Screening of Natural Molecules as Adjuvants to Topical Antibiotics to Treat Staphylococcus aureus from Diabetic Foot Ulcer Infections
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of the Phytochemicals and Derivatives
5.2. Preparation of Antibiotics
5.3. Bacterial Strains
5.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
5.5. Antibiotic/Phytochemical Dual Combination: Disc Diffusion Method
Classification
- ▪
- Potentiation (+++): (IZDa+p–IZDa) ≥ 6 mm;
- ▪
- Additive (++): 6 mm > (IZDa+p–IZDa) ≥ 4 mm;
- ▪
- Indifferent (+): 4 mm > (IZDa+p–IZDa) > −6 mm;
- ▪
- Negative (–): (IZDa+p–IZDa) ≤ −6 mm,
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in diabetic foot ulcers: Impact, risk factors and control strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199. [Google Scholar] [CrossRef]
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matta-Gutiérrez, G.; García-Morales, E.; García-álvarez, Y.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. The influence of multidrug-resistant bacteria on clinical outcomes of diabetic foot ulcers: A systematic review. J. Clin. Med. 2021, 10, 1948. [Google Scholar] [CrossRef] [PubMed]
- Banu, A.; Noorul Hassan, M.M.; Rajkumar, J.; Srinivasa, S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas. Med. J. 2015, 8, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Spichler, A.; Hurwitz, B.L.; Armstrong, D.G.; Lipsky, B.A. Microbiology of diabetic foot infections: From Louis Pasteur to “crime scene investigation”. BMC Med. 2015, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in diabetic foot ulcers: Significance and clinical relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kumar, A.; Singh, P.P.; Songachan, L.S. Antimicrobial and Antioxidant Properties of Phytochemicals: Current Status and Future Perspective; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128185933. [Google Scholar]
- Rosato, A.; Vitali, C.; De Laurentis, N.; Armenise, D.; Antonietta Milillo, M. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine 2007, 14, 727–732. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.O.; Rodrigues, F.F.G.; Campos, A.R.; Lima, S.G.; Da Costa, J.G.M. Chemical composition and synergistic interaction between aminoglycosides antibiotics and essential oil of Lantana montevidensis Briq. Nat. Prod. Res. 2013, 27, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.F.G.; Costa, J.G.M.; Coutinho, H.D.M. Synergy effects of the antibiotics gentamicin and the essential oil of Croton zehntneri. Phytomedicine 2009, 16, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Ruiz, R.M.; Negrín, Z.R.; Distinto, S.; Borges, F.; Simões, M. 2-(2-Methyl-2-Nitrovinyl)Furan But Not Furvina Interfere With Staphylococcus Aureus Agr Quorum-Sensing System and Potentiate the Action of Fusidic Acid Against Biofilms. Int. J. Mol. Sci. 2021, 22, 613. [Google Scholar] [CrossRef]
- Gibbons, S.; Oluwatuyi, M.; Kaatz, G.W. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J. Antimicrob. Chemother. 2003, 51, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti. Infect. Ther. 2012, 10, 775–790. [Google Scholar] [CrossRef]
- Fadli, M.; Chevalier, J.; Saad, A.; Mezrioui, N.E.; Hassani, L.; Pages, J.M. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2011, 38, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Zhou, L.L.; Wang, H.Y.; Huang, S.N.; Liu, Q.; Hu, S.L.; Li, T.R.; Chen, Y.B.; Jiang, J.X. The inhibitory effect of Zingiber corallinum Hance essential oil on drug-resistant bacteria and evaluation of its acute toxicity. Med. Sci. Monit. 2011, 17, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, M.; Nagasaki, S.; Ito, R.; Ohta, T. Sesquiterpene farnesol as a competitive inhibitor of lipase activity of Staphylococcus aureus. FEMS Microbiol. Lett. 2007, 273, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, H.; Oono, T.; Huh, W.-K.; Yamasaki, O.; Ogawa, S.; Katsuyama, M.; Ichikawa, H.; Iwatsuki, K. Actions of farnesol and xylitol against Staphylococcus aureus. Chemotherapy 2002, 48, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Masako, K.; Yusuke, K.; Hideyuki, I.; Atsuko, M.; Yoshiki, M.; Kayoko, M.; Makoto, K. A novel method to control the balance of skin microflora: Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. J. Dermatol. Sci. 2005, 38, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [Green Version]
- da Cruz, R.P.; de Freitas, T.S.; Costa, M.D.S.; Dos Santos, A.T.L.; Campina, F.F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; Araújo, A.A.D.S.; De Siqueira Júnior, J.P.; et al. Effect of α-bisabolol and its β-cyclodextrin complex as TetK and NorA efflux pump inhibitors in Staphylococcus aureus strains. Antibiotics 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The complex relationship between virulence and antibiotic resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Pérez, V.K.C.; da Costa, G.M.; Guimarães, A.S.; Heinemann, M.B.; Lage, A.P.; Dorneles, E.M.S. Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 792–802. [Google Scholar] [CrossRef]
- Fux, C.A.; Shirtliff, M.; Stoodley, P.; Costerton, J.W. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 2005, 13, 58–63. [Google Scholar] [CrossRef]
- Mocan, A.; Babotă, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical constituents and biologic activities of sage species: A comparison between salvia officinalis l., s. glutinosa l. and s. transsylvanica (schur ex griseb. & schenk) schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Araújo Delmondes, G.; Bezerra, D.S.; de Queiroz Dias, D.; de Souza Borges, A.; Araújo, I.M.; Lins da Cunha, G.; Bandeira, P.F.R.; Barbosa, R.; Melo Coutinho, H.D.; Felipe, C.F.B.; et al. Toxicological and pharmacologic effects of farnesol (C15H26O): A descriptive systematic review. Food Chem. Toxicol. 2019, 129, 169–200. [Google Scholar] [CrossRef] [PubMed]
- Mendanha, S.A.; Moura, S.S.; Anjos, J.L.V.; Valadares, M.C.; Alonso, A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. In Vitro 2013, 27, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Corpas-López, V.; Morillas-Márquez, F.; Navarro-Moll, M.C.; Merino-Espinosa, G.; Díaz-Sáez, V.; Martín-Sánchez, J. (-)-α-Bisabolol, a Promising Oral Compound for the Treatment of Visceral Leishmaniasis. J. Nat. Prod. 2015, 78, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Vingsbo Lundberg, C.; Frimodt-Møller, N. Efficacy of topical and systemic antibiotic treatment of meticillin-resistant Staphylococcus aureus in a murine superficial skin wound infection model. Int. J. Antimicrob. Agents 2013, 42, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Sousa, P.; Gaspar, A.; Vilar, S.; Borges, F.; Simões, M. Furvina inhibits the 3-oxo-C12-HSL-based quorum sensing system of Pseudomonas aeruginosa and QS-dependent phenotypes. Biofouling 2017, 33, 156–168. [Google Scholar] [CrossRef]
- Baptista, J.; Simões, M.; Borges, A. Effect of plant-based catecholic molecules on the prevention and eradication of Escherichia coli biofilms: A structure activity relationship study. Int. Biodeterior. Biodegrad. 2019, 141, 101–113. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Salgado, A.J.; Simões, M. Evaluation of the best method to assess antibiotic potentiation by phytochemicals against Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2014, 79, 125–134. [Google Scholar] [CrossRef] [Green Version]




| Class | Compound | Chemical Structure | |
|---|---|---|---|
| Phytochemicals | Phenolics | Chalcone |  |
| Juglone |  | ||
| Cinnamic acid |  | ||
| Alkaloid | Trigonelline |  | |
| Synthetic nitrovinylfuran | Furvina | 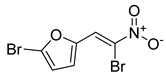 | |
| 2-nitrovinylfuran derivatives | Compound 1 | 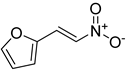 | |
| Compound 2 | 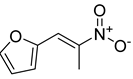 | ||
| Compound 3 | 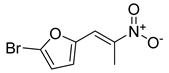 | ||
| Compound 4 | 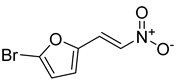 | ||
| Sesquiterpenoid constituents of essential oils | Guaiazulene | 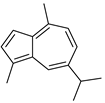 | |
| α-bisabolol | 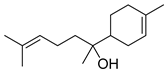 | ||
| Farnesol | 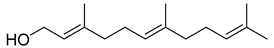 | ||
| Nerolidol |  | ||
| Antibiotics | Fusidane | Fusidic acid | 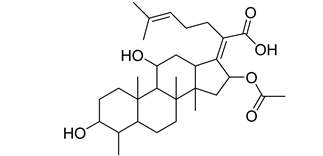 |
| Carboxylic acid | Mupirocin | 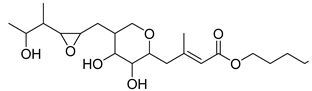 | |
| Penicillin | Methicillin |  | |
| Oxacillin |  | ||
| Aminoglycoside | Gentamicin | 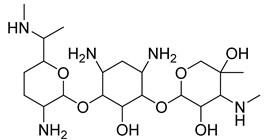 |
| CECT 976 | MJMC102 | MJMC109 | MJMC110 | MJMC111 | Xu212 | SA 1199B | RN4220 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Chalcone | 100 | >1000 | 200 | >1000 | 200 | >1000 | 200 | >1000 | 200 | >1000 | 200 | >1000 | 200 | >1000 | 200 | >1000 | |
| Juglone | 12.5 | 50 | 12.5–25 | 50 | 12.5–25 | 25 | 12.5 | 25 | 12.5 | 50 | 12.5 | 50 | 12.5–25 | 50 | 25 | 50 | |
| Cinnamic acid | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | |
| Trigonelline | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | |
| Furvina | 100 | 200 | 25 | 200 | 25 | 100 | 25 | 200 | 25 | 200 | 25 | 100 | 25 | 50 | 25 | 50 | |
| 2-nitrovinylfuran derivatives | Compound 1 | 50 | 400 | 100 | 1000 | 200 | 800 | 200 | >1000 | 200 | 800 | 200 | 800 | 200 | 800 | 50 | 400 |
| Compound 2 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | |
| Compound 3 | 400 | 800 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | |
| Compound 4 | >1000 | >1000 | 200 | 1000 | 200 | 800 | 200 | >1000 | 200 | 800 | 200 | 800 | 200 | 800 | 50 | 400 | |
| Guaiazulene | 200 | >1000 | 200 | >1000 | 200 | >1000 | 200 | >1000 | 200 | >1000 | 100 | >1000 | 100 | >1000 | 200 | >1000 | |
| α-Bisabolol | 50 | >1000 | 100 | >1000 | 50 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | |
| Farnesol | 25 | 50 | 100 | 800 | 25 | 800 | 25 | 800 | 50 | 800 | 25 | 25 | 25 | 25 | 100 | >1000 | |
| Nerolidol | 100 | >1000 | 100 | >1000 | 50 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | 100 | >1000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, D.; Borges, A.; Saavedra, M.J.; Borges, F.; Simões, M. Screening of Natural Molecules as Adjuvants to Topical Antibiotics to Treat Staphylococcus aureus from Diabetic Foot Ulcer Infections. Antibiotics 2022, 11, 620. https://doi.org/10.3390/antibiotics11050620
Oliveira D, Borges A, Saavedra MJ, Borges F, Simões M. Screening of Natural Molecules as Adjuvants to Topical Antibiotics to Treat Staphylococcus aureus from Diabetic Foot Ulcer Infections. Antibiotics. 2022; 11(5):620. https://doi.org/10.3390/antibiotics11050620
Chicago/Turabian StyleOliveira, Diana, Anabela Borges, Maria J. Saavedra, Fernanda Borges, and Manuel Simões. 2022. "Screening of Natural Molecules as Adjuvants to Topical Antibiotics to Treat Staphylococcus aureus from Diabetic Foot Ulcer Infections" Antibiotics 11, no. 5: 620. https://doi.org/10.3390/antibiotics11050620
APA StyleOliveira, D., Borges, A., Saavedra, M. J., Borges, F., & Simões, M. (2022). Screening of Natural Molecules as Adjuvants to Topical Antibiotics to Treat Staphylococcus aureus from Diabetic Foot Ulcer Infections. Antibiotics, 11(5), 620. https://doi.org/10.3390/antibiotics11050620










