Antimicrobial Susceptibility of Enterococcus Isolates from Cattle and Pigs in Portugal: Linezolid Resistance Genes optrA and poxtA
Abstract
:1. Introduction
2. Results
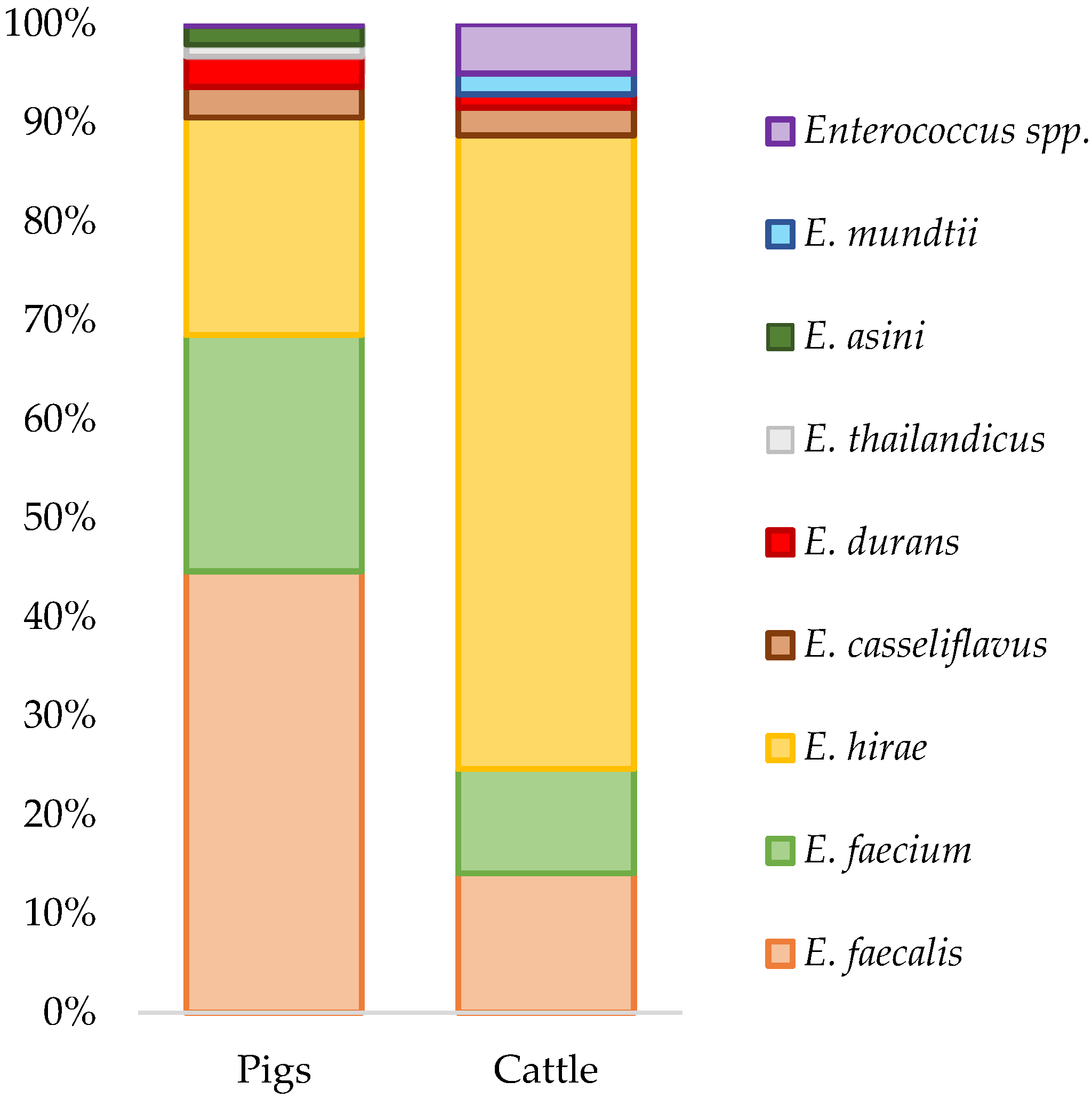
2.1. Enterococcus Isolation and Species Diversity
2.2. Antimicrobial Susceptibility Testing by Agar Dilution
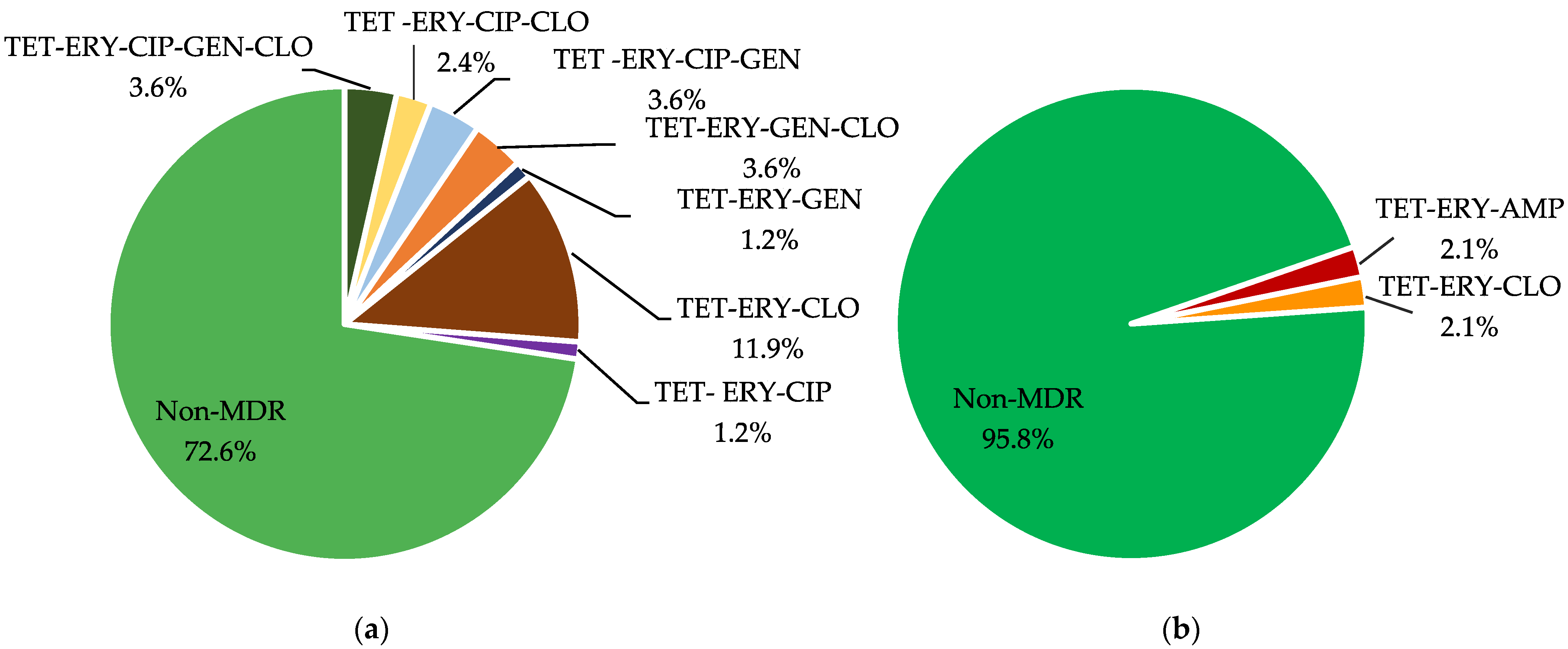
2.3. PCR Screening of Antimicrobial Resistance Determinants
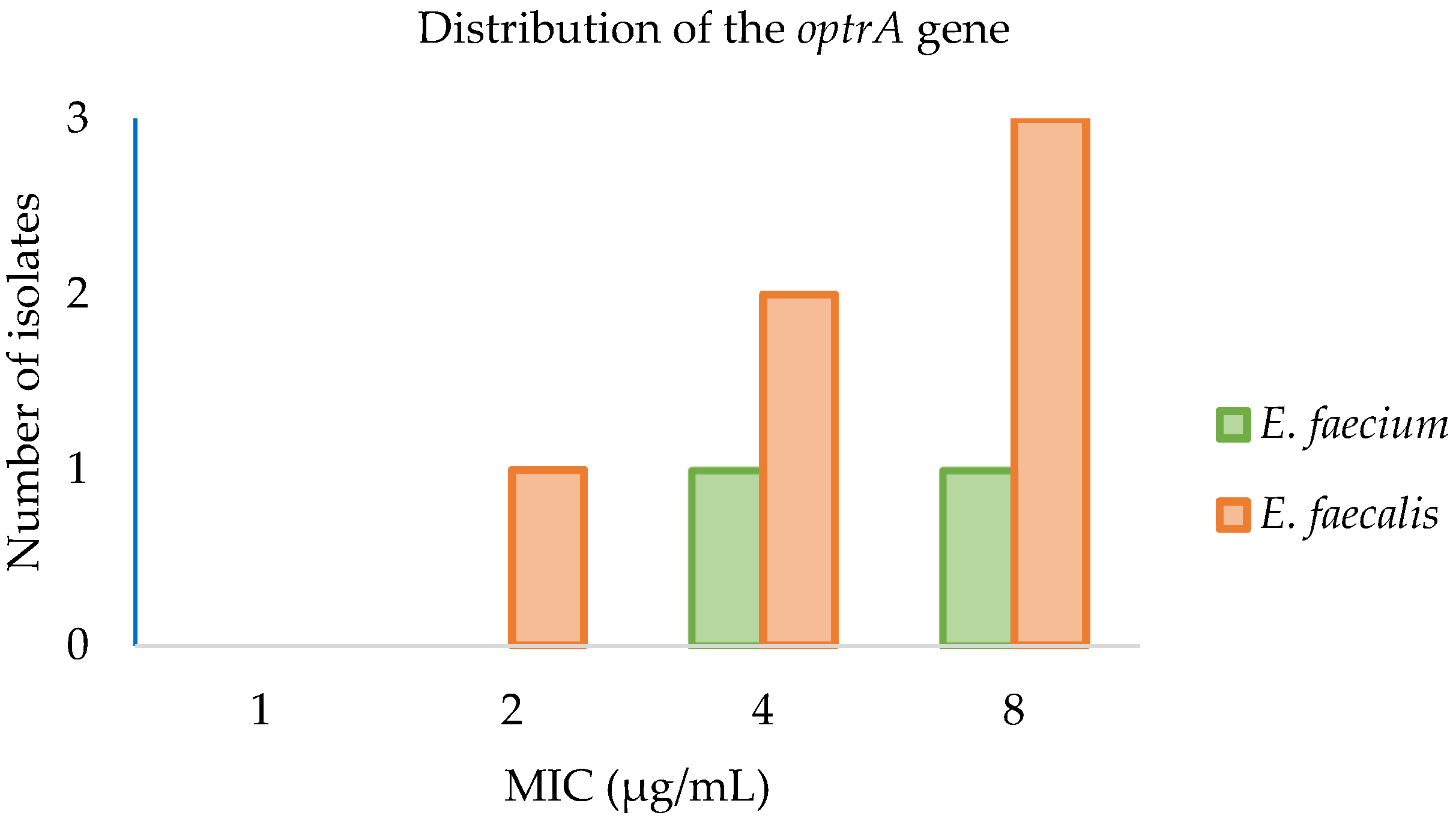
2.4. Antimicrobial Susceptibility Testing of optrA Positive Strains by Microdilution
2.5. Genomic Characterization of Isolates
2.5.1. Molecular Characterization of Linezolid Resistance Mechanisms
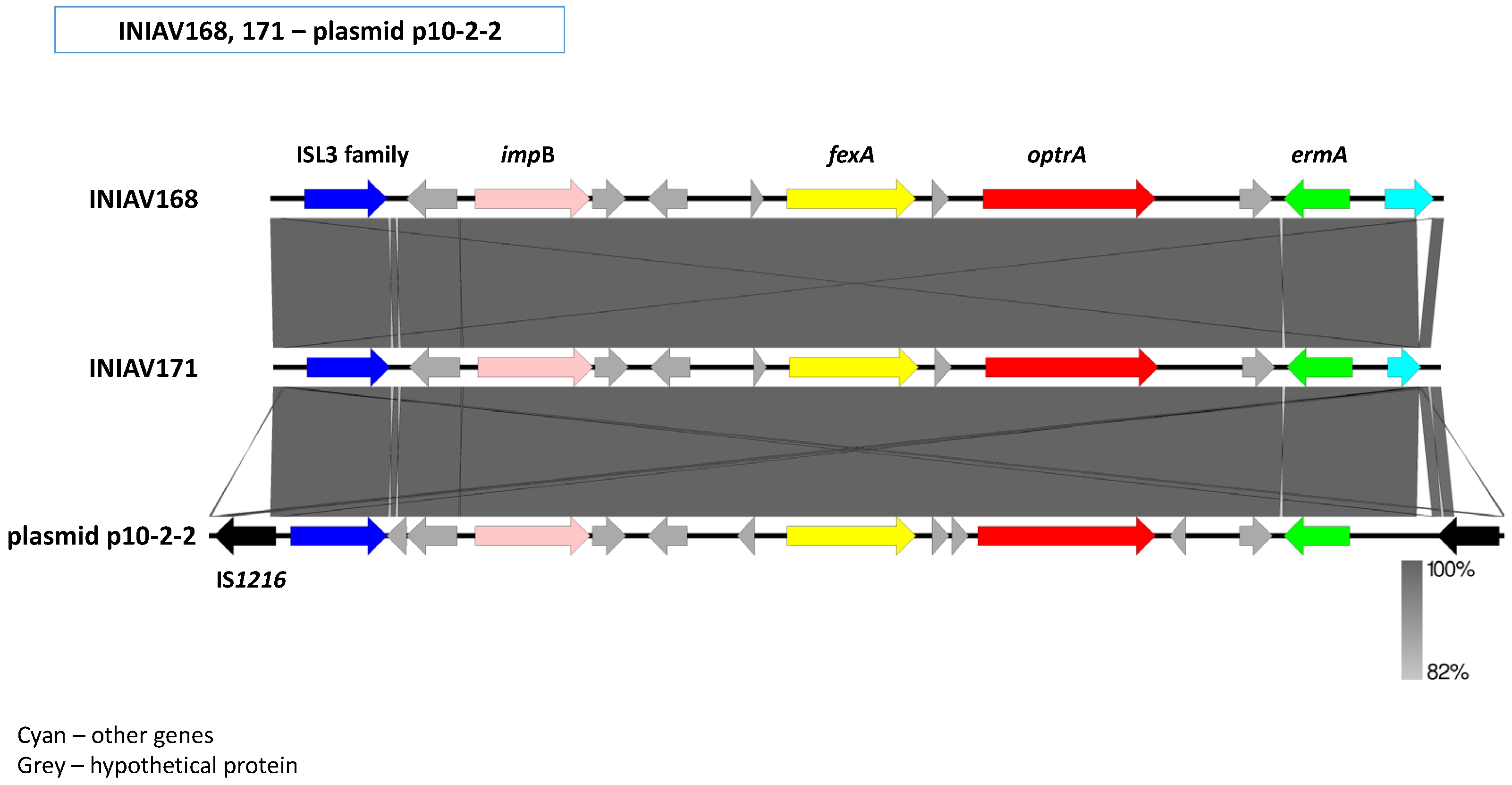
2.5.2. Genetic Environment of the optrA Gene
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolation and Species Identification
4.2. Antimicrobial Susceptibility Testing
4.3. PCR Screening of Antibiotic Resistance Genes
4.4. Whole-Genome Sequencing
4.5. Statistical Analysis
4.6. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Isolate | Species | Sample Source | Geographic Location | MLST | EUVENC MDR Profile | Acquired Antimicrobial Resistance Genes and Mutations | Plasmid Replicons | Virulence Genes | Accession Number |
|---|---|---|---|---|---|---|---|---|---|
| INIAV004 | E. faecium | Swine | 39.2333, −8.68333 | ST22 | TET-ERY-LZD-CLO | poxtA, optrA, fex(B), tet(M), tet(L), erm(A), pbp5 (T172A), pbp5 (L177I), pbp5 (A216S), pbp5 (P667S), pbp5 (D204G), pbp5 (K144Q), pbp5 (R34Q), pbp5 (S27G), pbp5 (E100Q), pbp5 (A499T), pbp5 (G66E), pbp5 (T324A), pbp5 (A68T), pbp5 (V24A), pbp5 (N496K), pbp5 (E525D), pbp5 (E85D) | rep29, rep33, repUS43, rep1, rep2, repUS15 | acm, efaAfm | ERS6142029 |
| INIAV173 | E. faecium | Swine | 38.9167, −9.2667 | ST2138 | TET-ERY-CLO | poxtA, optrA, fex(B), erm(A), ant(9)-Ia; | rep1; rep11c; rep18b; rep29; repUS15; repUS43 | acm; efaAfm | ERS11708758 |
| INIAV005 | E. faecalis | Swine | 38.9485, −9.1967 | ST93 | TET-ERY- DAP | aac(6′)-aph(2″), tet(M), tet(L), erm(B) | rep9a | elrA, srtA, ace, cCf10, cOB1, cad, camE, ebpA, ebpC, efaAfs, hylA, tpx, | ERS6142031 |
| INIAV168 | E. faecalis | Swine | 41.4124, −8.5206 | ST207 | TET -ERY-LZD-CLO | optrA; fex(A), tet(M), tet(L), erm(A), erm(B), clpL | repUS1; rep9b; repUS43 | elrA; srtA; ace; agg; cCF10; cOB1; cad; camE; ebpA; ebpC; efaAfs; fsrB; gelE; hylB; tpx | ERS11708754 |
| INIAV169 | E. faecalis | Swine | 41.4124, −8.5206 | ST474 | TET-ERY-CIP-GEN-CLO | optrA, fex(A), cat, aac(6′)-aph(2″), aph(3′)-III, tet(M), tet(L), erm(A), erm(B), dfrG, lnu(B); gyrA (E87G), parC (S80I), lsa(E) | rep9a; repUS43 | elrA; srtA; ace; cCF10; cOB1; cad; camE; ebpA; ebpC; efaAfs; fsrB; gelE; hylA; hylB; tpx | ERS11708755 |
| INIAV170 | E. faecalis | Swine | 38.7058, −8.97462 | ST474 | TET-ERY-CIP-GEN-CLO | optrA, fex(A), cat, aac(6′)-aph(2″), aph(3′)-III, tet(M), tet(L), erm(A), erm(B), dfrG, gyrA (E87G), parC (S80I), lnu(B), lsa(E) | rep9a; repUS43 | elrA; srtA; ace; cCF10; cOB1; cad; camE; ebpA; ebpC; efaAfs; fsrB; gelE; hylA; hylB; tpx | ERS11708756 |
| INIAV171 | E. faecalis | Swine | 39.4598, −8.6671 | ST16 | TET-ERY-LZD-CLO | optrA, fex(A), cat, aac(6′)-aph(2″), aph(3′)-III, str, tet(M), erm(A), erm(B), str, lnu(B), lsa(E) | rep6; rep9b; repUS43 | elrA; srtA; ace; agg; cCF10; cOB1; cad; camE; cylA; cylL; cylM; ebpA; ebpC; efaAfs; hylA; tpx | ERS11708757 |
| INIAV174 | E. faecalis | Swine | 38.9167, −9.2667 | ST1178 | TET-ERY-CIP-CLO | optrA, cat, ant(9)-Ia, tet(M), tet(L), erm(A), erm(B), gyrA (E87G), parC (S80I) | rep9a; repUS43 | elrA; srtA; ace; cCF10; cOB1; cad; camE; ebpA; ebpC; efaAfs; fsrB; gelE; hylA; hylB; tpx | ERS11708759 |
| INIAV175 | E. faecalis | Swine | 39.4598, −8.6671 | ST58 | TET-ERY-DAP | tet (M), erm(B), ant(6)-Ia, dfrG, lnu(B), lsa(E) | repUS43 | elrA; srtA; ace; cCF10; cOB1; cad; camE; ebpA; ebpC; efaAfs; fsrB; gelE; hylA; tpx | ERS11708760 |
References
- LPSN—List of Prokaryotic Names with Standing in Nomenclature. Genus Enterococcus. 1997. Available online: https://www.bacterio.net/genus/enterococcus (accessed on 20 December 2021).
- Staley, C.; Dunny, G.M.; Sadowsky, M.J. Environmental and Animal-Associated Enterococci. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 87, pp. 147–186. [Google Scholar] [CrossRef]
- Devriese, L.A.; Hommez, J.; Wijfels, R.; Haesebrouck, F. Composition of the Enterococcal and Streptococcal Intestinal Flora of Poultry. J. Appl. Bacteriol. 1991, 71, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, F.; Willems, R.; Gilmore, M. Enterococcus Diversity, Origins in Nature, and Gut Colonization. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Molina, M.; Cyterski, M.; Maimes, J.; Fisher, J.; Johnson, B. Comparison of the Temporal Variability of Enterococcal Clusters in Impacted Streams Using a Multiplex Polymerase Chain Reaction Procedure. In Proceedings of the 2007 Georgia Water Resources Conference, Athens, GA, USA, 27–29 March 2007. [Google Scholar]
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, J.K.; Cattoir, V.; Hegstad, K.; Sadowy, E.; Coque, T.M.; Westh, H.; Hammerum, A.M.; Schaffer, K.; Burns, K.; Murchan, S.; et al. Update on Prevalence and Mechanisms of Resistance to Linezolid, Tigecycline and Daptomycin in Enterococci in Europe: Towards a Common Nomenclature. Drug Resist. Updates 2018, 40, 25–39. [Google Scholar] [CrossRef]
- Advice on the Designation of Antimicrobials or Groups of Antimicrobials Reserved for Treatment of Certain Infections in Humans—In Relation to Implementing Measures under Article 37(5) of Regulation (EU) 2019/6 on Veterinary Medicinal Products EMA/CVMP/678496/2021Committee for Veterinary Medicinal Products (CVMP). Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/advice-designation-antimicrobials-groups-antimicrobials-reserved-treatment-certain-infections-humans/6-veterinary-medicinal-products_en.pdf (accessed on 16 February 2022).
- Hammerum, A.M. Enterococci of Animal Origin and Their Significance for Public Health. Clin. Microbiol. Infect. 2012, 18, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O. Vancomycin Resistant Enterococci in Farm Animals—Occurrence and Importance. Infect. Ecol. Epidemiol. 2012, 2, 16959. [Google Scholar] [CrossRef] [Green Version]
- EU Action on Antimicrobial Resistance. Available online: https://ec.europa.eu/health/antimicrobial-resistance/eu-action-antimicrobial-resistance_en (accessed on 15 December 2021).
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef] [Green Version]
- Bender, J.K.; Fleige, C.; Klare, I.; Fiedler, S.; Mischnik, A.; Mutters, N.T.; Dingle, K.E.; Werner, G. Detection of a Cfr(B) Variant in German Enterococcus faecium Clinical Isolates and the Impact on Linezolid Resistance in Enterococcus spp. PLoS ONE 2016, 11, e0167042. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A Novel Gene, OptrA, That Confers Transferable Resistance to Oxazolidinones and Phenicols and Its Presence in Enterococcus faecalis and Enterococcus faecium of Human and Animal Origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, A.; D’Andrea, M.M.; Brenciani, A.; Galeotti, C.L.; Morroni, G.; Pollini, S.; Varaldo, P.E.; Rossolini, G.M. Characterization of PoxtA, a Novel Phenicol–Oxazolidinone–Tetracycline Resistance Gene from an MRSA of Clinical Origin. J. Antimicrob. Chemother. 2018, 73, 1763–1769. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Munita, J.M.; Arias, C.A. Mechanisms of Drug Resistance: Daptomycin Resistance: Daptomycin Resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 32–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, O.E.; Pedersen, K.; Andersen, J.S.; Madsen, M. Vancomycin-Resistant Enterococci (VRE) in Broiler Flocks 5 Years after the Avoparcin Ban. Microb. Drug Resist. 2002, 8, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Coque, T.M.; Costa, M.J.; Sousa, J.C.; Baquero, F.; Peixe, L.V. High Occurrence and Persistence of Antibiotic-Resistant Enterococci in Poultry Food Samples in Portugal. J. Antimicrob. Chemother. 2005, 56, 1139–1143. [Google Scholar] [CrossRef]
- Sørum, M.; Johnsen, P.J.; Aasnes, B.; Rosvoll, T.; Kruse, H.; Sundsfjord, A.; Simonsen, G.S. Prevalence, Persistence, and Molecular Characterization of Glycopeptide-Resistant Enterococci in Norwegian Poultry and Poultry Farmers 3 to 8 Years after the Ban on Avoparcin. Appl. Environ. Microbiol. 2006, 72, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Migura, L.; Liebana, E.; Jensen, L.B.; Barnes, S.; Pleydell, E. A Longitudinal Study to Assess the Persistence of Vancomycin-Resistant Enterococcus faecium (VREF) on an Intensive Broiler Farm in the United Kingdom. FEMS Microbiol. Lett. 2007, 275, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, A.; Simjee, S.; Garch, F.E.; Moyaert, H.; Rose, M.; Youala, M.; Dry, M. Antimicrobial Susceptibility of Enterococci Recovered from Healthy Cattle, Pigs and Chickens in Nine EU Countries (EASSA Study) to Critically Important Antibiotics. Vet. Microbiol. 2018, 216, 168–175. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. EU Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2013. EFS2 2015, 13, 4036. [Google Scholar] [CrossRef] [Green Version]
- Timmermans, M.; Bogaerts, B.; Vanneste, K.; De Keersmaecker, S.C.J.; Roosens, N.H.C.; Kowalewicz, C.; Simon, G.; Argudín, M.A.; Deplano, A.; Hallin, M.; et al. Large Diversity of Linezolid-Resistant Isolates Discovered in Food-Producing Animals through Linezolid Selective Monitoring in Belgium in 2019. J. Antimicrob. Chemother. 2021, 77, 49–57. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Feßler, A.T.; Hanke, D.; Sanz, S.; Olarte, C.; Eichhorn, I.; Schwarz, S.; Torres, C. Detection of PoxtA- and OptrA-Carrying E. Faecium Isolates in Air Samples of a Spanish Swine Farm. J. Glob. Antimicrob. Resist. 2020, 22, 28–31. [Google Scholar] [CrossRef]
- Moure, Z.; Lara, N.; Marín, M.; Sola-Campoy, P.J.; Bautista, V.; Gómez-Bertomeu, F.; Gómez-Dominguez, C.; Pérez-Vázquez, M.; Aracil, B.; Campos, J.; et al. Interregional Spread in Spain of Linezolid-Resistant Enterococcus spp. Isolates Carrying the OptrA and PoxtA Genes. Int. J. Antimicrob. Agents 2020, 55, 105977. [Google Scholar] [CrossRef]
- Egan, S.A.; Shore, A.C.; O’Connell, B.; Brennan, G.I.; Coleman, D.C. Linezolid Resistance in Enterococcus faecium and Enterococcus faecalis from Hospitalized Patients in Ireland: High Prevalence of the MDR Genes OptrA and PoxtA in Isolates with Diverse Genetic Backgrounds. J. Antimicrob. Chemother. 2020, 75, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Guérin, F.; Zouari, A.; Beyrouthy, R.; Auzou, M.; Fines-Guyon, M.; Potrel, S.; Dejoies, L.; Collet, A.; Boukthir, S.; et al. Emergence of OptrA-Mediated Linezolid Resistance in Enterococci from France, 2006–2016. J. Antimicrob. Chemother. 2019, 74, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.P.; Parcell, B.J.; Pettigrew, K.A.; Toner, G.; Khatamzas, E.; Karcher, A.M.; Walker, J.; Weir, R.; Meunier, D.; Hopkins, K.L.; et al. Emergence of OptrA-Mediated Linezolid Resistance in Multiple Lineages and Plasmids of Enterococcus faecalis Revealed by Long Read Sequencing. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Kelesidis, T.; Humphries, R.; Uslan, D.Z.; Pegues, D.A. Daptomycin Nonsusceptible Enterococci: An Emerging Challenge for Clinicians. Clin. Infect. Dis. 2011, 52, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Ruzauskas, M.; Virgailis, M.; Šiugždinien, R. Antimicrobial Resistance of Enterococcus spp. Isolated from Livestock in Lithuania. Vet. Arch. 2009, 79, 439–449. [Google Scholar]
- DANMAP—Danish Integrated Antimicrobial Resistance Monitoring and Research Programm, 2019. Resistance in Indicator Bacteria. Available online: https://www.danmap.org (accessed on 3 December 2021).
- Morroni, G.; Brenciani, A.; Simoni, S.; Vignaroli, C.; Mingoia, M.; Giovanetti, E. Commentary: Nationwide Surveillance of Novel Oxazolidinone Resistance Gene OptrA in Enterococcus Isolates in China from 2004 to 2014. Front. Microbiol. 2017, 8, 1631. [Google Scholar] [CrossRef] [Green Version]
- Freitas, A.R.; Tedim, A.P.; Novais, C.; Lanza, V.F.; Peixe, L. Comparative Genomics of Global OptrA-Carrying Enterococcus Faecalis Uncovers a Common Chromosomal Hotspot for OptrA Acquisition within a Diversity of Core and Accessory Genomes. Microb. Genom. 2020, 6, e000350. [Google Scholar] [CrossRef]
- Almeida, L.M.; Lebreton, F.; Gaca, A.; Bispo, P.M.; Saavedra, J.T.; Calumby, R.N.; Grillo, L.M.; Nascimento, T.G.; Filsner, P.H.; Moreno, A.M.; et al. Transferable Resistance Gene OptrA in Enterococcus Faecalis from Swine in Brazil. Antimicrob. Agents Chemother. 2020, 64, e00142-20. [Google Scholar] [CrossRef]
- Silva, N.; Igrejas, G.; Gonçalves, A.; Poeta, P. Commensal Gut Bacteria: Distribution of Enterococcus Species and Prevalence of Escherichia coli Phylogenetic Groups in Animals and Humans in Portugal. Ann. Microbiol. 2012, 62, 449–459. [Google Scholar] [CrossRef]
- Kühn, I. Comparison of Enterococcal Populations in Animals, Humans, and the Environment—A European Study. Int. J. Food Microbiol. 2003, 88, 133–145. [Google Scholar] [CrossRef]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. Reveals Distinct Species and Antimicrobial Resistance Diversity across a One-Health Continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef] [PubMed]
- Novais, C. First Report of the Activity of Linezolid against Portuguese Enterococci from Human, Animal and Environmental Sources. J. Antimicrob. Chemother. 2003, 51, 1314–1315. [Google Scholar] [CrossRef] [PubMed]
- Beukers, A.G.; Zaheer, R.; Goji, N.; Amoako, K.K.; Chaves, A.V.; Ward, M.P.; McAllister, T.A. Comparative Genomics of Enterococcus Spp. Isolated from Bovine Feces. BMC Microbiol. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byappanahalli, M.N.; Nevers, M.B.; Korajkic, A.; Staley, Z.R.; Harwood, V.J. Enterococci in the Environment. Microbiol. Mol. Biol. Rev. 2012, 76, 685–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosecka-Strojek, M.; Wolska, M.; Żabicka, D.; Sadowy, E.; Międzobrodzki, J. Identification of Clinically Relevant Streptococcus and Enterococcus Species Based on Biochemical Methods and 16S RRNA, sodA, tuf, rpoB, and recA Gene Sequencing. Pathogens 2020, 9, 939. [Google Scholar] [CrossRef]
- European Medicines Agency; European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020 (EMA/58183/2021). 2021. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2019-2020-trends-2010-2020-eleventh_en.pdf (accessed on 6 April 2022).
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Editorial: Assessing the Antimicrobial Susceptibility of Bacteria Obtained from Animals. J. Antimicrob. Chemother. 2010, 65, 601–604. [Google Scholar] [CrossRef]
- Marinho, C.M.; Santos, T.; Gonçalves, A.; Poeta, P.; Igrejas, G. A Decade-Long Commitment to Antimicrobial Resistance Surveillance in Portugal. Front. Microbiol. 2016, 7, 1650. [Google Scholar] [CrossRef]
- Commission Directive 97/6/EC 30 January 1997 ammending Counsil Directive 70/524/EEC Concerning Additives in Feedingstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31997L0006 (accessed on 6 April 2022).
- Aasmäe, B.; Häkkinen, L.; Kaart, T.; Kalmus, P. Antimicrobial Resistance of Escherichia coli and Enterococcus spp. Isolated from Estonian Cattle and Swine from 2010 to 2015. Acta Vet. Scand. 2019, 61, 5. [Google Scholar] [CrossRef]
- APHIS–United States Department of Agriculture. Commensal Enterococcus on U.S. Swine Sites: Prevalence and Antimicrobial Drug Susceptibility. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth (accessed on 1 December 2021).
- Liu, Y.; Liu, K.; Lai, J.; Wu, C.; Shen, J.; Wang, Y. Prevalence and Antimicrobial Resistance of Enterococcus Species of Food Animal Origin from Beijing and Shandong Province, China. J. Appl. Microbiol. 2013, 114, 555–563. [Google Scholar] [CrossRef]
- Tan, S.C.; Chong, C.W.; Teh, C.S.J.; Ooi, P.T.; Thong, K.L. Occurrence of Virulent Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium in the Pigs, Farmers and Farm Environments in Malaysia. Peer J. 2018, 6, e5353. [Google Scholar] [CrossRef] [Green Version]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and Acquired Resistance Mechanisms in Enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUCAST—The European Committee on Antimicrobial Susceptibility Testing, 2020. EUCAST Expert Rules v 3.2 on Enterococcus spp. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/2020/ExpertRules_V3.2_20190515_Enterococcus_revision_20200224.pdf (accessed on 1 December 2021).
- Galloway-Peña, J.R.; Rice, L.B.; Murray, B.E. Analysis of PBP5 of Early U.S. Isolates of Enterococcus faecium: Sequence Variation Alone Does Not Explain Increasing Ampicillin Resistance over Time. Antimicrob. Agents Chemother. 2011, 55, 3272–3277. [Google Scholar] [CrossRef] [Green Version]
- Montealegre, M.C.; Roh, J.H.; Rae, M.; Davlieva, M.G.; Singh, K.V.; Shamoo, Y.; Murray, B.E. Differential Penicillin-Binding Protein 5 (PBP5) Levels in the Enterococcus faecium Clades with Different Levels of Ampicillin Resistance. Antimicrob. Agents Chemother. 2017, 61, e02034-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, L.B.; Bellais, S.; Carias, L.L.; Hutton-Thomas, R.; Bonomo, R.A.; Caspers, P.; Page, M.G.P.; Gutmann, L. Impact of Specific Pbp5 Mutations on Expression of β-Lactam Resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 2004, 48, 3028–3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietta, E.; Montealegre, M.C.; Roh, J.H.; Cocconcelli, P.S.; Murray, B.E. Enterococcus faecium PBP5-S/R, the Missing Link between PBP5-S and PBP5-R. Antimicrob. Agents Chemother. 2014, 58, 6978–6981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, X.; Fu, Y.; Chen, Y.; Wang, Y.; Ye, D.; Wang, C.; Hu, X.; Zhou, L.; Du, J.; et al. Association of Florfenicol Residues with the Abundance of Oxazolidinone Resistance Genes in Livestock Manures. J. Hazard. Mater. 2020, 399, 123059. [Google Scholar] [CrossRef]
- European Food Safety Authority. Report from the Task Force on Zoonoses Data Collection including guidance for harmonized monitoring and reporting of antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. from food animals. EFSA J. 2008, 141, 1–44. [Google Scholar]
- Càmara, J.; Camoez, M.; Tubau, F.; Pujol, M.; Ayats, J.; Ardanuy, C.; Domínguez, M.Á. Detection of the Novel OptrA Gene Among Linezolid-Resistant Enterococci in Barcelona, Spain. Microb. Drug Resist. 2019, 25, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Wang, Y.; Schwarz, S.; Lv, H.; Li, Y.; Liao, K.; Yu, S.; Zhao, K.; Gu, D.; Wang, X.; et al. Enterococcal Isolates Carrying the Novel Oxazolidinone Resistance Gene OptrA from Hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin. Microbiol. Infect. 2015, 21, e1–e1095. [Google Scholar] [CrossRef] [Green Version]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-Access Bacterial Population Genomics: BIGSdb Software, the PubMLST.Org Website and Their Applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Tamang, M.D.; Moon, D.C.; Kim, S.-R.; Kang, H.Y.; Lee, K.; Nam, H.-M.; Jang, G.-C.; Lee, H.-S.; Jung, S.-C.; Lim, S.-K. Detection of Novel Oxazolidinone and Phenicol Resistance Gene OptrA in Enterococcal Isolates from Food Animals and Animal Carcasses. Vet. Microbiol. 2017, 201, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Vorobieva, V.; Roer, L.; Justesen, U.S.; Hansen, F.; Frimodt-Møller, N.; Hasman, H.; Hammerum, A.M. Detection of the OptrA Gene in a Clinical ST16 Enterococcus faecalis Isolate in Denmark. J. Glob. Antimicrob. Resist. 2017, 10, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Tsilipounidaki, K.; Gerontopoulos, A.; Papagiannitsis, C.; Petinaki, E. First Detection of an OptrA-Positive, Linezolid-Resistant ST16 Enterococcus faecalis from Human in Greece. New Microbes New Infect. 2019, 29, 100515. [Google Scholar] [CrossRef]
- Chen, M.; Pan, H.; Lou, Y.; Wu, Z.; Zhang, J.; Huang, Y.; Yu, W.; Qiu, Y. Epidemiological Characteristics and Genetic Structure of Linezolid-Resistant Enterococcus faecalis. Infect. Drug Resist. 2018, 11, 2397–2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Yang, Y.; Ding, L.; Xu, X.; Lin, D. Molecular Investigations of Linezolid Resistance in Enterococci OptrA Variants from a Hospital in Shanghai. Infect. Drug Resist. 2020, 13, 2711–2716. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Freitas, A.R.; Tedim, A.P.; Duarte, B.; Elghaieb, H.; Abbassi, M.S.; Hassen, A.; Read, A.; Alves, V.; Novais, C.; Peixe, L. Linezolid-Resistant (Tn 6246::FexB—PoxtA) Enterococcus faecium Strains Colonizing Humans and Bovines on Different Continents: Similarity without Epidemiological Link. J. Antimicrob. Chemother. 2020, 75, 2416–2423. [Google Scholar] [CrossRef]
- Cai, J.; Schwarz, S.; Chi, D.; Wang, Z.; Zhang, R.; Wang, Y. Faecal Carriage of OptrA-Positive Enterococci in Asymptomatic Healthy Humans in Hangzhou, China. Clin. Microbiol. Infect. 2019, 25, e1–e630. [Google Scholar] [CrossRef]
- He, T.; Shen, Y.; Schwarz, S.; Cai, J.; Lv, Y.; Li, J.; Feßler, A.T.; Zhang, R.; Wu, C.; Shen, J.; et al. Genetic Environment of the Transferable Oxazolidinone/Phenicol Resistance Gene OptrA in Enterococcus faecalis Isolates of Human and Animal Origin. J. Antimicrob. Chemother. 2016, 71, 1466–1473. [Google Scholar] [CrossRef] [Green Version]
- Zankari, E.; Hasman, H.; Kaas, R.S.; Seyfarth, A.M.; Agerso, Y.; Lund, O.; Larsen, M.V.; Aarestrup, F.M. Genotyping Using Whole-Genome Sequencing Is a Realistic Alternative to Surveillance Based on Phenotypic Antimicrobial Susceptibility Testing. J. Antimicrob. Chemother. 2013, 68, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, R.L. Enterococcus faecalis: Nuevas perspectivas sobre la estructura poblacional y el impacto de los elementos genéticos móviles en la evolución. Ph.D. Thesis, Universidad Complutense, Faculdad de Farmacia, Madrid, Spain, 2017. [Google Scholar]
- Kim, Y.B.; Seo, H.J.; Seo, K.W.; Jeon, H.Y.; Kim, D.K.; Kim, S.W.; Lim, S.-K.; Lee, Y.J. Characteristics of High-Level Ciprofloxacin-Resistant Enterococcus faecalis and Enterococcus faecium from Retail Chicken Meat in Korea. J. Food Prot. 2018, 81, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Huang, X.; Wen, Y.; Zhao, Q.; Huang, X.; Xia, J.; Huang, Y.; Cao, S.; Du, S.; et al. Molecular Characterization of Antimicrobial Resistance and Virulence Factors of Enterococcus faecalis from Ducks at Slaughterhouses. Poult. Sci. 2022, 101, 101646. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.L.; Bisgaard, M.; Son, N.T.; Trung, N.V.; An, H.M.; Dalsgaard, A. Enterococcus faecalis Clones in Poultry and in Humans with Urinary Tract Infections, Vietnam. Emerg. Infect. Dis. 2012, 18, 1096–1100. [Google Scholar] [CrossRef]
- Kim, E.B.; Marco, M.L. Nonclinical and Clinical Enterococcus Faecium Strains, but Not Enterococcus faecalis Strains, Have Distinct Structural and Functional Genomic Features. Appl. Environ. Microbiol. 2014, 80, 154–165. [Google Scholar] [CrossRef] [Green Version]
- McBride, S.M.; Fischetti, V.A.; LeBlanc, D.J.; Moellering, R.C.; Gilmore, M.S. Genetic Diversity among Enterococcus faecalis. PLoS ONE 2007, 2, e582. [Google Scholar] [CrossRef]
- Elghaieb, H.; Freitas, A.R.; Abbassi, M.S.; Novais, C.; Zouari, M.; Hassen, A.; Peixe, L. Dispersal of linezolid-resistant enterococci carrying PoxtA or OptrA in retail meat and food-producing animals from Tunisia. J. Antimicrob. Chemother. 2019, 74, 2865–2869. [Google Scholar] [CrossRef]
- Deasy, B.M.; Rea, M.C.; Fitzgerald, G.F.; Cogan, T.M.; Beresford, T.P. A Rapid PCR Based Method to Distinguish between Lactococcus and Enterococcus. Syst. Appl. Microbiol. 2000, 23, 510–522. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B. Use of a Genus- and Species-Specific Multiplex PCR for Identification of Enterococci. J. Clin. Microbiol. 2004, 42, 3558–3565. [Google Scholar] [CrossRef] [Green Version]
- Basic Local Alignment Search Tool. 2014. Available online: https://blast.ncbi.nlm.nih.gov/BlastAlign.cgi (accessed on 10 January 2022).
- CLSI Standard M07; Chapter 3—Broth and Agar Dilution Susceptibility Testing Process. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 15–53.
- Aarestrup, F.M.; Ahrens, P.; Madsen, M.; Pallesen, L.V.; Poulsen, R.L.; Westh, H. Glycopeptide Susceptibility among Danish Enterococcus faecium and Enterococcus faecalis Isolates of Animal and Human Origin and PCR Identification of Genes within the VanA Cluster. Antimicrob. Agents Chemother. 1996, 40, 1938–1940. [Google Scholar] [CrossRef] [Green Version]
- Depardieu, F.; Perichon, B.; Courvalin, P. Detection of the van Alphabet and Identification of Enterococci and Staphylococci at the Species Level by Multiplex PCR. J. Clin. Microbiol. 2004, 42, 5857–5860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehrenberg, C.; Schwarz, S. Distribution of Florfenicol Resistance Genes FexA and Cfr among Chloramphenicol-Resistant Staphylococcus Isolates. Antimicrob. Agents Chemother. 2006, 50, 1156–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 6 April 2022).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelsky, A.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling Genomes and Mini-Metagenomes from Highly Chimeric Reads. In Research in Computational Molecular Biology: 17th Annual International Conference, RECOMB 2013, Beijing, China, 7–10 April 2013; Deng, M., Jiang, R., Sun, F., Zhang, X., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 158–170. Available online: http://link.springer.com/chapter/10.1007/978-3-642-37195-0_13 (accessed on 10 January 2022).
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.F.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.R. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garcìa-Fernandez, A.; Larsen, M.; Lund, O.; Voldby Villa, L.; Møller Aarestrup, F.; Hasman, H. PlasmidFinder and pMLST: In silico detection and typing of plasmids. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [Green Version]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F.J. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus Sequence Typing of Total Genome Sequenced Bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- CGE—Center for Genomic Epidemiology. 2020. Available online: https://cge.cbs.dtu.dk/services/ (accessed on 29 December 2020).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
| Antimicrobial | Criteria (a) | E. faecalis (n = 84) | E. faecium (n = 48) | ||||
|---|---|---|---|---|---|---|---|
| (T)ECOFF (b) | Cattle (n = 18) | Pigs (n = 66) | (T)ECOFF (b) | Cattle (n = 12) | Pigs (n = 36) | ||
| Vancomycin | MIC50 | 4 | ≤1 | ≤1 | 4 | ≤1 | ≤1 |
| MIC90 | 4 | 2 | ≤1 | ≤1 | |||
| % DS | 0 | 0 | 0 | 0 | |||
| Teicoplanin | MIC50 | 2 | ≤0.5 | ≤0.5 | 2 | ≤0.5 | ≤0.5 |
| MIC90 | ≤0.5 | ≤0.5 | 1 | ≤0.5 | |||
| % DS | 0 | 0 | 0 | 0 | |||
| Tetracycline | MIC50 | 4 | ≤1 | 128 | 4 | 16 | 128 |
| MIC90 | 64 | 128 | 128 | >128 | |||
| % DS | 44 (c) | 98 (c) | 58 | 78 | |||
| Ciprofloxacin | MIC50 | 4 | 1 | 1 | 8 | 2 | 1 |
| MIC90 | 2 | 4 | 4 | 2 | |||
| % DS | 0 | 9 | 0 | 0 | |||
| Erythromycin | MIC50 | 4 | ≤1 | >128 | 4 | ≤1 | >128 |
| MIC90 | >128 | >128 | 2 | >128 | |||
| % DS | 17 (c) | 86 (c) | 0 (c) | 58 (c) | |||
| Linezolid | MIC50 | ND | 2 | 1 | 4 | 2 | 2 |
| MIC90 | 2 | 2 | 2 | 2 | |||
| % DS | - | - | 0 | 0 | |||
| Gentamicin | MIC50 | 64 | ≤8 | ≤8 | 32 | ≤8 | ≤8 |
| MIC90 | ≤8 | 128 | ≤8 | ≤8 | |||
| % DS | 0 | 11 | 0 | 0 | |||
| Ampicillin | MIC50 | 4 | ≤0.5 | 1 | 8 | 1 | 1 |
| MIC90 | 2 | 2 | 1 | 8 | |||
| % DS | 0 | 0 | 0 | 6 | |||
| Chloramphenicol | MIC50 | 32 | ≤4 | 8 | 32 | ≤4 | ≤4 |
| MIC90 | 8 | 64 | ≤4 | 16 | |||
| % DS | 0 (c) | 27 (c) | 8 | 3 | |||
| Strain | Species | Linezolid Susceptibility | OptrA Variant | ||||
|---|---|---|---|---|---|---|---|
| EUVENC MIC (µg/mL) | Interpretation (a) | Morroni et al. [32] | Freitas et al. [33] | Almeida et al. [34] | Amino Acid Substitutions | ||
| INIAV173 | E. faecium | 4 | S | DVD (b) | OptrA_28 (b) | V12 | Y176D, A350V, G393D |
| INIAV004 | E. faecium | 8 | R | WT | OptrA_1 | V19 | None |
| INIAV168 | E. faecalis | 8 | R | DP | OptrA_8 | V22 | Y176D, T481P |
| INIAV169 | E. faecalis | 4 | S | EDD | OptrA_7 | V34 | K3E, Y176D, G393D |
| INIAV170 | E. faecalis | 4 | S | EDD | OptrA_7 | V34 | K3E, Y176D, G393D |
| INIAV171 | E. faecalis | 8 | R | DP | OptrA_8 | V22 | Y176D, T481P |
| INIAV174 | E. faecalis | 2 | S | EDD | OptrA_7 | V34 | K3E, Y176D, G393D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gião, J.; Leão, C.; Albuquerque, T.; Clemente, L.; Amaro, A. Antimicrobial Susceptibility of Enterococcus Isolates from Cattle and Pigs in Portugal: Linezolid Resistance Genes optrA and poxtA. Antibiotics 2022, 11, 615. https://doi.org/10.3390/antibiotics11050615
Gião J, Leão C, Albuquerque T, Clemente L, Amaro A. Antimicrobial Susceptibility of Enterococcus Isolates from Cattle and Pigs in Portugal: Linezolid Resistance Genes optrA and poxtA. Antibiotics. 2022; 11(5):615. https://doi.org/10.3390/antibiotics11050615
Chicago/Turabian StyleGião, Joana, Célia Leão, Teresa Albuquerque, Lurdes Clemente, and Ana Amaro. 2022. "Antimicrobial Susceptibility of Enterococcus Isolates from Cattle and Pigs in Portugal: Linezolid Resistance Genes optrA and poxtA" Antibiotics 11, no. 5: 615. https://doi.org/10.3390/antibiotics11050615
APA StyleGião, J., Leão, C., Albuquerque, T., Clemente, L., & Amaro, A. (2022). Antimicrobial Susceptibility of Enterococcus Isolates from Cattle and Pigs in Portugal: Linezolid Resistance Genes optrA and poxtA. Antibiotics, 11(5), 615. https://doi.org/10.3390/antibiotics11050615






