, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Qiu, Z.; Shen, Z.; Zhao, H.; Jin, M.; Li, H.; Liu, W.; Li, J.-W. The Occurrence of the Colistin Resistance Gene mcr-1 in the Haihe River (China). Int. J. Environ. Res. Public Health 2017, 14, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Wen, Q.; Ma, Y.Q.; Yang, C.; Liu, Z.C. Antibiotics pollution in Gonghu Bay in the period of water diversion from Yangtze River to Taihu Lake. Environ. Earth Sci. 2018, 77, 419. [Google Scholar] [CrossRef]
- He, T.; Wang, R.; Liu, D.; Walsh, T.R.; Wang, Y. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Séveno, N.A.; Kallifidas, D.; Smalla, K.; van Elsas, J.D.; Collard, J.-M.; Karagouni, A.D.; Wellington, E.M. Occurrence and reservoirs of antibiotic resistance genes in the environment. Rev. Med. Microbiol. 2002, 13, 15–27. [Google Scholar] [CrossRef]
- Senka, D.; Vladimir, B. Horizontal gene transfer—Emerging multidrug resistance in hospital bacteria. Acta Pharm. Sin. 2003, 024, 519–526. [Google Scholar]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Timmis, K.; Ramos, J.L. The soil crisis: The need to treat as a global health problem and the pivotal role of microbes in prophylaxis and therapy. Microb. Biotechnol. 2021, 14, 769–797. [Google Scholar] [CrossRef]
- Kav, A.B.; Benhar, I.; Mizrahi, I. A method for purifying high quality and high yield plasmid DNA for metagenomic and deep sequencing approaches. J. Microbiol. Methods 2013, 95, 272–279. [Google Scholar]
- Jones, B.V.; Marchesi, J.R. Transposon-aided capture (TRACA) of plasmids resident in the human gut mobile metagenome. Nat. Methods 2007, 4, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dib, J.R.; Wagenknecht, M.; Farias, M.E.; Meinhardt, F. Strategies and approaches in plasmidome studies—uncovering plasmid diversity disregarding of linear elements? Front. Microbiol. 2015, 6, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kav, A.B.; Sasson, G.; Jami, E.; Doron-Faigenboim, A.; Benhar, I.; Mizrahi, I. Insights into the bovine rumen plasmidome. Proc. Natl. Acad. Sci. USA 2012, 109, 5452–5457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.L.; Norman, A.; Hansen, L.H.; Srensen, S.J. Metamobilomics—Expanding our knowledge on the pool of plasmid encoded traits in natural environments using high-throughput sequencing. Clin. Microbiol. Infect. 2012, 18, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Jrgensen, T.S.; Xu, Z.; Hansen, M.A.; Srensen, S.J.; Hansen, L.H. Hundreds of Circular Novel Plasmids and DNA Elements Identified in a Rat Cecum Metamobilome. PLoS ONE 2014, 9, e87924. [Google Scholar] [CrossRef] [Green Version]
- Lopatkin, A.J.; Meredith, H.R.; Srimani, J.K.; Pfeiffer, C.; Durrett, R.; You, L. Persistence and reversal of plasmid-mediated antibiotic resistance. Nat. Commun. 2017, 8, 1689. [Google Scholar] [CrossRef]
- Von, W.; John, P.; Van, N.; Mills, N.D.; Snehali, M.; Van, A.; Savelkoul, P.; Wolffs, P. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar]
- Jacquiod, S.; Brejnrod, A.; Morberg, S.M.; Al-Soud, W.A.; SøRensen, S.J.; Riber, L. Deciphering conjugative plasmid permissiveness in wastewater microbiomes. Mol. Ecol. 2017, 26, 3556–3571. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Zhang, J.; Liang, P.; Huang, X. Conjugative potential of antibiotic resistance plasmids to activated sludge bacteria from wastewater treatment plants. Int. Biodeterior. Biodegrad. 2019, 138, 33–40. [Google Scholar] [CrossRef]
- Li, L.; Dechesne, A.; He, Z.; Madsen, J.S.; Smets, B.F. Estimating the Transfer Range of Plasmids Encoding Antimicrobial Resistance in a Wastewater Treatment Plant Microbial Community. Environ. Sci. Technol. Lett. 2018, 5, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Kholodii, G.; Mindlin, S.; Petrova, M.; Minakhina, S. Tn 5060 from the Siberian permafrost is most closely related to the ancestor of Tn 21 prior to integron acquisition. FEMS Microbiol. Lett. 2003, 226, 251–255. [Google Scholar] [CrossRef] [Green Version]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D.; Poinar, H. Antibiotic resistance is ancient: Implications for drug discovery. Trends Microbiol. 2012, 20, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 2013, 10, 3983–3996. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Juneau, P.; Huang, R.; He, Z.; Liang, Y. Coexistence between antibiotic resistance genes and metal resistance genes in manure-fertilized soils. Geoderma 2021, 382, 114760. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Gogry, F.A.; Siddiqui, M.T.; Sultan, I.; Haq, Q.M.R. Current Update on Intrinsic and Acquired Colistin Resistance Mechanisms in Bacteria. Front. Med. 2021, 8, 677720. [Google Scholar] [CrossRef]
- Nicoloff, H.; Hjort, K.; Levin, B.R.; Andersson, D.I. The high prevalence of antibiotic heteroresistance in pathogenic bacteria is mainly caused by gene amplification. Nat. Microbiol. 2019, 4, 504–514. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2015, 387, 176–187. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2015, 16, 161–168. [Google Scholar] [CrossRef]
- Kudinova, A.G.; Soina, V.S.; Maksakova, S.A.; Petrova, M.A. Basic Antibiotic Resistance of Bacteria Isolated from Different Biotopes. Microbiology 2019, 88, 739–746. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.; Huang, R.; Cui, Y.; Li, Q.; Zhao, Y.; Wang, X.; Mao, D.; Luo, Y.; Ren, H. Antibiotic Resistance Gene-Carrying Plasmid Spreads into the Plant Endophytic Bacteria using Soil Bacteria as Carriers. Environ. Sci. Technol. 2021, 55, 10462–10470. [Google Scholar] [CrossRef] [PubMed]
- Willms, I.M.; Kamran, A.; Aßmann, N.F.; Krone, D.; Bolz, S.H.; Fiedler, F.; Nacke, H. Discovery of Novel Antibiotic Resistance Determinants in Forest and Grassland Soil Metagenomes. Front. Microbiol. 2019, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Song, D.; Jiang, L.; Zhang, D.; Zhang, G. Large-scale biogeographical patterns of antibiotic resistome in the forest soils across China. J. Hazard. Mater. 2021, 403, 123990. [Google Scholar] [CrossRef] [PubMed]
- Shawver, S.; Wepking, C.; Ishii, S.; Strickland, M.S.; Badgley, B.D. Application of manure from cattle administered antibiotics has sustained multi-year impacts on soil resistome and microbial community structure. Soil Biol. Biochem. 2021, 157, 108252. [Google Scholar] [CrossRef]
- Pu, Q.; Zhao, L.X.; Li, Y.T.; Su, J.Q. Manure fertilization increase antibiotic resistance in soils from typical greenhouse vegetable production bases, China. J. Hazard. Mater. 2020, 391, 122267. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, T.; Cao, Z.; Zhong, S.; Wen, X.; Mi, J.; Ma, B.; Zou, Y.; Zhang, N.; Liao, X.; et al. Antibiotic resistance genes in layer farms and their correlation with environmental samples. Poult. Sci. 2021, 100, 101485. [Google Scholar] [CrossRef]
- Laconi, A.; Mughini-Gras, L.; Tolosi, R.; Grilli, G.; Trocino, A.; Carraro, L.; Di Cesare, F.; Cagnardi, P.; Piccirillo, A. Microbial community composition and antimicrobial resistance in agricultural soils fertilized with livestock manure from conventional farming in Northern Italy. Sci. Total Environ. 2021, 760, 143404. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kim, S.; Lee, Y.M.; Shin, S.C. Characterization of antimicrobial resistance genes and virulence factor genes in an Arctic permafrost region revealed by metagenomics. Environ. Pollut. 2022, 294, 118634. [Google Scholar] [CrossRef]
- Mootapally, C.; Nathani, N.M.; Poriya, P.; Beleem, I.; Dabhi, J.C.; Gadhvi, I.R.; Joshi, C.G. Antibiotic Resistome Biomarkers associated to the Pelagic Sediments of the Gulfs of Kathiawar Peninsula and Arabian Sea. Sci. Rep. 2019, 9, 17281. [Google Scholar] [CrossRef] [Green Version]
- Nathani, N.M.; Mootapally, C.; Dave, B.P. Antibiotic resistance genes allied to the pelagic sediment microbiome in the Gulf of Khambhat and Arabian Sea. Sci. Total Environ. 2018, 653, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Fu, B.; Shi, X.; Sun, C.; Wu, C. Contaminated in-house environment contributes to the persistence and transmission of NDM-producing bacteria in a Chinese poultry farm. Environ. Int. 2020, 139, 105715. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.G.; Zhang, R.M.; Wang, L.L.; Sun, R.Y.; Bai, S.C.; Han, L.; Fang, L.X.; Sun, J.; Liu, Y.H.; Liao, X.P. Molecular epidemiology of carbapenemase-producing Escherichia coli from duck farms in south-east coastal China. J. Antimicrob. Chemother. 2020, 76, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Hassan, B.; Martins, W.M.; Li, R.; Abdullah, S.; Sands, K.; Walsh, T.R. Emergence of plasmid-mediated tigecycline resistance tet(X4) gene in Escherichia coli isolated from poultry, food and the environment in South Asia. Sci. Total Environ. 2021, 787, 147613. [Google Scholar] [CrossRef] [PubMed]
- Cwiek, K.; Wozniak-Biel, A.; Karwanska, M.; Siedlecka, M.; Lammens, C.; Rebelo, A.R.; Hendriksen, R.S.; Kuczkowski, M.; Chmielewska-Wladyka, M.; Wieliczko, A. Phenotypic and genotypic characterization of mcr-1-positive multidrug-resistant Escherichia coli ST93, ST117, ST156, ST10, and ST744 isolated from poultry in Poland. Braz. J. Microbiol. 2021, 52, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Gong, X.; Sun, Y. Characteristics of two transferable aminoglycoside resistance plasmids in Escherichia coli isolated from pig and chicken manure. Front. Environ. Sci. Eng. 2019, 13, 15. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Wang, J.; Wang, J.; Zhu, L.; Ge, W. Macrolide- and quinolone-resistant bacteria and resistance genes as indicators of antibiotic resistance gene contamination in farmland soil with manure application. Ecol. Indic. 2019, 106, 105456. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Zhu, L.; Wang, J. Field-based evidence for enrichment of antibiotic resistance genes and mobile genetic elements in manure-amended vegetable soils. Sci. Total Environ. 2019, 654, 906–913. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total Environ. 2017, 621, 990–999. [Google Scholar] [CrossRef]
- Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Hong, C.J.C.E.J. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar]
- Bahram, M.; Hildebrand, F.; Forslund, S.K. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, A.L.; Ermakova, A.Y.; Beletsky, A.V.; Petrova, M.; Mardanov, A.V.; Ravin, N.V. Genome Analysis of Acinetobacter lwoffii Strains Isolated from Permafrost Soils Aged from 15 Thousand to 1.8 Million Years Revealed Their Close Relationships with Present-Day Environmental and Clinical Isolates. Biology 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Belov, A.A.; Cheptsov, V.S.; Manucharova, N.A.; Ezhelev, Z.S. Bacterial Communities of Novaya Zemlya Archipelago Ice and Permafrost. Geosciences 2020, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Paun, V.I.; Lavin, P.; Chifiriuc, M.C.; Purcarea, C. First report on antibiotic resistance and antimicrobial activity of bacterial isolates from 13,000-year old cave ice core. Sci. Rep. 2021, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Boeckel, T.V.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Szekeres, E.; Baricz, A.; Chiriac, C.M.; Farkas, A.; Opris, O.; Soran, M.L.; Andrei, A.S.; Rudi, K.; Luis Balcazar, J.; Dragos, N. Abundance of antibiotics, antibiotic resistance genes and bacterial community composition in wastewater effluents from different Romanian hospitals. Environ. Pollut. 2017, 225, 304–315. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, Y.H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef]
- Osińska, A.; Harnisz, M.; Korzeniewska, E. Prevalence of plasmid-mediated multidrug resistance determinants in fluoroquinolone-resistant bacteria isolated from sewage and surface water. Environ. Sci. Pollut. Res. 2016, 23, 10818–10831. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Osinska, A.; Korzeniewska, E.; Harnisz, M.; Niestepski, S. The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Sci. Total Environ. 2017, 577, 367. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Larsson, D. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson-Palme, J.; Hammaren, R.; Pal, C.; Östman, M.; Björlenius, B.; Flach, C.F.; Fick, J.; Kristiansson, E.; Tysklind, M.; Joakim Larsson, D.G. Elucidating selection processes for antibiotic resistance in sewage treatment plants using metagenomics. Sci. Total Environ. 2016, 572, 697–712. [Google Scholar] [CrossRef]
- Karkman, A.; Johnson, T.A.; Lyra, C.; Stedtfeld, R.D.; Tamminen, M.; Tiedje, J.M.; Virta, M. High-throughput quantification of antibiotic resistance genes from an urban wastewater treatment plant. FEMS Microbiol. Ecol. 2016, 92, 14. [Google Scholar] [CrossRef] [Green Version]
- Cen, D.J.; Sun, R.Y.; Mai, J.L.; Jiang, Y.W.; Fang, L.X. Occurrence and transmission of blaNDM-producing Enterobacteriaceae from geese and the surrounding environment on a commercial goose farm. Appl. Environ. Microbiol. 2021, 87, 11–21. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zheng, N.; Ge, C.; Yao, H. Occurrence and distribution of antibiotics and antibiotic resistance genes in the guts of shrimp from different coastal areas of China. Sci. Total Environ. 2022, 815, 152756. [Google Scholar] [CrossRef]
- Jo, H.; Raza, S.; Farooq, A.; Kim, J.; Unno, T. Fish Farm Effluents as a Source of Antibiotic Resistance Gene Dissemination on Jeju Island, South Korea. Eeviron. Pollut. 2021, 276, 116764. [Google Scholar] [CrossRef]
- Tamminen, M.; Karkman, A.; Corander, J.; Paulin, L.; Virta, M. Differences in bacterial community composition in Baltic Sea sediment in response to fish farming. Aquaculture 2011, 313, 15–23. [Google Scholar] [CrossRef]
- Muziasari, W.I.; Pärnänen, K.; Johnson, T.A.; Lyra, C.; Karkman, A.; Stedtfeld, R.D.; Tamminen, M.; Tiedje, J.M.; Virta, M. Aquaculture changes the profile of antibiotic resistance and mobile genetic element associated genes in Baltic Sea sediments. FEMS Microbiol. Ecol. 2016, 92, 54. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Lou, C.; Zhai, W.; Tang, X.; Hashmi, M.Z.; Murtaza, R.; Yong, L.; Liu, X.; Xu, J. Increased occurrence of heavy metals, antibiotics and resistance genes in surface soil after long-term application of manure. Sci. Total Environ. 2018, 635, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Feng, Y.; Wang, Y.; Guo, X.; Chu, H.; Lin, X. Prevalence of antibiotic resistance genes in soils after continually applied with different animal manure for 30 years. J. Hazard. Mater. 2017, 340, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Hu, H.-W.; Gou, M.; Wang, J.-T.; Chen, D.; He, J.-Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics—ScienceDirect. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xin, Z.; Zhang, Y.; Chen, J.; Yan, J.; Li, H.; Hu, H. Long-term manure application increased the levels of antibiotics and antibiotic resistance genes in a greenhouse soil. Appl. Soil Ecol. 2017, 121, 193–200. [Google Scholar] [CrossRef]
- Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: A review on their distribution and environmental risk assessment. Trends Environ. Anal. Chem. 2021, 30, e00125. [Google Scholar] [CrossRef]
- Markowicz, A.; Bondarczuk, K.; Wiekiera, A.; Suowicz, S. Is sewage sludge a valuable fertilizer? A soil microbiome and resistome study under field conditions. J. Soils Sediments 2021, 721, 2882–2895. [Google Scholar] [CrossRef]
- Iwu, C.D.; Plessis, E.D.; Korsten, L.; Okoh, A.I. Antibiogram imprints of E. coli O157:H7 recovered from irrigation water and agricultural soil samples collected from two district municipalities in South Africa. Int. J. Environ. Stud. 2021, 213, 1–14. [Google Scholar] [CrossRef]
- Talukder, A.; Rahman, M.M.; Chowdhury, M.M.H.; Mobashshera, T.A.; Islam, N.N. Plasmid profiling of multiple antibiotic-resistant Pseudomonas aeruginosa isolated from soil of the industrial area in Chittagong, Bangladesh. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 44. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, X.; Lin, H.; Wang, J. Antibiotics and Antibiotic Resistance Genes in Sediment of Honghu Lake and East Dongting Lake, China. Microb. Ecol. 2016, 72, 791–801. [Google Scholar] [CrossRef]
- Chen, B.; Liang, X.; Nie, X.; Huang, X.; Zou, S.; Li, X. The role of class I integrons in the dissemination of sulfonamide resistance genes in the Pearl River and Pearl River Estuary, South China. J. Hazard. Mater. 2014, 282, 61–67. [Google Scholar] [CrossRef]
- Li, A.; Chen, L.; Zhang, Y.; Tao, Y.; Xie, H.; Li, S.; Sun, W.; Pan, J.; He, Z.; Mai, C.; et al. Occurrence and distribution of antibiotic resistance genes in the sediments of drinking water sources, urban rivers, and coastal areas in Zhuhai, China. Environ. Sci. Pollut. Res. 2018, 25, 26209–26217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Su, Z.; Dai, T.; Huang, B.; Mu, Q.; Zhang, Y.; Wen, D. Occurrence and distribution of antibiotic resistance genes in the sediments of the East China Sea bays. J. Environ. Sci. 2019, 81, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sun, W.; Zhang, Z.; Chapman, S.J.; Freitag, T.E.; Fu, J.; Zhang, X.; Ma, J. Effects of manure and mineral fertilization strategies on soil antibiotic resistance gene levels and microbial community in a paddy–upland rotation system. Eeviron. Pollut. 2016, 211, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Wang, J.T.; Jing, L.; Shi, X.Z.; Ma, Y.B.; Chen, D.; He, J.Z. Long-Term Nickel Contamination Increases the Occurrence of Antibiotic Resistance Genes in Agricultural Soils. Environ. Sci. Technol. 2017, 51, 790. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, J.; Mao, D.; Luo, Y. Effect of the selective pressure of sub-lethal level of heavy metals on the fate and distribution of ARGs in the catchment scale. Environ. Pollut. 2017, 220, 900–908. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Guo, J. Copper nanoparticles and copper ions promote horizontal transfer of plasmid-mediated multi-antibiotic resistance genes across bacterial genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef]
- Pu, Q.; Fan, X.; Sun, A.; Pan, T.; Su, J.Q. Co-effect of cadmium and iron oxide nanoparticles on plasmid-mediated conjugative transfer of antibiotic resistance genes. Environ. Int. 2021, 152, 106453. [Google Scholar] [CrossRef]
- Klümper, U.; Dechesne, A.; Riber, L.; Brandt, K.K.; Gülay, A.; SøRensen, S.R.J.; Smets, B.F. Metal stressors consistently modulate bacterial conjugal plasmid uptake potential in a phylogenetically conserved manner. ISME J. 2017, 11, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Wu, Y.; Huang, Q.; Cai, P. Insights into conjugative transfer of antibiotic resistance genes affected by soil minerals. Eur. J. Soil Sci. 2020, 72, 1143–1153. [Google Scholar] [CrossRef]
- Li, X.; Wen, C.; Liu, C.; Lu, S.; Xu, Z.; Yang, Q.; Chen, Z.; Liao, H.; Zhou, S. Herbicide promotes the conjugative transfer of multi-resistance genes by facilitating cellular contact and plasmid transfer. J. Environ. Sci. 2022, 115, 363–373. [Google Scholar] [CrossRef]
- Liao, H.; Li, X.; Yang, Q.; Bai, Y.; Zhu, Y.G. Herbicide selection promotes antibiotic resistance in soil microbiomes. Mol. Biol. Evol. 2021, 38, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Valera, F.; Martin-Cuadrado, A.B.; Rodriguez-Brito, B.; Pasic, L.; Thingstad, T.F.; Rohwer, F.; Mira, A. Explaining microbial population genomics through phage predation. Nat. Rev. Microbiol. 2009, 7, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Brigulla, M.; Wackernagel, W. Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues. Appl. Microbiol. Biotechnol. 2010, 86, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Jeon, J.H.; Kang, I.; Park, K.S.; Cho, J.C. Freshwater viral metagenome reveals novel and functional phage-borne antibiotic resistance genes. Microbiome 2020, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Picazo, P.; Roscales, G.; Toribio-Avedillo, D.; Gomez-Gomez, C.; Avila, C.; Balleste, E.; Muniesa, M.; Rodriguez-Rubio, L. Antibiotic Resistance Genes in Phage Particles from Antarctic and Mediterranean Seawater Ecosystems. Microorganisms 2020, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Bearson, B.L.; Brunelle, B.W. Fluoroquinolone induction of phage-mediated gene transfer in multidrug-resistant Salmonella. Int. J. Antimicrob. Agents 2015, 46, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colomer-Lluch, M.; Imamovic, L.; Jofre, J.; Muniesa, M. Bacteriophages carrying antibiotic resistance genes in fecal waste from cattle, pigs, and poultry. Antimicrob Agents Chemother 2011, 55, 4908–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quirós, P.; Colomer-Lluch, M.; Martínez-Castillo, A.; Miró, E.; Argente, M.; Jofre, J.; Navarro, F.; Muniesa, M. Antibiotic Resistance Genes in the Bacteriophage DNA Fraction of Human Fecal Samples. Antimicrob. Agents Chemother. 2014, 58, 606–609. [Google Scholar] [CrossRef] [Green Version]
- WHO. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.un-dp.org/content/undp/en/home/sustainable-development-goals.html (accessed on 20 June 2021).
- Graham, D.W.; Knapp, C.W.; Christensen, B.T.; Mccluskey, S.; Dolfing, J. Appearance of β-lactam Resistance Genes in Agricultural Soils and Clinical Isolates over the 20th Century. Sci. Rep. 2016, 6, 21550. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Xu, M.; Stedtfeld, R.D.; Sheng, H.; Fan, J.; Liu, M.; Chai, B.; de Carvalho, T.S.; Li, H.; Li, Z.; et al. Long-term Effect of Different Fertilization and Cropping Systems on the Soil Antibiotic Resistome. Environ. Sci. Technol. 2018, 52, 13037–13046. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2014, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef] [PubMed]
- Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar]
- Redondo-Salvo, S.; Fernández-López, R.; Ruiz, R.; Vielva, L.; Toro, M.; Rocha, E.; Garcillán-Barcia, M.; Cruz, F. Pathways for horizontal gene transfer in bacteria revealed by a global map of their plasmids. Nat. Commun. 2020, 11, 3602. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Phan, H.T.; Findlay, J.; Stoesser, N.; Pankhurst, L.; Navickaite, I.; De Maio, N.; Eyre, D.W.; Toogood, G.; Orsi, N.M.; et al. Covert dissemination of carbapenemase-producing Klebsiella pneumoniae (KPC) in a successfully controlled outbreak: Long- and short-read whole-genome sequencing demonstrate multiple genetic modes of transmission. J. Antimicrob. Chemother. 2017, 72, 3025–3034. [Google Scholar] [CrossRef]
- Schweizer, C.; Bischoff, P.; Bender, J.; Kola, A.; Gastmeier, P.; Hummel, M.; Klefisch, F.R.; Schoenrath, F.; Fruhauf, A.; Pfeifer, Y. Plasmid-Mediated Transmission of KPC-2 Carbapenemase in Enterobacteriaceae in Critically Ill Patients. Front. Microbiol. 2019, 10, 276. [Google Scholar] [CrossRef] [Green Version]
| ARGs | Antibiotic Types | Origin | References |
|---|---|---|---|
| nonmobile dihydropteroate synthase (DHPS) genes | sulfonamides | Beech and pine forest soils | [33] |
| qnrB, aacC, blaOXY, sulI, sulII, sulIII, tetD, tetA, tetM01, tetW, tetR | quinolone, aminoglycoside, beta-lactam, sulfonamide, tetracycline | Primeval forest soil | [34] |
| tetA, tetL, addD, merA, blaSHV | aminoglycoside, sulfonamides, tetracycline | Manure-amended agricultural soil | [35] |
| aadA, acrA, ampC, blaTEM, blaCTX, ermC, vanTC, vanRA, tetT, tetL | aminoglycoside, beta-lactam, sulfonamides, tetracycline, vancomycin | Greenhouse vegetable production bases | [36] |
| tetA, tetQ, tetX, tetM, blaTEM, sul1, sul2, strB, qnrS, ermB, ermC, oqxB, cfr | quinolone, beta-lactam, sulfonamide, tetracycline, chloromycetin, streptomycin | Layer farm soil | [37,38] |
| rpoB2, rpoB, rphA, mdtB, mdtC, vanRO | rifamycin, aminocoumarin, glycopeptide | Arctic permafrost zone | [39] |
| carA, macB, bcrA, taeA, srmB, tetA, oleC, sav1866, tlrC | macrolides, glycopeptides, tetracyclines | Deep-sea sediments | [40,41] |
| blaNDM, blaTEM, tet (X4), tetA, tetB, sulI, sulII, sulIII | beta-lactam, tetracycline, sulfonamides | Farm, Aquaculture wastewater | [42,43,44,45] |
| arr-3, aacA, qnrS, ermB, tetW, tetO, sulI, sulII | aminoglycoside, macrolides, quinolone, tetracycline, sulfonamide | Domestic wastewater, Medical wastewater | [46,47,48,49,50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, M.; Li, Y.; Yao, H. Plasmid-Mediated Transfer of Antibiotic Resistance Genes in Soil. Antibiotics 2022, 11, 525. https://doi.org/10.3390/antibiotics11040525
Meng M, Li Y, Yao H. Plasmid-Mediated Transfer of Antibiotic Resistance Genes in Soil. Antibiotics. 2022; 11(4):525. https://doi.org/10.3390/antibiotics11040525
Chicago/Turabian StyleMeng, Miaoling, Yaying Li, and Huaiying Yao. 2022. "Plasmid-Mediated Transfer of Antibiotic Resistance Genes in Soil" Antibiotics 11, no. 4: 525. https://doi.org/10.3390/antibiotics11040525
APA StyleMeng, M., Li, Y., & Yao, H. (2022). Plasmid-Mediated Transfer of Antibiotic Resistance Genes in Soil. Antibiotics, 11(4), 525. https://doi.org/10.3390/antibiotics11040525






