Antibiotic Resistance of Salmonella Typhimurium Monophasic Variant 1,4,[5],12:i:-in China: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
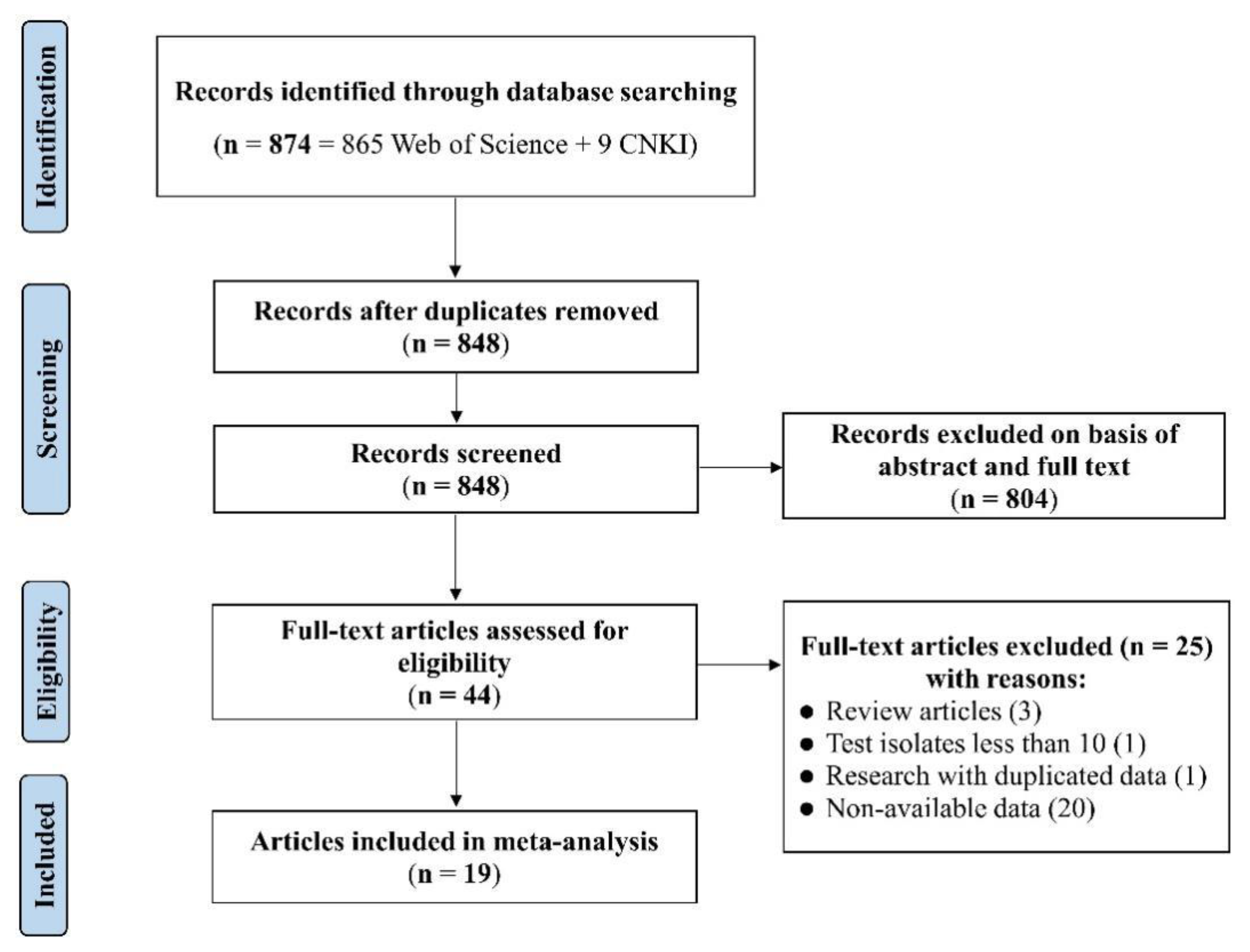
2.1. Characteristics of Eligible Studies and Datasets
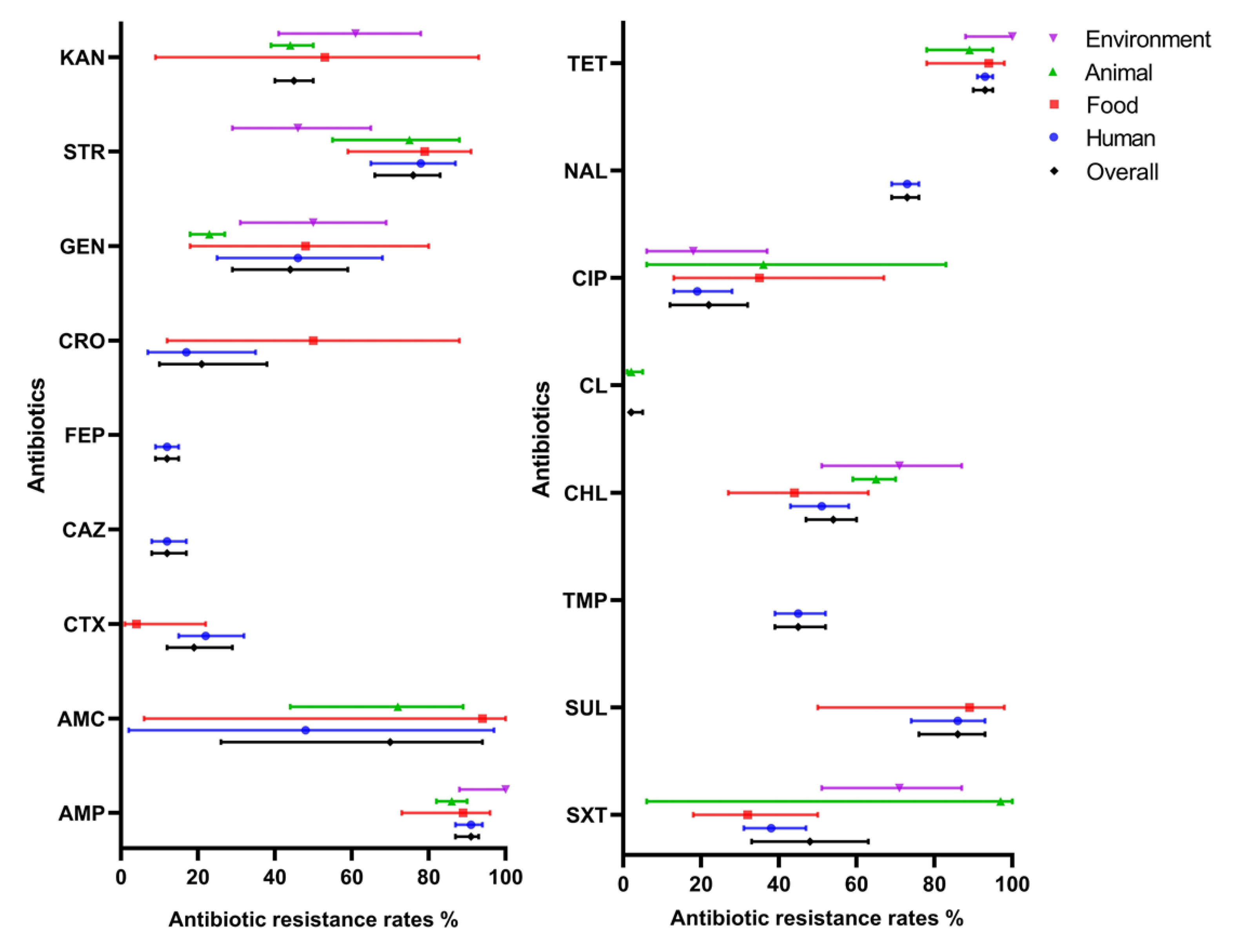
2.2. Pooled Prevalence of Antibiotic Resistance in S. 1,4,[5],12:i:-
2.3. Pooled Prevalence of Multiple-Drug Resistance in S. 1,4,[5],12:i:-
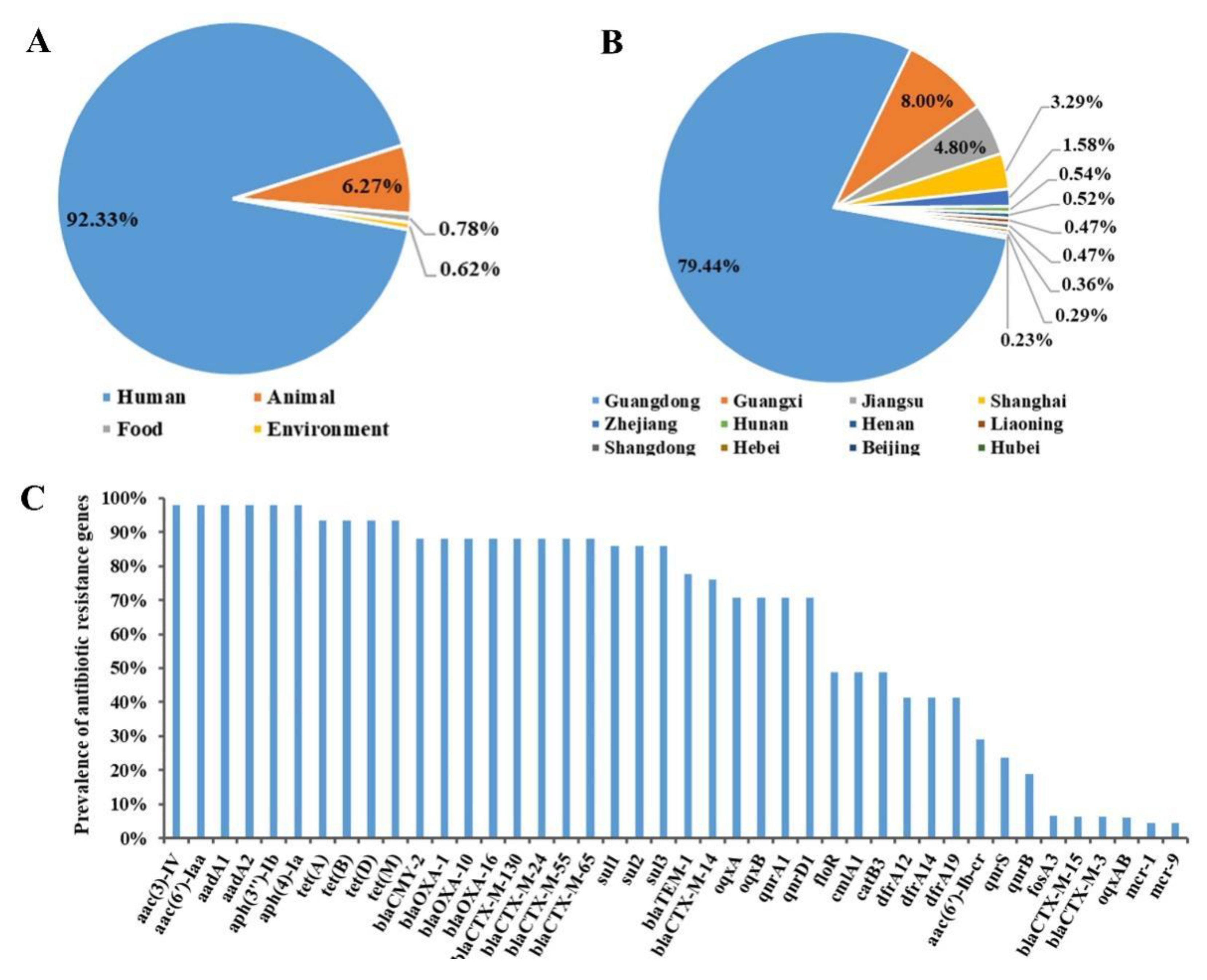
2.4. Prevalence of Antibiotic-Resistant S. 1,4,[5],12:i:- from Different Isolated Sources
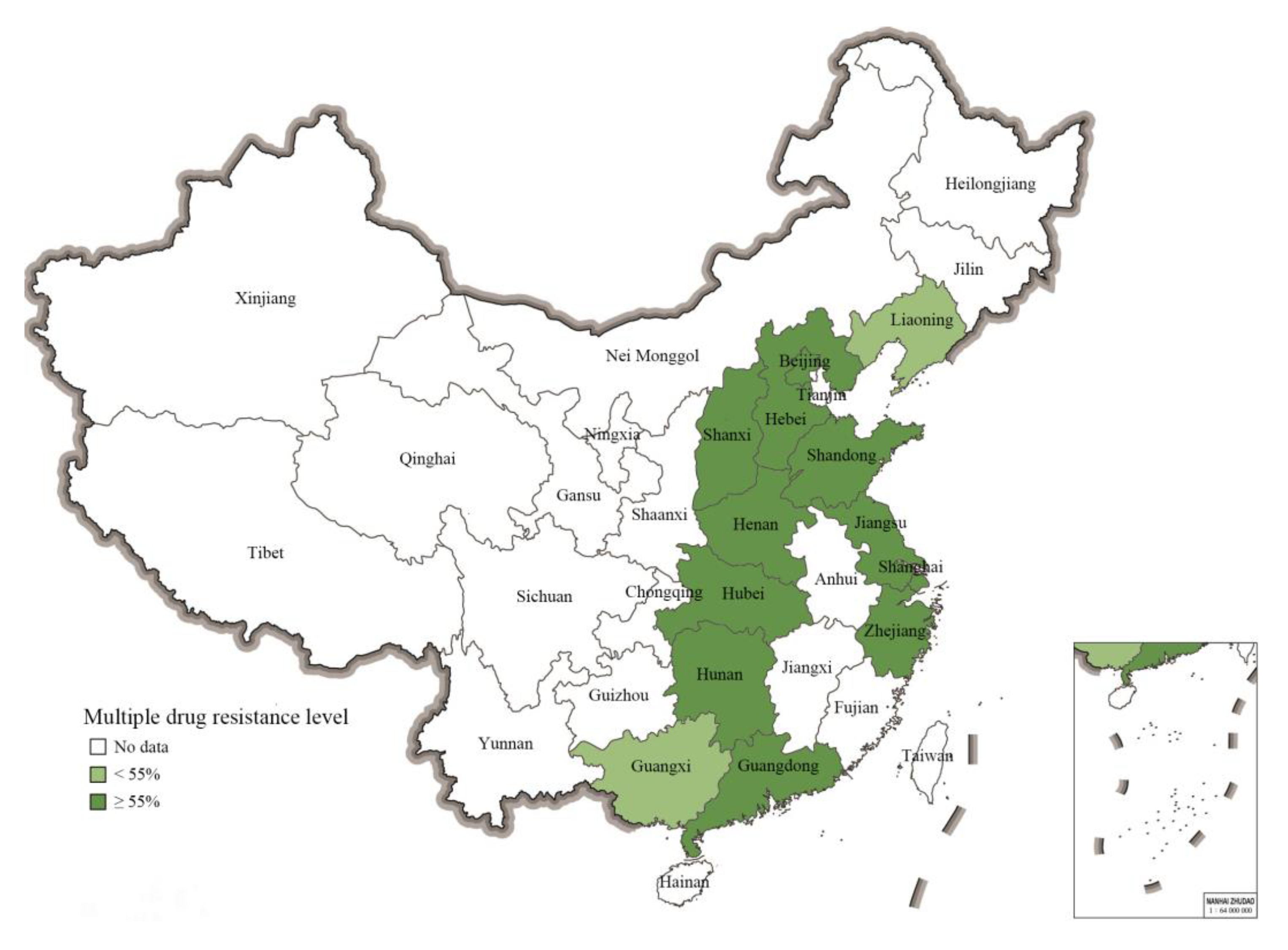
2.5. Prevalence of Multiple-Resistant S. 1,4,[5],12:i:- from Different Geographical Regions in China
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Selection Criteria
4.3. Data Extraction
4.4. Meta-Analysis and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seif, Y.; Kavvas, E.; Lachance, J.C.; Yurkovich, J.T.; Nuccio, S.P.; Fang, X.; Catoiu, E.; Raffatellu, M.; Palsson, B.O.; Monk, J.M. Genome-scale metabolic reconstructions of multiple Salmonella strains reveal serovar-specific metabolic traits. Nat. Commun. 2018, 9, 3771. [Google Scholar] [CrossRef] [PubMed]
- Sholpan, A.; Lamas, A.; Cepeda, A.; Franco, C.M. Salmonella spp. quorum sensing: An overview from environmental persistence to host cell invasion. AIMS Microbiol. 2021, 7, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microb. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Center for Disease Prevention and Control. The European Union one health 2018 zoonoses report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- Switt, A.I.M.; Soyer, Y.; Warnick, L.D.; Wiedmann, M. Emergence, distribution, and molecular and phenotypic characteristics of Salmonella enterica serotype 4,5,12: I:-. Foodborne Pathog. Dis. 2009, 6, 407–415. [Google Scholar] [CrossRef]
- Ido, N.; Lee, K.; Iwabuchi, K.; Izumiya, H.; Uchida, I.; Kusumoto, M.; Iwata, T.; Ohnishi, M.; Akiba, M. Characteristics of Salmonella enterica Serovar 4,[5],12: I:- as a monophasic variant of serovar Typhimurium. PLoS ONE 2014, 9, e104380. [Google Scholar] [CrossRef][Green Version]
- Crayford, G.; Coombes, J.L.; Humphrey, T.J.; Wigley, P. Monophasic expression of FliC by Salmonella 4,[5],12:i:- DT193 does not alter its pathogenicity during infection of porcine intestinal epithelial cells. Microbiology 2014, 160, 2507–2516. [Google Scholar] [CrossRef]
- Andreoli, G.; Merla, C.; Valle, C.D.; Corpus, F.; Morganti, M.; D’incau, M.; Colmegna, S.; Marone, P.; Fabbi, M.; Barco, L.; et al. Foodborne salmonellosis in Italy: Characterization of Salmonella enterica serovar Typhimurium and monophasic variant 4,[5],12:i- isolated from salami and human patients. J. Food Prot. 2017, 80, 632–639. [Google Scholar] [CrossRef]
- Sun, H.H.; Wan, Y.P.; Du, P.C.; Bai, L. The epidemiology of monophasic Salmonella Typhimurium. Foodborne Pathog. Dis. 2020, 17, 87–97. [Google Scholar] [CrossRef]
- Molina-lópez, R.A.; Vidal, A.; Obón, E.; Martín, M.; Darwich, L. Multidrug-resistant Salmonella enterica serovar Typhimurium monophasic variant 4,12:i:- isolated from asymptomatic wildlife in a Catalonian Wildlife Rehabilitation Center, Spain. J. Wildl. Dis. 2015, 51, 759–763. [Google Scholar] [CrossRef]
- Proroga, Y.T.R.; Mancusi, A.; Peruzy, M.F.; Carullo, M.R.; Montone, A.M.I.; Fulgione, A.; Capuano, F. Characterization of Salmonella Typhimurium and its monophasic variant 1,4,[5],12:i:- isolated from different sources. Folia. Microbiol. 2019, 64, 711–718. [Google Scholar] [CrossRef]
- Elnekave, E.; Hong, S.; Taylor, A.; Boxrud, D.; Rovira, A.; Alvarez, J. Tracing the evolutionary history of an emerging Salmonella 4,[5],12:i:- clone in the United States. Virus Evol. 2019, 5, 19–20. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Gu, D.; Wang, F.; Liu, B.W.; Li, J.W.; Kang, X.L.; Meng, C.; Jiao, X.A.; Pan, Z.M. Prevalence and characteristics of Salmonella spp. from a pig farm in Shanghai, China. Foodborne Pathog. Dis. 2021, 18, 477–488. [Google Scholar] [CrossRef]
- Barco, L.; Barrucci, F.; Cortini, E.; Ramon, E.; Olsen, J.E.; Luzzi, I.; Lettini, A.A.; Ricci, A. Ascertaining the relationship between Salmonella Typhimurium and Salmonella 4,[5],12:i:- by MLVA and inferring the sources of human salmonellosis due to the two serovars in Italy. Front Microbiol. 2015, 6, 301. [Google Scholar] [CrossRef]
- Marder, E.P.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Hurd, S.; Jervis, R.; Lathrop, S.; Muse, A.; Ryan, P.; Smith, K.; et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. Sites, 2006–2017. Mmwr. Morb. Mortal. Wkly. Rep. 2018, 67, 324–328. [Google Scholar] [CrossRef]
- Yang, X.J.; Wu, Q.P.; Zhang, J.M.; Huang, J.H.; Chen, L.; Wu, S.; Zeng, H.Y.; Wang, J.; Chen, M.T.; Wu, H.M.; et al. Prevalence, bacterial load, and antimicrobial resistance of Salmonella serovars isolated from retail meat and meat products in China. Front. Microbiol. 2019, 10, 2121. [Google Scholar] [CrossRef]
- Poonchareon, K.; Pulsrikarn, C.; Nuanmuang, N.; Khamai, P. Effectiveness of BOX-PCR in differentiating genetic relatedness among Salmonella enterica serotype 4,[5],12:i:- isolated from hospitalized patients and minced pork samples in northern Thailand. Int. J. Microbiol. 2019, 2019, 5086240. [Google Scholar] [CrossRef]
- Mossong, J.; Margues, P.; Raqimbeau, C.; Huberty-Krau, P.; Losch, S.; Meyer, G.; Moris, G.; Strottner, C.; Rabsch, W.; Schneider, F. Outbreaks of monophasic Salmonella enterica serovar 4,[5],12:i:- in Luxembourg, 2006. Eurosurveillance 2007, 12, 156–158. [Google Scholar] [CrossRef]
- Hauser, E.; Tietze, E.; Helmuth, R.; Junker, E.; Blank, K.; Prager, R.; Rabsch, W.; Appel, B.; Fruth, A.; Malorny, B. Pork contaminated with Salmonella enterica serovar 4,[5],12:i:-, an emerging health risk for humans. Appl. Environ. Microb. 2010, 76, 4601–4610. [Google Scholar] [CrossRef]
- Clemente, L.; Manageiro, V.; Ferreira, E.; Jonesdias, D.; Correia, I.; Themudo, P.; Albuquerque, T.; Canica, M. Occurrence of extended-spectrum β-lactamases among isolates of Salmonella enterica subsp. enterica from food-producing animals and food products, in Portugal. Int. J. Microbiol. 2013, 167, 221–228. [Google Scholar] [CrossRef]
- Fois, F.; Piras, F.; Torpdahl, M.; Mazza, R.; Consolati, S.G.; Spanu, C.; Scarano, C.; De Santis, E.P.L. Occurrence, characterization, and antimicrobial susceptibility of Salmonella enterica in slaughtered pigs in Sardinia. J. Food Sci. 2017, 82, 969–976. [Google Scholar] [CrossRef]
- Centers for Diseases Control and Prevention. National Enteric Disease Surveillance (NEDS): Salmonella Annual Report, 2016; CDC: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/nationalsurveillance/pdfs/2016-Salmonella-report-508.pdf (accessed on 17 July 2021).
- Deng, X.L.; Ran, L.; Wu, S.Y.; Ke, B.X.; He, D.M.; Yang, X.F.; Zhang, Y.H.; Ke, C.W.; Klena, J.D.; Yan, M.Y.; et al. Laboratory-based surveillance of non-typhoidal Salmonella infections in Guangdong province, China. Foodborne Pathog. Dis. 2012, 9, 305–312. [Google Scholar] [CrossRef]
- Arguello, H.; Sørensen, G.; Carvajal, A.; Baggesen, D.L.; Rubio, P.; Pedersen, K. Characterization of the emerging Salmonella 4,[5],12:i:- in Danish animal production. Foodborne Pathog. Dis. 2014, 11, 366–372. [Google Scholar] [CrossRef]
- Alessiani, A.; Sacchini, L.; Pontieri, E.; Gavini, J.; Giannatale, E.D. Molecular typing of Salmonella enterica subspecies enterica serovar Typhimurium isolated in Abruzzo region (Italy) from 2008 to 2010. Vet. Ital. 2014, 50, 31–39. [Google Scholar] [CrossRef]
- Seixas, R.; Santos, T.R.; Machado, J.; Tavares, L.; Bernardo, F.; Semedo-Lemsaddek, T.; Oliveira, M. Phenotypic and molecular characterization of Salmonella 1,4,[5],12:i:- R-Type ASSuT isolates from humans, animals, and environment in Portugal, 2006-2011. Foodborne Pathog. Dis. 2016, 13, 633–641. [Google Scholar] [CrossRef]
- Possebon, F.S.; Casas, M.R.T.; Nero, L.A.; Yamatogi, R.S.; Araújo, J.P.; Pinto, J. Prevalence, antibiotic resistance, PFGE and MLST characterization of Salmonella in swine mesenteric lymph nodes. Prev. Vet. Med. 2020, 179, 105024. [Google Scholar] [CrossRef]
- Bone, A.; Noel, H.; Hello, S.L.; Pihier, N.; Jourdan-da Silva, N. Nationwide outbreak of Salmonella enterica serotype 4,12:i:- infections in France, linked to dried pork sausage, March-May 2010. Eurosurveillance 2010, 15, 19592. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Finley, R.; Allen, V.; Ang, L.; Bekal, S.; Bailey, S.E.; Haldane, D.; Hoang, L.; Horsman, G.; Louie, M.; et al. Emergence of multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- involving human cases in Canada: Results from the Canadian Integrated Program on Antibiotic Resistance Surveillance (CIPARS), 2003–2010. J. Antimicrob. Chemoth. 2013, 9, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lv, B.; Zhang, X.; Yan, H.Q.; Huang, Y.; Qian, H.K.; Pang, B.; Jia, L.; Kan, B.; Wang, Q.Y. Prevalence and antibiotic resistance of bacterial pathogens isolated from childhood diarrhea in Beijing, China (2010–2014). Gut Pathog. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Mechesso, A.F.; Moon, D.C.; Kim, S.J.; Song, H.J.; Kang, H.Y.; Na, S.H.; Choi, J.H.; Kim, H.Y.; Yoon, S.S.; Lim, S.K. Nationwide surveillance on serotype distribution and antimicrobial resistance profiles of non-typhoidal Salmonella serovars isolated from food-producing animals in South Korea. Int. J. Food Microbiol. 2020, 355, 108893. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Guerra, B.; Bances, M.; Mendoza, M.C.; Rodicio, M.R. IncA/C plasmids mediate antimicrobial resistance linked to virulence genes in the Spanish clone of the emerging Salmonella enterica serotype 4,[5],12:i:-. J. Antimicrob. Chemoth. 2011, 66, 543–549. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Kirchner, M.; Guerra, B.; Granier, S.A.; Lucarelli, C.; Porrero, M.C.; Jakubczak, A.; Threlfall, E.J.; Mevius, D.J. Multiresistant Salmonella enterica serovar 4,[5],12:i:- in Europe: A new pandemic strain? Eurosurveillance 2010, 15, 19580. [Google Scholar] [CrossRef]
- Su, J.H.; Zhu, Y.H.; Ren, T.Y.; Guo, L.; Yang, G.Y.; Jiao, L.G.; Wang, J.F. Distribution and antimicrobial resistance of Salmonella isolated from pigs with diarrhea in China. Microorganisms 2018, 6, 117. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Ma, K.; Du, J.L.; Zhou, Y.J.; Wu, G.L.; Qiao, X.; Wang, Y.M.; Ni, Y.L.; Fu, J.J.; Huo, X. Characterization of human origin Salmonella serovar 1,4,[5],12:i:- in Eastern China, 2014 to 2018. Foodborne Pathog. Dis. 2021, 18, 790–797. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Lv, S.L.; Qu, C.; Lan, L.; Tan, D.M.; Li, X.G.; Bai, L. Serotypes, antibiotic resistance, and molecular characterization of non-typhoidal salmonella isolated from diarrheic patients in Guangxi Zhuang Autonomous Region, China, 2014-2017. Food Control. 2021, 120, 107478. [Google Scholar] [CrossRef]
- Lucarelli, C.; Dionisi, A.M.; Torpdahl, M.; Villa, L.; Graziani, C.; Hopkins, K.; Threlfall, J.; Caprioli, A.; Luzzi, I. Evidence for a second genomic island conferring multidrug resistance in a clonal group of strains of Salmonella enterica serovar Typhimurium and its monophasic variant circulating in Italy, Denmark, and the United Kingdom. J. Clin. Microbiol. 2010, 48, 2103–2109. [Google Scholar] [CrossRef]
- Arnott, A.; Wang, Q.N.; Bachmann, N.; Sadsad, R.; Biswas, C.; Sotomayor, C.; Howard, P.; Rockett, R.; Wiklendt, A.; Iredell, J.R.; et al. Multidrug-resistant Salmonella enterica 4,[5],12:i:- sequence type 34, new south wales, Australia, 2016-2017. Emerg Infect. Dis. 2018, 24, 751–753. [Google Scholar] [CrossRef]
- Rodríguez, I.; Jahn, S.; Schroeter, A.; Malorny, B.; Helmuth, R.; Guerra, B. Extended-spectrum β-lactamases in German isolates belonging to the emerging monophasic Salmonella enterica subsp. Enterica serovar Typhimurium 4,[5],12:i:- European clone. J. Antimicrob. Chemoth. 2012, 67, 505–508. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, X.N.; Li, R.H.; Xue, W.C.; Zhai, R.B.; Liu, L.W.; Liu, Y.; Jin, H. Prevalence, resistance, and extended-spectrum beta-lactamases among Salmonella from patients in northeast China. J. Infect. Dev. Countr. 2017, 11, 470–478. [Google Scholar] [CrossRef]
- Carroll, L.M.; Zurfluh, K.; Jang, H.; Gopinath, G.; Nuesch-Inderbinen, M.; Poirel, L.; Nordmann, P.; Stephan, R.; Guldimann, C. First report of an mcr-1 harboring Salmonella enterica subsp. Enterica serotype 4,5,12:i:- strain isolated from blood of a patient in Switzerland. Int. J. Antimicrob. Agents 2018, 52, 740–741. [Google Scholar] [CrossRef]
- Portes, A.B.; Rodrigues, G.; Leitão, M.P.; Ferrari, R.; Junior, C.A.C.; Panzenhagen, P. Global distribution of plasmid-mediated colistin resistance mcr gene in Salmonella: A systematic review. J. Appl. Microbiol. 2022, 132, 872–889. [Google Scholar] [CrossRef]
- Naberhaus, S.A.; Krull, A.C.; Bradner, L.K.; Harmon, K.M.; Arruda, P.; Arruda, B.L.; Sahin, O.; Burrough, E.R.; Schwartz, K.J.; Kreuder, A.J. Emergence of Salmonella enterica serovar 4,[5],12:i:- as the primary serovar identified from swine clinical samples and development of a multiplex real-time PCR for improved Salmonella serovar-level identification. J. Vet. Diagn. Investig. 2019, 31, 818–827. [Google Scholar] [CrossRef]
- Kawakami, V.M.; Bottichio, L.; Angelo, K.; Linton, N.; Kissler, B.; Basler, C.; Lloyd, J.; Inouye, W.; Gonzales, E.; Rietberg, K.; et al. Outbreak of multidrug-resistant Salmonella infections linked to pork-Washington, 2015. Mmwr. Morb. Mortal. Wkly. 2016, 65, 379–381. [Google Scholar] [CrossRef]
- Xie, X.L.; Wang, Z.Y.; Zhang, K.; Li, Y.; Hu, Y.C.; Pan, Z.M.; Chen, X.; Li, Q.C.; Jiao, X.A. Pig as a reservoir of CRISPR type TST4 Salmonella enterica serovar Typhimurium monophasic variant during 2009–2017 in China. Emerg. Microbes Infec. 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Bonardi, S.; Alpigiani, I.; Bruini, I.; Barilli, E.; Brindani, F.; Morganti, M.; Cavallini, P.; Bolzoni, L.; Pongolini, S. Detection of Salmonella enterica in pigs at slaughter and comparison with human isolates in Italy. Int. J. Food Microbiol. 2016, 218, 44–50. [Google Scholar] [CrossRef]
- Thai, T.H.; Hirai, T.; Lan, N.T.; Yamaguchi, R. Antibiotic resistance profiles of Salmonella serovars isolated from retail pork and chicken meat in North Vietnam. Int. J. Food Microbiol. 2012, 156, 147–151. [Google Scholar] [CrossRef]
- He, J.J.; Sun, F.; Sun, D.W.; Wang, Z.Y.; Jin, S.S.; Pan, Z.M.; Xu, Z.Z.; Chen, X.; Jiao, X.A. Multidrug resistance and prevalence of quinolone resistance genes of Salmonella enterica serotypes 4,[5],12:i:- in China. Int. J. Food Microbiol. 2020, 330, 108692. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Caffrey, N.P.; Diego, B. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, 316–327. [Google Scholar] [CrossRef]
- Campos, J.; Mourão, J.; Peixe, L.; Antunes, P. Non-typhoidal Salmonella in the pig production chain: A comprehensive analysis of its impact on human health. Pathogens 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E. Multidrug-resistant pathogens in the food supply. Foodborne Pathog. Dis. 2015, 12, 261–279. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wezel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial resistance to antimicrobial agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Hao, H.; Sander, P.; Iqbal, Z.; Wang, Y.; Cheng, G.; Yuan, Z. The risk of some veterinary antimicrobial agents on public health associated with antimicrobial resistance and their molecular basis. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Vtnair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-resistance Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Fernandes, L.; Centeno, M.M.; Couto, N.; Nunes, T.; Almeida, V.; Alban, L.; Pomba, C. Longitudinal characterization of monophasic Salmonella Typhimurium throughout the pig’s life cycle. Vet. Microbiol. 2016, 192, 231–237. [Google Scholar] [CrossRef]
- Mourão, J.; Machado, J.; Novais, C.; Antunes, P.; Peixe, L. Characterization of the emerging clinically-relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- (monophasic variant of S. Typhimurium) clones. Eur. J. Clin. Microbiol. 2014, 33, 2249–2257. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Q.; Zhang, J.; Huang, J.; Guo, W.; Cai, S. Prevalence and characterization of monophasic Salmonella Serovar 1,4,[5],12:i:- of food origin in China. PLoS ONE 2015, 10, e0137967. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Brit. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, W.; Wang, H.Q.; Yu, H.; Pan, J.C.; Chen, Q.; Liu, X.D. Characteristics and molecular subtyping of multidrug resistant Salmonella in Hangzhou. Microbiol. China 2021, 48, 536–544. (In Chinese) [Google Scholar]
- Huang, J.M.; Ke, B.X.; Li, B.S.; He, D.M.; Liu, Z.; Li, Z.C.; Deng, X.L. Molecular epidemiological characteristics and antibiotic resistance of multi-drug resistant Salmonella 1,4,[5],12:i:- in Guangdong. Dis. Surveill. 2021, 36, 501–508. (In Chinese) [Google Scholar]
- Zeng, X.Y.; Lv, S.L.; Qu, C.; Lan, L.; Tan, D.M.; Li, X.G. The drug resistance and molecular characteristics of Salmonella typhimurium 1,4,[5],12:i:- in Guangxi. Chin. J. Food Hygiene 2021, 33, 558–565. (In Chinese) [Google Scholar]
- Yuan, Z.H.; Zheng, Y.K.; Wu, C.Q.; Liu, Q.M.; Qiu, Q.L.; Yang, S.H. Serotype distribution and drug resistance analysis of 540 Salmonella spp. isolated from diarrhea cases from Zhongshan City. Chin. J. Food Hygiene 2020, 32, 134–138. (In Chinese) [Google Scholar]
- Huang, S.F.; Peng, S.P.; Xu, M.Q.; Liao, G.D. Epidemiological characteristics and drug resistance analysis of Salmonella 1,4,[5],12:i:- in Maoming City from 2017 to 2019. Chin. J. Food Hygiene 2020, 32, 681–685. (In Chinese) [Google Scholar]
- Wu, W.; Tu, L.H.; Zhang, W.X.; Zhang, X.; Chen, M. Identification and antimicrobial susceptibility of Salmonella typhimurium monophasic variant in Shanghai. Lab. Med. 2018, 33, 798–802. (In Chinese) [Google Scholar]
- Ke, B.X.; Zeng, H.H.; He, D.M.; Tan, H.L.; Li, B.S.; Liang, Y.H.; Ke, C.W. Circulation and etiological characterization of Salmonella enterica serotype in human in Guangdong province, 2007–2016. Chin. J. Epidemiol. 2018, 39, 64–66. (In Chinese) [Google Scholar]
- He, X.H.; Xu, X.B.; Li, K.; Liu, B.; Yue, T.L. Identification of Salmonella enterica Typhimurium and variants using a novel multiplex PCR assay. Food Control 2016, 65, 152–159. [Google Scholar] [CrossRef]
- Liang, Z.M.; Ke, B.X.; Deng, X.L.; Liang, J.H.; Ran, L.; Lu, L.L.; He, D.M.; Huang, Q.; Ke, C.W.; Li, Z.J.; et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009–2012. BMC Infect. Dis. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.X.; Sun, J.F.; He, D.M.; Li, X.C.; Liang, Z.M.; Ke, C. Serovar distribution, antimicrobial resistance profiles, and PFGE typing of Salmonella enterica strains isolated from 2007–2012 in Guangdong, China. BMC Infect. Dis. 2014, 14, 338. [Google Scholar] [CrossRef] [PubMed]
| Antibiotics | Total Number of Isolates | Resistance Rate | Heterogeneity | |||
|---|---|---|---|---|---|---|
| Mean of Pooled Resistance Rate % | 95% CI (%) 1 | I2(%) 2 | Q-p3 | |||
| β-Lactams | Ampicillin | 4536 | 91 | 83–94 | 63 | <0.1 |
| Amoxicillin–clavulanic acid | 579 | 70 | 26–94 | 96 | <0.1 | |
| Cefotaxime | 4151 | 19 | 12–29 | 89 | <0.1 | |
| Ceftazidime | 4128 | 10 | 7–15 | 74 | <0.1 | |
| Cefepime | 498 | 12 | 9–15 | 0 | 0.98 | |
| Ceftriaxone | 33 | 21 | 10–38 | 1 | 0.36 | |
| Aminoglycosides | Gentamicin | 4520 | 44 | 29–59 | 91 | <0.1 |
| Streptomycin | 1701 | 76 | 66–83 | 79 | <0.1 | |
| Kanamycin | 363 | 45 | 40–50 | 20 | 0.28 | |
| Sulfonamides | Trimethoprim–sulfamethoxazole | 3179 | 48 | 33–63 | 86 | <0.1 |
| Sulfisoxazole | 1347 | 86 | 76–93 | 83 | <0.1 | |
| Trimethoprim | 1340 | 45 | 39–52 | 83 | <0.1 | |
| Phenicols | Chloramphenicol | 4443 | 54 | 47–60 | 88 | <0.1 |
| Polymyxins | Colistin | 255 | 2 | 1–5 | — | — |
| Quinolones | Ciprofloxacin | 4532 | 22 | 15–32 | 90 | <0.1 |
| Nalidixic acid | 498 | 73 | 69–76 | 0 | 0.35 | |
| Tetracyclines | Tetracycline | 4447 | 93 | 90–95 | 46 | <0.1 |
| Multiple | Total Number of Isolates | MDR Rate | Heterogeneity | ||
|---|---|---|---|---|---|
| Mean of Pooled MDR Rate % | 95% CI (%) 1 | I2(%) 2 | Q-p3 | ||
| ≥3 | 2251 | 86 | 78–92 | 87 | <0.1 |
| ASSuT | 681 | 70 | 66–73 | 40 | 0.19 |
| ACSSuT | 319 | 29 | 18–43 | 74 | <0.1 |
| ACSuGSTTm | 616 | 27 | 24–31 | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Yang, M.; Cai, H.; Liu, Y.; Gorris, L.; Aslam, M.Z.; Jia, K.; Sun, T.; Wang, X.; Dong, Q. Antibiotic Resistance of Salmonella Typhimurium Monophasic Variant 1,4,[5],12:i:-in China: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 532. https://doi.org/10.3390/antibiotics11040532
Qin X, Yang M, Cai H, Liu Y, Gorris L, Aslam MZ, Jia K, Sun T, Wang X, Dong Q. Antibiotic Resistance of Salmonella Typhimurium Monophasic Variant 1,4,[5],12:i:-in China: A Systematic Review and Meta-Analysis. Antibiotics. 2022; 11(4):532. https://doi.org/10.3390/antibiotics11040532
Chicago/Turabian StyleQin, Xiaojie, Mingzhe Yang, Hua Cai, Yangtai Liu, Leon Gorris, Muhammad Zohaib Aslam, Kai Jia, Tianmei Sun, Xiang Wang, and Qingli Dong. 2022. "Antibiotic Resistance of Salmonella Typhimurium Monophasic Variant 1,4,[5],12:i:-in China: A Systematic Review and Meta-Analysis" Antibiotics 11, no. 4: 532. https://doi.org/10.3390/antibiotics11040532
APA StyleQin, X., Yang, M., Cai, H., Liu, Y., Gorris, L., Aslam, M. Z., Jia, K., Sun, T., Wang, X., & Dong, Q. (2022). Antibiotic Resistance of Salmonella Typhimurium Monophasic Variant 1,4,[5],12:i:-in China: A Systematic Review and Meta-Analysis. Antibiotics, 11(4), 532. https://doi.org/10.3390/antibiotics11040532







