Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Profile
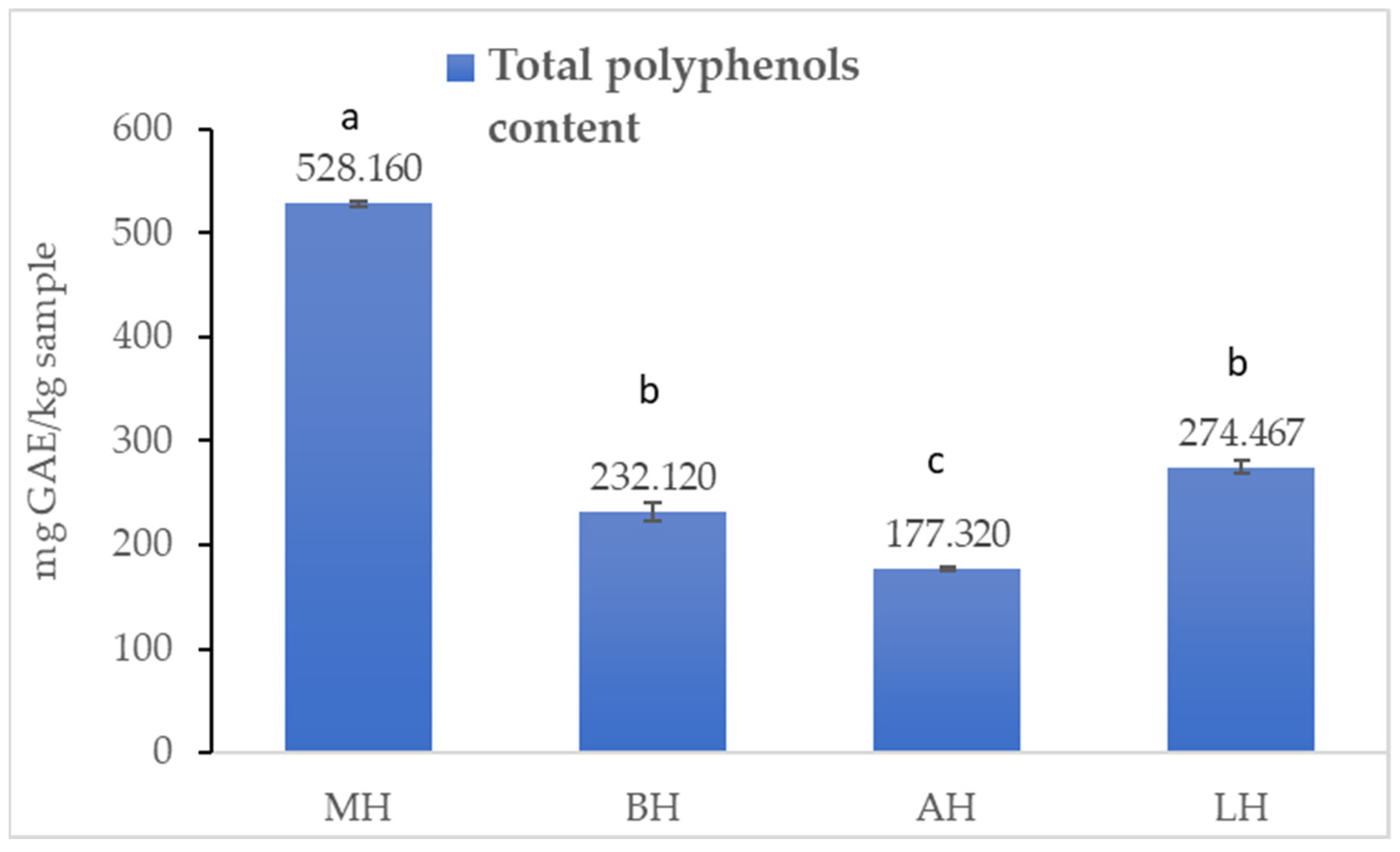
2.1.1. Total Polyphenol Content
2.1.2. Total Flavonoids Content (TFC)
2.1.3. Antioxidant Capacity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
2.2. Antimicrobial Activity
3. Discussion
3.1. Antioxidant Profile
3.2. Antimicrobial Activity
- -
- S. pyogenes and TPC (r = −0.776), DPPH (r = −0.733);
- -
- H. influenzae and TPC (r = −0.890), TFC (r = −0.884) and DPPH (r = −0.889);
- -
- C. parapsilopsis and TPC (r = −0.808), TFC (r = −0.713) and DPPH (r = −0.780);
- -
- S. aureus and TPC (r = −0.686) and DPPH (r = −0.620);
- -
- S. flexneri and TFC (r = −0.539).
- -
- P. aeruginosa and TFC (r = 0.532).
4. Materials and Methods
4.1. Antioxidant Profile
4.1.1. Preparation of the Alcoholic Extract
4.1.2. Determination of Total Polyphenol Content by Folin–Ciocâlteu Assay
4.1.3. Determination of Total Flavonoid Content (TFC)
4.1.4. Antioxidant Capacity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
- Acontrol—the control absorbance values;
- Asample—the absorbance values tested of the samples.
4.2. Antimicrobial Activity
4.2.1. Bacterial Culture
- ODsample—optical density at 540 nm as the mean value of triplicate readings for EOs in the presence of the selected bacteria;
- ODnegative control—optical density at 540 nm as the mean value of triplicate readings for the selected bacteria in BHI.
4.2.2. Fungal Culture
- ODsample—optical density at 540 nm as the mean value of triplicate readings for EOs in the presence of the selected fungi;
- ODnegative control—optical density at 540 nm as the mean value of triplicate readings for the selected fungi in BHI.
4.2.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Tirziu, E.; Herman, V.; Nichita, I.; Morar, A.; Imre, M.; Ban-Cucerzan, A.; Bucur, I.; Tirziu, A.; Mateiu-Petrec, O.C.; Imre, K. Diversity and Antibiotic Resistance Profiles of Listeria monocytogenes Serogroups in Different Food Products from the Transylvania Region of Central Romania. J. Food Prot. 2021, 85, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Morar, A.; Ban-Cucerzan, A.; Herman, V.; Tîrziu, E.; Sallam, K.I.; Abd-Elghany, S.M.; Imre, K. Multidrug Resistant Coagulase-Positive Staphylococcus aureus and Their Enterotoxins Detection in Traditional Cheeses Marketed in Banat Region, Romania. Antibiotics 2021, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Imre, K.; Herman, V.; Morar, A. Scientific Achievements in the Study of the Occurrence and Antimicrobial Susceptibility Profile of Major Foodborne Pathogenic Bacteria in Foods and Food Processing Environments in Romania: Review of the Last Decade. BioMed Res. Int. 2020, 2020, 5134764. [Google Scholar] [CrossRef] [PubMed]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Bardanca, M.G.; Ambroa, A.; López, M.; Bou, G.; Tomás, M. Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics 2020, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Herman, V.; Roșiu, D.; Cătana, N.; Degi, J.; Iancu, I.; Mițiți, I.; Ciobanu, G.; Grema, C.F.; Pascu, C. Evaluation of Propolis for Antibacterial Activity In Vitro. Rev. Romana De Med. Vet. 2018, 28, 13–17. [Google Scholar]
- Mernea, A.I.; Hulea, A.; Gartner, A.; Pascu, C.; Herman, V. Case report related to local efficacy of propolis and beeswax. Lucr. Stiintifice-Univ. De Stiinte Agric. A Banat. Timis. Med. Vet. 2017, 50, 87–92. [Google Scholar]
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. 2010, 29, 1237–1241. [Google Scholar] [CrossRef] [Green Version]
- Beteg, F.; Vieilly, V.; Fiț, N.; Mureșan, C.; Gal, A.; Stancu, B.; Pascu, C.; Herman, V. Propolis-an ancient remedy or a new paradigm for wound healing: In-vivo preclinical evaluation, antimicrobial activity and histopathologic aspects. Rev. Română De Med. Vet. 2019, 29, 12–17. [Google Scholar]
- Mathew, C.; Tesfaye, W.; Rasmussen, P.; Peterson, G.M.; Bartholomaeus, A.; Sharma, M.; Thomas, J. Mānuka Oil—A Review of Antimicrobial and Other Medicinal Properties. Pharmaceuticals 2020, 13, 343. [Google Scholar] [CrossRef]
- Mederle, N.; Kaya, A.; Kaya, M.A.; Pătrașcu, M.; Chiș, C.; Gartner, A.; Frăsia, D.; Buzatu, R.; Kumbakisaka, S. Therapeutic efficacy testing of demosimcan gel and canider ointment used in dry canine demodicosis lesions. Preliminary study. Lucr. Stiintifice-Univ. Stiinte Agric. A Banat. Timis. Med. Vet. 2017, 50, 322–330. [Google Scholar]
- Vallianou, N.G. Honey and its Anti-Inflammatory, Anti-Bacterial and Anti-Oxidant Properties. Gen. Med. Open Access 2014, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Pătruică, S.; Simiz, E.; Peț, I.; Ștef, L. Some correlations between environmental parameters and the foraging behavior of honeybees (Apismellifera) on oilseed rape (Brassica napusoleifera), Scientific papers, Series D. Anim. Sci. 2019, 2, 180–184. [Google Scholar]
- Abd-El Aal, A.M.; El-Hadidy, M.R.; El-Mashad, N.B.; El-Sebaie, A.H. Antimicrobial effect of bee honey in comparison to antibiotics on organisms isolated from infected burns. Ann. Burn. Fire Disasters 2007, 20, 83. [Google Scholar]
- Bang, K.W.; Lewis, G.; Villas-Boas, S.G. Leptospermum scoparium (Mānuka) and Cryptomeria japonica (Sugi) Leaf Essential Oil Seasonal Chemical Variation and Their Effect on Antimicrobial Activity. Preprints 2020, 2020080623. [Google Scholar] [CrossRef]
- Lee, H.; Churey, J.J.; Worobo, R.W. Antimicrobial activity of bacterial isolates from different floral sources of honey. Int. J. Food Microbiol. 2008, 126, 240–244. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Hegazi, N.M.; Elghani, G.E.A.; Farag, M.A. Manuka honey, a unique mono-floral honey. A comprehensive review of its bioactives, metabolism, action mechanisms, and therapeutic merits. Food Biosci. 2021, 42, 101038. [Google Scholar] [CrossRef]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Azim, S.G.; Abdel-Azim, A.G.; Piasecki, B.P.; Abdel-Azim, G.A. Characterization of the gain and loss of resistance to antibiotics versus tolerance to honey as an antimutagenic and antimicrobial medium in extended-time serial transfer experiments. Pharmacogn. Res. 2019, 11, 147. [Google Scholar] [CrossRef]
- Bazzi, A.M.; Rabaan, A.; Al-Tawfiq, J.A.; Shannak, B.M. Comparison of Effectiveness of Germania Honey Compared to Manuka Honey in Methicillin-Resistant Staphylococcus aureus (MRSA) Killing. Open Microbiol. J. 2019, 13, 21–27. [Google Scholar] [CrossRef]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2019, 57, 1269–1277. [Google Scholar] [CrossRef]
- Matzen, R.D.; Leth-Espensen, J.Z.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect In Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Prac. 2018, 2018, 7021713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morroni, G.; Alvarez-Suarez, J.M.; Brenciani, A.; Simoni, S.; Fioriti, S.; Pugnaloni, A.; Giampieri, F.; Mazzoni, L.; Gasparrini, M.; Marini, E.; et al. Comparison of the Antimicrobial Activities of Four Honeys from Three Countries (New Zealand, Cuba, and Kenya). Front. Microbiol. 2018, 9, 1378. [Google Scholar] [CrossRef] [Green Version]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Elborai, A. Antibacterial and Antioxidant Activities of Different Varieties of Locally Produced Egyptian Honey. Egypt. J. Bot. 2018, 58, 97–107. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Si, J.-J.; Li, S.-S.; Zhang, G.-Z.; Wang, S.; Zheng, H.-Q.; Hu, F.-L. Chemical Analyses and Antimicrobial Activity of Nine Kinds of Unifloral Chinese Honeys Compared to Manuka Honey (12+ and 20+). Molecules 2021, 26, 2778. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Pang, E.C.K.; Mantri, N. Agastache honey has superior antifungal activity in comparison with important commercial honeys. Sci. Rep. 2019, 9, 18197. [Google Scholar] [CrossRef] [Green Version]
- De Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.J.; Chowdhary, A.; Meis, J.F. Antifungal Activity of a Medical-Grade Honey Formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef]
- Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012, 2, 554–557. [Google Scholar] [CrossRef] [Green Version]
- Majtan, J.; Bohova, J.; Horniackova, M.; Klaudiny, J.; Majtan, V. Anti-biofilm Effects of Honey Against Wound Pathogens Proteus mirabilis and Enterobacter cloacae. Phytother. Res. 2014, 28, 69–75. [Google Scholar] [CrossRef]
- Tan, H.-T.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Bs, K.-K. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Kwiecińska-Piróg, J.; Przekwas, J.; Majkut, M.; Skowron, K.; Gospodarek-Komkowska, E. Biofilm Formation Reducing Properties of Manuka Honey and Propolis in Proteus mirabilis Rods Isolated from Chronic Wounds. Microorganisms 2020, 8, 1823. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.L.; Powell, L.C.; Pritchard, M.F.; Thomas, D.; Jenkins, R. Anti-pseudomonad Activity of Manuka Honey and Antibiotics in a Specialized ex vivo Model Simulating Cystic Fibrosis Lung Infection. Front. Microbiol. 2019, 10, 869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, R.; Wootton, M.; Howe, R.; Cooper, R. A demonstration of the susceptibility of clinical isolates obtained from cystic fibrosis patients to manuka honey. Arch. Microbiol. 2015, 197, 597–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, A.K.; Kumar, A.; Mittal, S.; Romesh, H.; Varshney, S.; Malhotra, M. Efficacy of medical grade manuka honey in acute otitis externa: A pilot study. Indian J. Otol. 2020, 26, 151. [Google Scholar] [CrossRef]
- Henatsch, D.; Nabuurs, C.H.; Van De Goor, R.M.; Wolffs, P.F.; Stokroos, R.J. Treatment of Recurrent Eczematous External Otitis with Honey Eardrops: A Proof-of-Concept Study. Otolaryngol. Neck Surg. 2017, 157, 696–699. [Google Scholar] [CrossRef]
- Maruhashi, E.; Braz, B.S.; Nunes, T.; Pomba, C.; Belas, A.; Duarte-Correia, J.H.; Lourenço, A.M. Efficacy of medical grade honey in the management of canine otitis externa—A pilot study. Veter. Dermatol. 2016, 27, 93-e27. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abde-Llah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and Antioxidant Potency of Floral Honeys from Different Botanical and Geographical Origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [Green Version]
- Bundit, T.; Anothai, T.; Pattaramart, P.; Roongpet, T.; Chuleeporn, S. Comparison of Antioxidant Contents of Thai Honeys. Mal. J. Nutr. 2016, 22, 413–420. [Google Scholar]
- Smetanska, I.; Alharthi, S.S.; Selim, K.A. Physicochemical, antioxidant capacity and color analysis of six honeys from different origin. J. King Saud Univ. Sci. 2021, 33, 101447. [Google Scholar] [CrossRef]
- Wilczyńska, A. Phenolic Content and Antioxidant Activity of Different Types of Polish, Honey—A Short Report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Dobre, I.; Escuredo, O.; Rodriguez-Flores, S.; Seijo, M.C. Evaluation of Several Romanian Honeys Based on their Palynological and Biochemical Profiles. Int. J. Food Prop. 2014, 17, 1850–1860. [Google Scholar] [CrossRef]
- Yao, L.; Datta, N.; Tomas-Barberan, F.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Tan, T.T.E. Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef]
- Masalha, M.; Abu-Lafi, S.; Abu-Farich, B.; Rayan, M.; Issa, N.; Zeidan, M.; Rayan, A. A New Approach for Indexing Honey for Its Heath/Medicinal Benefits: Visualization of the Concept by Indexing Based on Antioxidant and Antibacterial Activities. Medicine 2018, 5, 135. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.D.; Kalluru, R.S.; Ahmed, S.; Abhilashini, A.; Jayaprakash, T.; Garlapati, R.; Sowmya, B.; Reddy, K.N. Comparison of the Antibacterial Efficacy of Manuka Honey Against E. faecalis and E. coli—An In Vitro Study. JCDR 2014, 8, ZC29–ZC31. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Worobo, R.W.; Szweda, P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef] [Green Version]
- Gkoutzouvelidou, M.; Panos, G.; Xanthou, M.; Papachristoforou, A.; Giaouris, E. Comparing the Antimicrobial Actions of Greek Honeys from the Island of Lemnos and Manuka Honey from New Zealand against Clinically Important Bacteria. Foods 2021, 10, 1402. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Ribeiro, H.; Oliveira, A.; Silva, A.S.; Freitas, A.; Henriques, M.; Rodrigues, M.E. Portuguese honeys as antimicrobial agents against Candida species. J. Tradit. Complement. Med. 2021, 11, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Lopez, M.S.; Rowlands, R.S.; Cooper, R.A. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 2012, 158, 781–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzo, D.; Cokcetin, N.N.; Li, L.; Ballerin, G.; Bottomley, A.L.; Lazenby, J.; Whitchurch, C.B.; Paulsen, I.T.; Hassan, K.A.; Harry, E.J. Characterizing the Mechanism of Action of an Ancient Antimicrobial, Manuka Honey, against Pseudomonas aeruginosa Using Modern Transcriptomics. mSystems 2020, 5, e00106-20. [Google Scholar] [CrossRef]
- Mandal, S.; DebMandal, M.; Pal, N.K.; Saha, K. Antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa and Salmonella enterica serovar Typhi. Asian Pac. J. Trop. Med. 2010, 3, 961–964. [Google Scholar] [CrossRef] [Green Version]
- Guadarrama, D. The Effect of Manuka Honey’s effect on Ampicillin-Resistant Escherichia coli HB101 K-12 Compared to other Antibiotics. FASEB J. 2019, 33, 483–488. [Google Scholar] [CrossRef]
- Idris, A.R.; Afegbua, S.L. Single and joint antibacterial activity of aqueous garlic extract and Manuka honey on extended-spectrum beta-lactamase-producing Escherichia coli. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 472–478. [Google Scholar] [CrossRef]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.; Jenkins, R. 119 Manuka honey reduces viability and virulence in Burkholderia cenocepacia. J. Cyst. Fibros. 2017, 16, S95–S96. [Google Scholar] [CrossRef]
- Carter, D.A.; Blair, S.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E. Therapeutic Manuka Honey: No Longer So Alternative. Front. Microbiol. 2016, 7, 569. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.M.P.; Devesa, J.S.P.; Hill, P.B.; Silva, V.; Poeta, P. In Vitro Efficacy of Manuka Honey against Staphylococcus pseudintermedius and Malassezia pachydermatis. J. Am. Holist. Vet. Med. Assoc. 2018, 51, 34–41. [Google Scholar]
- Mračević, S.Đ.; Krstić, M.; Lolić, A.; Ražić, S. Comparative study of the chemical composition and biological potential of honey from different regions of Serbia. Microchem. J. 2020, 152, 104420. [Google Scholar] [CrossRef]
- Farkasovska, J.; Bugarova, V.; Godocikova, J.; Majtan, V.; Majtan, J. The role of hydrogen peroxide in the antibacterial activity of different floral honeys. Eur. Food Res. Technol. 2019, 245, 2739–2744. [Google Scholar] [CrossRef]
- Bagheri, A.; Koohsari, H.; Sayyed, A.S.Z. Antibacterial And Antioxidant Activity of Four Types of Honey with Different Floral Origion. Int. J. Mol. Clin. Microbiol. 2016, 6, 670–677. [Google Scholar]
- Balázs, V.; Nagy-Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, Á. In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods 2021, 10, 1632. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Szweda, P.; Jerkovic, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Gobin, I.; Goranka, C.; Magdalenić, M.; Begić, G.; Babić, A.; Lušić, D.; Vučković, D. Antibacterial potential of Croatian honey against antibiotic resistant pathogenic bacteria. Med. Glas. 2018, 15, 139–144. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- Aurongzeb, M.; Rashid, Y.; Azim, M.K. Antifungal Activity of Unifloral and Multifloral Pakistani Honeys against Clinically Isolated Candida species. Pak. J. Biochem. Mol. Biol. 2021, 52, 105–117. [Google Scholar]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedűş-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Obistioiu, D.; Cocan, I.; Tîrziu, E.; Herman, V.; Negrea, M.; Cucerzan, A.; Neacsu, A.-G.; Cozma, A.; Nichita, I.; Hulea, A.; et al. Phytochemical Profile and Microbiological Activity of Some Plants Belonging to the Fabaceae Family. Antibiotics 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Al-Amri, A.; Al-Hadhrami, A.; Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018, 4, e00874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Mehta, A.; Berenbaum, M.; Zangerl, A.R.; Engeseth, N.J. Honeys from Different Floral Sources as Inhibitors of Enzymatic Browning in Fruit and Vegetable Homogenates. J. Agric. Food Chem. 2000, 48, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Duca, A.; Sturza, A.; Moacă, E.-A.; Negrea, M.; Lalescu, V.-D.; Lungeanu, D.; Dehelean, C.-A.; Muntean, D.-M.; Alexa, E. Identification of Resveratrol as Bioactive Compound of Propolis from Western Romania and Characterization of Phenolic Profile and Antioxidant Activity of Ethanolic Extracts. Molecules 2019, 24, 3368. [Google Scholar] [CrossRef] [Green Version]
- Beicu, R.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Imbrea, F.; Pop, G.; Circioban, D.; Moisa, C.; Lupitu, A.; Copolovici, L.; et al. Antimicrobial Potential and Phytochemical Profile of Wild and Cultivated Populations of Thyme (Thymus sp.) Growing in Western Romania. Plants 2021, 10, 1833. [Google Scholar] [CrossRef]
- Cocan, I.; Negrea, M.; Cozma, A.; Alexa, E.; Poiana, M.-A.; Raba, D.; Danciu, C.; Popescu, I.; Cadariu, A.I.; Obistioiu, D.; et al. Chili and Sweet Pepper Seed Oil Used as a Natural Antioxidant to Improve the Thermo-Oxidative Stability of Sunflower Oil. Agronomy 2021, 11, 2579. [Google Scholar] [CrossRef]
- Cocan, I.; Alexa, E.; Danciu, C.; Radulov, I.; Galuscan, A.; Obistioiu, D.; Morvay, A.A.; Sumalan, R.M.; Poiana, M.-A.; Pop, G.; et al. Phytochemical screening and biological activity of Lamiaceae family plant extracts. Exp. Ther. Med. 2017, 15, 1863–1870. [Google Scholar] [CrossRef] [Green Version]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef] [Green Version]











| The Concentration of Honey % | S. pyogenes (ATCC 19615) | S. aureus (ATCC 25923) | S. flexneri (ATCC 12022) | P. aeruginosa (ATCC 27853) | E. coli (ATCC 25922) | S. typhimurium (ATCC 14028) | H. influenzae (ATCC 10211) | C. parapsilopsis (ATCC 22019) | C. albicans (ATCC 10231) |
|---|---|---|---|---|---|---|---|---|---|
| MH 10 | 0.626 ± 0.006 | 0.552 ± 0.002 | 0.923 ± 0.002 | 1.006 ± 0.002 | 1.262 ± 0.002 | 0.815 ± 0.004 | 0.773 ± 0.008 | 0.821 ± 0.007 | 0.939 ± 0.003 |
| MH 15 | 0.604 ± 0.004 | 0.504 ± 0.003 | 0.902 ± 0.002 | 1.189 ± 0.002 | 0.985 ± 0.004 | 0.898 ± 0.004 | 0.934 ± 0.005 | 0.870 ± 0.007 | 0.879 ± 0.003 |
| MH 20 | 0.530 ± 0.004 | 0.484 ± 0.002 | 0.865 ± 0.002 | 1.211 ± 0.004 | 0.869 ± 0.003 | 0.900 ± 0.004 | 1.007 ± 0.005 | 0.935 ± 0.007 | 0.743 ± 0.003 |
| BH 10 | 0.717 ± 0.004 | 0.618 ± 0.005 | 0.913 ± 0.002 | 0.993 ± 0.002 | 0.980 ± 0.003 | 0.905 ± 0.004 | 1.119 ± 0.005 | 0.922 ± 0.005 | 0.829 ± 0.003 |
| BH 15 | 0.743 ± 0.004 | 0.624 ± 0.003 | 0.859 ± 0.007 | 0.865 ± 0.003 | 1.091 ± 0.003 | 0.921 ± 0.004 | 1.221 ± 0.005 | 1.113 ± 0.005 | 0.966 ± 0.003 |
| BH 20 | 0.763 ± 0.005 | 0.656 ± 0.008 | 0.738 ± 0.003 | 0.784 ± 0.005 | 1.237 ± 0.005 | 0.977 ± 0.004 | 1.315 ± 0.005 | 1.216 ± 0.005 | 1.006 ± 0.003 |
| AH 10 | 0.814 ± 0.003 | 0.614 ± 0.003 | 1.006 ± 0.002 | 1.195 ± 0.003 | 1.295 ± 0.003 | 1.222 ± 0.004 | 1.305 ± 0.005 | 1.305 ± 0.005 | 0.773 ± 0.003 |
| AH 15 | 0.775 ± 0.007 | 0.682 ± 0.004 | 1.189 ± 0.002 | 0.976 ± 0.002 | 0.969 ± 0.005 | 1.134 ± 0.004 | 1.359 ± 0.005 | 1.206 ± 0.005 | 0.820 ± 0.003 |
| AH 20 | 0.697 ± 0.005 | 0.706 ± 0.006 | 1.211 ± 0.004 | 0.912 ± 0.003 | 0.905 ± 0.003 | 0.925 ± 0.003 | 1.361 ± 0.005 | 1.111 ± 0.005 | 0.903 ± 0.003 |
| LH 10 | 0.881 ± 0.006 | 0.728 ± 0.005 | 0.879 ± 0.004 | 1.194 ± 0.005 | 1.135 ± 0.004 | 1.427 ± 0.004 | 1.059 ± 0.005 | 1.059 ± 0.005 | 1.134 ± 0.003 |
| LH 15 | 0.803 ± 0.004 | 0.775 ± 0.004 | 0.828 ± 0.003 | 1.202 ± 0.005 | 1.224 ± 0.003 | 1.218 ± 0.014 | 1.132 ± 0.005 | 1.125 ± 0.016 | 1.090 ± 0.077 |
| LH 20 | 0.757 ± 0.004 | 0.794 ± 0.006 | 0.791 ± 0.003 | 1.214 ± 0.003 | 1.240 ± 0.003 | 1.049 ± 0.004 | 1.218 ± 0.003 | 1.183 ± 0.032 | 1.039 ± 0.005 |
| C | 0.783 ± 0.004 | 0.626 ± 0.005 | 0.961 ± 0.005 | 1.005 ± 0.005 | 1.314 ± 0.004 | 1.043 ± 0.004 | 1.434 ± 0.005 | 0.926 ± 0.005 | 1.019 ± 0.004 |
| Honey Concentration% | E. coli (BIDLMV 2023) | E. coli (BIDLMV 2067) | E. coli (BIDLMV 21117) | B. cepacia (BIDLMV 219) | B. cepacia (BIDLMV 21952) | P. vulgaris (BID-LMV 20624) | P. vulgaris (BIDLMV 20124) | P. vulgaris (BIDLMV 2147) | P. aeruginosa (BIDLMV 19117) | P. aeruginosa (BIDLMV 19121) | P. aeruginosa (BIDLMV 20129) | P. aeruginosa (BIDLMV 20722) | P. aeruginosa (BIDLMV 21615) | M. pachydermatis (BIDLMV 20127) | M. pachydermatis (BIDLMV 21521) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MH 10 | 1.577 ± 0.005 | 1.721 ± 0.004 | 1.436 ± 0.007 | 2.045 ± 0.004 | 1.925 ± 0.006 | 1.889 ± 0.008 | 1.270 ± 0.003 | 1.326 ± 0.005 | 1.367 ± 0.005 | 1.568 ± 0.002 | 1.634 ± 0.004 | 1.332 ± 0.003 | 1.285 ± 0.005 | 1.771 ± 0.007 | 1.672 ± 0.004 |
| MH 15 | 1.087 ± 0.006 | 1.433 ± 0.006 | 1.006 ± 0.009 | 1.564 ± 0.006 | 1.575 ± 0.007 | 1.786 ± 0.005 | 0.846 ± 0.004 | 1.153 ± 0.004 | 1.131 ± 0.007 | 1.548 ± 0.008 | 1.577 ± 0.003 | 1.238 ± 0.003 | 1.244 ± 0.004 | 0.929 ± 0.002 | 1.125 ± 0.006 |
| MH 20 | 0.466 ± 0.004 | 0.964 ± 0.004 | 0.735 ± 0.008 | 1.281 ± 0.006 | 1.182 ± 0.006 | 1.790 ± 0.004 | 0.454 ± 0.003 | 0.986 ± 0.004 | 0.976 ± 0.003 | 1.465 ± 0.005 | 1.271 ± 0.003 | 0.993 ± 0.005 | 0.897 ± 0.005 | 0.432 ± 0.003 | 0.830 ± 0.003 |
| BH 10 | 1.932 ± 0.005 | 1.932 ± 0.007 | 1.817 ± 0.053 | 1.896 ± 0.005 | 1.883 ± 0.004 | 1.914 ± 0.005 | 0.911 ± 0.003 | 1.337 ± 0.005 | 1.454 ± 0.005 | 1.808 ± 0.004 | 1.913 ± 0.005 | 1.463 ± 0.005 | 1.513 ± 0.006 | 1.220 ± 0.005 | 1.247 ± 0.040 |
| BH 15 | 1.404 ± 0.004 | 1.623 ± 0.009 | 1.433 ± 0.006 | 1.734 ± 0.003 | 1.853 ± 0.005 | 1.747 ± 0.005 | 0.614 ± 0.005 | 1.010 ± 0.004 | 0.946 ± 0.006 | 1.603 ± 0.004 | 1.797 ± 0.007 | 0.923 ± 0.004 | 1.125 ± 0.005 | 0.881 ± 0.003 | 1.123 ± 0.006 |
| BH 20 | 1.008 ± 0.007 | 1.433 ± 0.005 | 1.110 ± 0.005 | 1.370 ± 0.005 | 1.544 ± 0.005 | 1.304 ± 0.006 | 0.686 ± 0.005 | 0.894 ± 0.003 | 0.653 ± 0.003 | 0.995 ± 0.004 | 1.006 ± 0.004 | 0.553 ± 0.003 | 0.955 ± 0.005 | 0.858 ± 0.003 | 0.973 ± 0.007 |
| AH 10 | 1.636 ± 0.006 | 1.617 ± 0.006 | 1.602 ± 0.009 | 1.961 ± 0.008 | 1.735 ± 0.006 | 1.855 ± 0.006 | 1.855 ± 0.004 | 1.918 ± 0.006 | 1.105 ± 0.009 | 1.812 ± 0.003 | 1.894 ± 0.005 | 1.419 ± 0.004 | 1.402 ± 0.004 | 0.871 ± 0.007 | 1.172 ± 0.007 |
| AH 15 | 1.508 ± 0.006 | 1.432 ± 0.006 | 1.485 ± 0.007 | 1.548 ± 0.008 | 1.660 ± 0.006 | 1.698 ± 0.005 | 1.091 ± 0.006 | 1.212 ± 0.006 | 1.062 ± 0.004 | 1.586 ± 0.003 | 1.554 ± 0.005 | 1.093 ± 0.005 | 1.127 ± 0.004 | 0.767 ± 0.004 | 0.887 ± 0.006 |
| AH 20 | 0.841 ± 0.009 | 0.920 ± 0.008 | 1.237 ± 0.006 | 1.271 ± 0.007 | 1.214 ± 0.005 | 1.409 ± 0.006 | 0.948 ± 0.003 | 0.988 ± 0.005 | 0.416 ± 0.005 | 1.502 ± 0.012 | 1.509 ± 0.008 | 0.614 ± 0.004 | 0.821 ± 0.004 | 0.705 ± 0.003 | 0.805 ± 0.008 |
| LH 10 | 1.815 ± 0.006 | 1.851 ± 0.007 | 1.584 ± 0.018 | 1.954 ± 0.008 | 1.926 ± 0.007 | 1.626 ± 0.005 | 1.076 ± 0.006 | 1.326 ± 0.002 | 1.885 ± 0.004 | 1.958 ± 0.004 | 1.953 ± 0.009 | 1.837 ± 0.003 | 1.733 ± 0.008 | 0.975 ± 0.004 | 1.123 ± 0.004 |
| LH 15 | 1.182 ± 0.007 | 1.325 ± 0.005 | 1.276 ± 0.007 | 1.762 ± 0.009 | 1.864 ± 0.004 | 1.321 ± 0.003 | 1.002 ± 0.005 | 1.122 ± 0.006 | 1.376 ± 0.005 | 1.585 ± 0.005 | 1.689 ± 0.003 | 1.393 ± 0.006 | 1.595 ± 0.006 | 0.802 ± 0.007 | 0.736 ± 0.345 |
| LH 20 | 0.910 ± 0.008 | 0.993 ± 0.006 | 0.986 ± 0.007 | 1.414 ± 0.008 | 1.299 ± 0.004 | 1.339 ± 0.003 | 0.702 ± 0.008 | 0.809 ± 0.008 | 0.529 ± 0.003 | 1.396 ± 0.005 | 1.405 ± 0.004 | 0.826 ± 0.005 | 0.999 ± 0.006 | 0.779 ± 0.005 | 0.882 ± 0.007 |
| C | 1.859 ± 0.005 | 1.897 ± 0.005 | 1.620 ± 0.008 | 1.522 ± 0.003 | 1.429 ± 0.002 | 1.427 ± 0.005 | 1.532 ± 0.005 | 1.487 ± 0.005 | 1.337 ± 0.002 | 1.605 ± 0.006 | 1.719 ± 0.003 | 1.336 ± 0.002 | 1.292 ± 0.002 | 1.499 ± 0.004 | 1.397 ± 0.005 |
| Pyogenes | Staphilococcus | Shigella | Pseudomonas | E. coli | Salmonella | H. inflenzae | Parapsilopsis | C. albicans | TPC | TFC | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyogenes | 1 | |||||||||||
| Staphilococcus | 0.774 | 1.000 | ||||||||||
| Shigella | −0.001 | 0.006 | 1.000 | |||||||||
| Pseudomonas | −0.025 | 0.009 | −0.163 | 1.000 | ||||||||
| E coli | 0.513 | 0.364 | −0.470 | 0.090 | 1.000 | |||||||
| Salmonella | 0.823 | 0.588 | 0.014 | 0.430 | 0.339 | 1.000 | ||||||
| H. influenzae | 0.523 | 0.531 | 0.357 | −0.377 | −0.038 | 0.292 | 1.000 | |||||
| Parapsilopsis | 0.696 | 0.608 | 0.130 | −0.127 | 0.366 | 0.546 | 0.876 | 1.000 | ||||
| C albicans | 0.556 | 0.681 | −0.462 | 0.011 | 0.497 | 0.434 | −0.058 | 0.128 | 1.000 | |||
| TPC | −0.776 | −0.686 | −0.321 | 0.367 | −0.145 | −0.457 | −0.890 | −0.808 | −0.150 | 1.000 | ||
| TFC | −0.522 | −0.361 | −0.539 | 0.532 | 0.036 | −0.197 | −0.884 | −0.713 | 0.207 | 0.908 | 1.000 | |
| DPPH | −0.733 | −0.620 | −0.305 | 0.448 | −0.119 | −0.380 | −0.889 | −0.780 | −0.103 | 0.993 | 0.931 | 1.000 |
| E. coli (BIDLMV 2023) | E. coli (BIDLMV 2067) | E. coli (BIDLMV 21117) | B. cepacia (BIDLMV 219) | B. cepacian (BIDLMV 21952) | P. vulgaris (BID-LMV 20624) | P. vulgaris (BIDLMV 20124) | P. vulgaris (BIDLMV 2147) | P. aeruginosa (BIDLMV 19117) | P. aeruginosa (BIDLMV 19121) | P. aeruginosa (BIDLMV 20129) | P. aeruginosa (BIDLMV 20722) | P. aeruginosa (BIDLMV 21615) | M. pachydermatis (BIDLMV 20127) | M. pachydermatis (BIDLMV 21521) | TPC | TFC | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli (BIDLMV 2023) | 1 | |||||||||||||||||
| E. coli (BIDLMV 2067) | 0.932 | 1 | ||||||||||||||||
| E. coli (BIDLMV 21117) | 0.949 | 0.826 | 1 | |||||||||||||||
| E. coli (BIDLMV 21117) | 0.689 | 0.723 | 0.578 | 1 | ||||||||||||||
| B. cepacia (BIDLMV 21952) | 0.661 | 0.750 | 0.528 | 0.919 | 1 | |||||||||||||
| P. vulgaris (BID-LMV 20624) | 0.761 | 0.746 | 0.700 | 0.575 | 0.619 | 1 | ||||||||||||
| P. vulgaris (BIDLMV 20124) | 0.718 | 0.748 | 0.666 | 0.475 | 0.461 | 0.907 | 1 | |||||||||||
| P. vulgaris (BIDLMV 2147) | 0.337 | 0.400 | 0.296 | 0.240 | 0.511 | 0.542 | 0.401 | 1 | ||||||||||
| P. aeruginosa (BIDLMV 19117) | 0.649 | 0.504 | 0.645 | 0.502 | 0.491 | 0.529 | 0.304 | 0.167 | 1 | |||||||||
| P. aeruginosa (BIDLMV 19121) | 0.703 | 0.634 | 0.683 | 0.426 | 0.534 | 0.658 | 0.428 | 0.487 | 0.903 | 1 | ||||||||
| P. aeruginosa (BIDLMV 20129) | 0.707 | 0.763 | 0.569 | 0.474 | 0.476 | 0.750 | 0.715 | 0.409 | 0.372 | 0.553 | 1 | |||||||
| P. aeruginosa (BIDLMV 20722) | 0.652 | 0.521 | 0.649 | 0.224 | 0.301 | 0.691 | 0.512 | 0.538 | 0.485 | 0.662 | 0.718 | 1 | ||||||
| P. aeruginosa (BIDLMV 21615) | 0.770 | 0.646 | 0.775 | 0.372 | 0.405 | 0.773 | 0.641 | 0.460 | 0.541 | 0.674 | 0.684 | 0.943 | 1 | |||||
| M. pachydermatis (BIDLMV 20127) | 0.706 | 0.687 | 0.580 | 0.417 | 0.440 | 0.786 | 0.653 | 0.441 | 0.516 | 0.681 | 0.948 | 0.843 | 0.794 | 1 | ||||
| M. pachydermatis (BIDLMV 21521 | 0.735 | 0.717 | 0.641 | 0.395 | 0.338 | 0.822 | 0.791 | 0.237 | 0.464 | 0.584 | 0.900 | 0.731 | 0.770 | 0.926 | 1 | |||
| TPC | −0.319 | −0.085 | −0.517 | 0.254 | 0.356 | −0.002 | −0.104 | 0.366 | −0.261 | −0.133 | 0.206 | −0.101 | −0.213 | 0.146 | 0.062 | 1 | ||
| TFC | −0.278 | −0.075 | −0.507 | 0.216 | 0.231 | 0.061 | −0.016 | 0.124 | −0.324 | −0.238 | 0.320 | −0.043 | −0.140 | 0.269 | 0.136 | 0.908 | 1 | |
| DPPH | −0.328 | 0.121 | −0.531 | 0.230 | 0.316 | 0.003 | −0.116 | 0.322 | −0.227 | −0.125 | 0.224 | −0.067 | −0.192 | 0.183 | −0.029 | 0.993 | 0.931 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. https://doi.org/10.3390/antibiotics11050595
Hulea A, Obiștioiu D, Cocan I, Alexa E, Negrea M, Neacșu A-G, Hulea C, Pascu C, Costinar L, Iancu I, et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics. 2022; 11(5):595. https://doi.org/10.3390/antibiotics11050595
Chicago/Turabian StyleHulea, Anca, Diana Obiștioiu, Ileana Cocan, Ersilia Alexa, Monica Negrea, Alina-Georgeta Neacșu, Călin Hulea, Corina Pascu, Luminita Costinar, Ionica Iancu, and et al. 2022. "Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential" Antibiotics 11, no. 5: 595. https://doi.org/10.3390/antibiotics11050595
APA StyleHulea, A., Obiștioiu, D., Cocan, I., Alexa, E., Negrea, M., Neacșu, A.-G., Hulea, C., Pascu, C., Costinar, L., Iancu, I., Tîrziu, E., & Herman, V. (2022). Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics, 11(5), 595. https://doi.org/10.3390/antibiotics11050595









