Decreased Antibiotic Consumption Coincided with Reduction in Bacteremia Caused by Bacterial Species with Respiratory Transmission Potential during the COVID-19 Pandemic
Abstract
1. Introduction
2. Methods
2.1. Setting
2.2. Wholesale Supply of Antibiotics in Hong Kong
2.3. Data Source
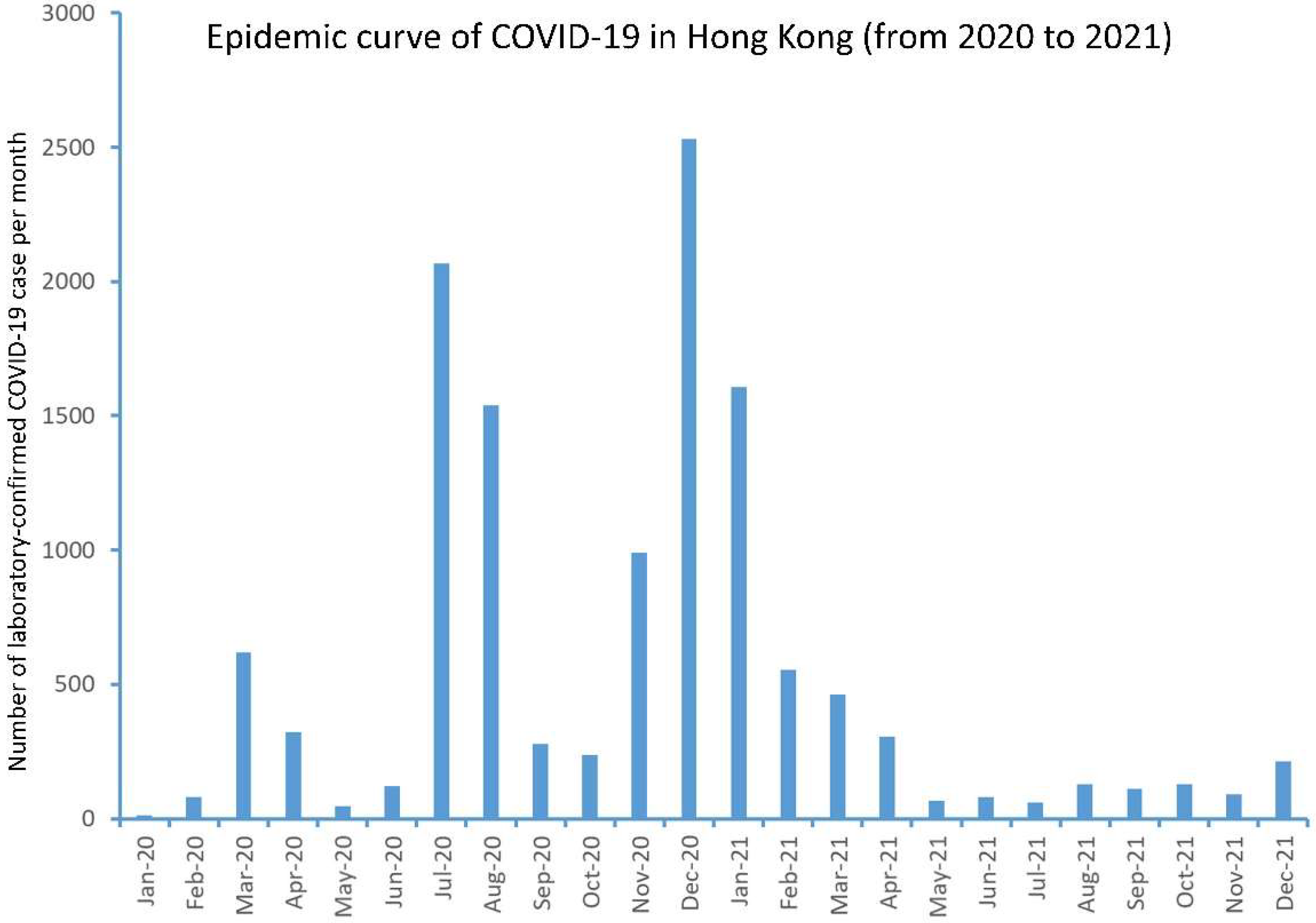
2.4. Overview of COVID-19 in Hong Kong
2.5. Number of Patient Admissions and Blood Culture Requests before and during COVID-19
2.6. Community-Onset Bacteremia before and during COVID-19
2.7. Hospital-Onset Bacteremia before and during COVID-19
2.8. Statistical Analysis
3. Results
3.1. Overview of COVID-19 in Hong Kong
3.2. Wholesale Supply of Antibiotics in Hong Kong
3.3. Number of Patient Admissions and Blood Culture Requests before and during COVID-19
3.4. Community-Onset Bacteremia before and during COVID-19
3.5. Hospital-Onset Bacteremia before and during COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, V.C.; Lau, S.K.; Woo, P.C.; Yuen, K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef]
- Cheng, V.C.; To, K.K.; Tse, H.; Hung, I.F.; Yuen, K.Y. Two years after pandemic influenza A/2009/H1N1: What have we learned? Clin. Microbiol. Rev. 2012, 25, 223–263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Tai, J.W.; Li, W.; Chau, P.H.; So, S.Y.; Wong, L.M.; Ching, R.H.; Ng, M.M.; Ho, S.K.; Lee, D.W.; et al. Implementation of directly observed patient hand hygiene for hospitalized patients by hand hygiene ambassadors in Hong Kong. Am. J. Infect. Control 2016, 44, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.C.; Wong, S.C.; Wong, S.C.Y.; Yuen, K.Y. Directly observed hand hygiene—From healthcare workers to patients. J. Hosp. Infect. 2019, 101, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Wong, S.-C.; Wong, I.W.; Chau, P.H.; So, S.Y.; Wong, S.C.; Chen, J.H.; Lee, W.-M.; Tai, J.W.; Chau, C.-H.; et al. The challenge of patient empowerment in hand hygiene promotion in health care facilities in Hong Kong. Am. J. Infect. Control 2017, 45, 562–565. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tai, J.W.; Wong, L.M.; Ching, R.H.; Ng, M.M.; Ho, S.K.; Lee, D.W.; Li, W.S.; Lee, W.M.; Sridhar, S.; et al. Effect of proactive infection control measures on benchmarked rate of hospital outbreaks: An analysis of public hospitals in Hong Kong over 5 years. Am. J. Infect. Control 2015, 43, 965–970. [Google Scholar] [CrossRef]
- Lynch, J.B.; Davitkov, P.; Anderson, D.J.; Bhimraj, A.; Cheng, V.C.C.; Guzman-Cottrill, J.; Dhindsa, J.; Duggal, A.; Jain, M.K.; Lee, G.M.; et al. Infectious Diseases Society of America Guidelines on Infection Prevention for Healthcare Personnel Caring for Patients with Suspected or Known COVID-19. Clin. Infect. Dis. 2021, ciab953. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, S.C.; Chuang, V.W.; So, S.Y.; Chen, J.H.; Sridhar, S.; To, K.K.; Chan, J.F.; Hung, I.F.; Ho, P.L.; et al. Absence of nosocomial transmission of coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in the prepandemic phase in Hong Kong. Am. J. Infect. Control 2020, 48, 890–896. [Google Scholar] [CrossRef]
- Cheng, V.C.-C.; Wong, S.-C.; Tong, D.W.-K.; Chuang, V.W.-M.; Chen, J.H.-K.; Lee, L.L.-Y.; To, K.K.-W.; Hung, I.F.-N.; Ho, P.-L.; Yeung, D.T.-K.; et al. Multipronged infection control strategy to achieve zero nosocomial coronavirus disease 2019 (COVID-19) cases among Hong Kong healthcare workers in the first 300 days of the pandemic. Infect. Control Hosp. Epidemiol. 2022, 43, 334–343. [Google Scholar] [CrossRef]
- Wong, S.C.; AuYeung, C.Y.; Lam, G.M.; Leung, E.L.; Chan, V.M.; Yuen, K.Y.; Cheng, V.C. Is it possible to achieve 100 percent hand hygiene compliance during the coronavirus disease 2019 (COVID-19) pandemic? J. Hosp. Infect. 2020, 105, 779–781. [Google Scholar] [CrossRef]
- Wong, S.C.; Lam, G.K.M.; AuYeung, C.H.Y.; Chan, V.W.M.; Wong, N.L.D.; So, S.Y.C.; Chen, J.H.K.; Hung, I.F.N.; Chan, J.F.W.; Yuen, K.Y.; et al. Absence of nosocomial influenza and respiratory syncytial virus infection in the coronavirus disease 2019 (COVID-19) era: Implication of universal masking in hospitals. Infect. Control Hosp. Epidemiol. 2021, 42, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.S.; Leung, C.C.; Lee, S.S. Abrupt Subsidence of Seasonal Influenza after COVID-19 Outbreak, Hong Kong, China. Emerg. Infect. Dis. 2020, 26, 2753–2755. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Hong, K.; Sohn, S.; Kim, J.; Chun, B.C. Trends in Viral Respiratory Infections during COVID-19 Pandemic, South Korea. Emerg. Infect. Dis. 2021, 27, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, K.; Koolhof, I.S.; Shobugawa, Y.; Saito, R. Decreased human respiratory syncytial virus activity during the COVID-19 pandemic in Japan: An ecological time-series analysis. BMC Infect. Dis. 2021, 21, 734. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.Y.; Thoon, K.C.; Loo, L.H.; Chan, K.S.; Oon, L.L.E.; Ramasamy, A.; Maiwald, M. Trends in Respiratory Virus Infections During the COVID-19 Pandemic in Singapore, 2020. JAMA Netw. Open. 2021, 4, e2115973. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Buda, S.; Biere, B.; Reiche, J.; Schlosser, F.; Duwe, S.; Wedde, M.; von Kleist, M.; Mielke, M.; Wolff, T.; et al. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January–September 2020: Analysis of national surveillance data. Lancet Reg. Health Eur. 2021, 6, 100112. [Google Scholar] [CrossRef]
- Groves, H.E.; Piché-Renaud, P.-P.; Peci, A.; Farrar, D.S.; Buckrell, S.; Bancej, C.; Sevenhuysen, C.; Campigotto, A.; Gubbay, J.B.; Morris, S.K. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg. Health Am. 2021, 1, 100015. [Google Scholar]
- Haddadin, Z.; Schuster, J.E.; Spieker, A.J.; Rahman, H.; Blozinski, A.; Stewart, L.; Campbell, A.P.; Lively, J.Y.; Michaels, M.G.; Williams, J.V.; et al. Acute Respiratory Illnesses in Children in the SARS-CoV-2 Pandemic: Prospective Multicenter Study. Pediatrics. 2021, 148, e2021051462. [Google Scholar] [CrossRef]
- Varela, F.H.; Scotta, M.C.; Polese-Bonatto, M.; Sartor, I.T.S.; Ferreira, C.F.; Fernandes, I.R.; Zavaglia, G.O.; de Almeida, W.A.F.; Arakaki-Sanchez, D.; Pinto, L.A.; et al. Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: Likely role of lower transmission in the community. J. Glob. Health 2021, 11, 05007. [Google Scholar] [CrossRef]
- Högberg, L.D.; Vlahović-Palčevski, V.; Pereira, C.; Weist, K.; Monnet, D.L.; ESAC-Net study group; ESAC-Net study group participants. Decrease in community antibiotic consumption during the COVID-19 pandemic, EU/EEA, 2020. Eurosurveillance 2021, 26, 2101020. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, X.; Liu, X.; Wen, X.; Wu, R.; Cui, D.; Mao, Z. Antibiotic Use in China’s Public Healthcare Institutions During the COVID-19 Pandemic: An Analysis of Nationwide Procurement Data, 2018–2020. Front. Pharmacol. 2022, 13, 813213. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, A.B.; van Rensburg, M.J.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.; van der Linden, M.P.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [PubMed]
- Amin-Chowdhury, Z.; Aiano, F.; Mensah, A.; Sheppard, C.L.; Litt, D.; Fry, N.K.; Andrews, N.; Ramsay, M.E.; Ladhani, S.N. Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Invasive Pneumococcal Disease and Risk of Pneumococcal Coinfection With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Prospective National Cohort Study, England. Clin. Infect. Dis. 2021, 72, e65–e75. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.L.L.; Fok, K.M.N.; Lin, K.P.K.; Chan, E.; Ma, Y.; Lau, S.K.P.; Woo, P.C.Y. Substantial Decline in Invasive Pneumococcal Disease During Coronavirus Disease 2019 Pandemic in Hong Kong. Clin. Infect. Dis. 2022, 74, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Ma, T.F.; Ip, M.S.; Ho, P.L. Invasive pneumococcal disease, pneumococcal pneumonia and all-cause pneumonia in Hong Kong during the COVID-19 pandemic compared with the preceding 5 years: A retrospective observational study. BMJ Open 2021, 11, e055575. [Google Scholar] [CrossRef]
- Statistics on Antimicrobial Resistance Control. Centre for Health Protection. Department of Health, The Government of the Hong Kong Special Administrative Region. Available online: https://www.chp.gov.hk/en/statistics/data/10/100044/6960.html?msclkid=346ba6d7c6c211ecb6eed0867e6a2fe4 (accessed on 28 April 2022).
- The Centre for Health Protection Closely Monitors Cluster of Pneumonia Cases on Mainland. Press Release of the Department of Health, Hong Kong Special Administrative Region. Available online: https://www.info.gov.hk/gia/general/201912/31/P2019123100667.htm (accessed on 28 April 2022).
- Cheng, V.C.C.; Wong, S.C.; To, K.K.W.; Ho, P.L.; Yuen, K.Y. Preparedness and proactive infection control measures against the emerging novel coronavirus in China. J. Hosp. Infect. 2020, 104, 254–255. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, S.C.; Chen, J.H.; Yip, C.C.; Chuang, V.W.; Tsang, O.T.; Sridhar, S.; Chan, J.F.; Ho, P.L.; Yuen, K.Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020, 41, 493–498. [Google Scholar] [CrossRef]
- Wong, S.-C.; Yuen, L.L.-H.; Chan, V.W.-M.; Chen, J.H.-K.; To, K.K.-W.; Yuen, K.-Y.; Cheng, V.C.-C. Airborne transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): What is the implication of hospital infection control? Infect. Control Hosp. Epidemiol. 2021, 1–2. [Google Scholar] [CrossRef]
- Wong, S.C.; Chen, H.; Lung, D.C.; Ho, P.L.; Yuen, K.Y.; Cheng, V.C. To prevent SARS-CoV-2 transmission in designated quarantine hotel for travelers: Is the ventilation system a concern? Indoor Air 2021, 31, 1295–1297. [Google Scholar] [CrossRef]
- Wong, S.C.; Au, A.K.W.; Chen, H.; Yuen, L.L.H.; Li, X.; Lung, D.C.; Chu, A.W.H.; Ip, J.D.; Chan, W.M.; Tsoi, H.W.; et al. Transmission of Omicron (B.1.1.529)—SARS-CoV-2 Variant of Concern in a designated quarantine hotel for travelers: A challenge of elimination strategy of COVID-19. Lancet Reg. Health West Pac. 2022, 18, 100360. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Siu, G.K.H.; Wong, S.C.; Au, A.K.W.; Ng, C.S.F.; Chen, H.; Li, X.; Lee, L.K.; Leung, J.S.L.; Lu, K.K.; et al. Complementation of contact tracing by mass testing for successful containment of beta COVID-19 variant (SARS-CoV-2 VOC B.1.351) epidemic in Hong Kong. Lancet Reg. Health West Pac. 2021, 17, 100281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.-C.; Wong, S.-C.; Chuang, V.W.-M.; So, S.Y.-C.; Chen, J.H.-K.; Sridhar, S.; To, K.K.-W.; Chan, J.F.-W.; Hung, I.F.-N.; Ho, P.-L.; et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidem.mic due to SARS-CoV-2. J. Infect. 2020, 81, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Leung, M.; Tong, D.W.K.; Lee, L.L.Y.; Leung, W.L.H.; Chan, F.W.K.; Chen, J.H.K.; Hung, I.F.N.; Yuen, K.Y.; Yeung, D.T.K.; et al. Infection control challenges in setting up community isolation and treatment facilities for patients with coronavirus disease 2019 (COVID-19): Implementation of directly observed environmental disinfection. Infect. Control Hosp. Epidemiol. 2021, 42, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Leung, M.; Lee, L.L.; Chung, K.L.; Cheng, V.C. Infection control challenge in setting up a temporary test centre at Hong Kong International Airport for rapid diagnosis of COVID-19 due to SARS-CoV-2. J. Hosp. Infect. 2020, 105, 571–573. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Tsang, O.T.Y.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.Y.; Cai, J.P.; Chan, J.M.C.; Chik, T.S.H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Number of Notifiable Infectious Diseases by Month. Centre for Health Protection. Department of Health, The Government of the Hong Kong Special Administrative Region. Available online: https://www.chp.gov.hk/en/static/24012.html (accessed on 27 April 2022).
- Cozorici, D.; Măciucă, R.A.; Stancu, C.; Tihăuan, B.M.; Uță, R.B.; Codrea, C.I.; Matache, R.; Pop, C.E.; Wolff, R.; Fendrihan, S. Microbial Contamination and Survival Rate on Different Types of Banknotes. Int. J. Environ. Res. Public Health 2022, 19, 4310. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, G.A.; Lee, S.H.; Park, Y.H. A systematic review on the causes of the transmission and control measures of outbreaks in long-term care facilities: Back to basics of infection control. PLoS ONE 2020, 15, e0229911. [Google Scholar] [CrossRef]
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; Pneumococcal Carriage Group. The fundamental link between pneumococcal carriage and disease. Expert. Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef]
- Gray, B.M.; Converse, G.M., 3rd; Dillon, H.C., Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: Acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 1980, 142, 923–933. [Google Scholar] [CrossRef]
- Gwaltney, J.M., Jr.; Sande, M.A.; Austrian, R.; Hendley, J.O. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J. Infect. Dis. 1975, 132, 62–68. [Google Scholar] [CrossRef]
- Cordery, R.; Purba, A.K.; Begum, L.; Mills, E.; Mosavie, M.; Vieira, A.; Jauneikaite, E.; Leung, R.C.Y.; Siggins, M.K.; Ready, D.; et al. Frequency of transmission, asymptomatic shedding, and airborne spread of Streptococcus pyogenes in schoolchildren exposed to scarlet fever: A prospective, longitudinal, multicohort, molecular epidemiological, contact-tracing study in England, UK. Lancet Microbe 2022, 3, e366–e375. [Google Scholar] [CrossRef]
- McCullers, J.A.; McAuley, J.L.; Browall, S.; Iverson, A.R.; Boyd, K.L.; Henriques Normark, B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 2010, 202, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Melamed, R.; Greenberg, D.; Landau, D.; Khvatskin, S.; Shany, E.; Dagan, R. Neonatal nosocomial pneumococcal infections acquired by patient-to-patient transmission. Scand. J. Infect. Dis. 2002, 34, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Jauneikaite, E.; Khan-Orakzai, Z.; Kapatai, G.; Bloch, S.; Singleton, J.; Atkin, S.; Shah, V.; Hatcher, J.; Samarasinghe, D.; Sheppard, C.; et al. Nosocomial Outbreak of Drug-Resistant Streptococcus pneumoniae Serotype 9V in an Adult Respiratory Medicine Ward. J. Clin. Microbiol. 2017, 55, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.O.; Górny, R.L. Haemophilus influenzae as an airborne contamination in child day care centers. Am. J. Infect. Control 2013, 41, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.L.; Martin, L.E.; Stephens, D.S. Environmental survival of Neisseria meningitidis. Epidemiol. Infect. 2014, 142, 187–190. [Google Scholar] [CrossRef]
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Frank, D.N.; Bessesen, M.T. Genomic evolution of Staphylococcus aureus isolates colonizing the nares and progressing to bacteremia. PLoS ONE 2018, 13, e0195860. [Google Scholar] [CrossRef]
- McCullers, J.A. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 2006, 19, 571–582. [Google Scholar] [CrossRef]
- Ni Lee, L.; Dias, P.; Han, D.; Yoon, S.; Shea, A.; Zakharov, V.; Parham, D.; Sarawar, S.R. mouse model of lethal synergism between influenza virus and Haemophilus influenzae. Am. J. Pathol. 2010, 176, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Kawabata, S.; Nakagawa, I.; Okuno, Y.; Goto, T.; Sano, K.; Hamada, S. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J. Virol. 2003, 77, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.E.; McLoughlin, R.M. Staphylococcus aureus and Influenza A Virus: Partners in Coinfection. mBio 2016, 7, e02068-16. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Poudel, A.N.; Alhusein, N.; Wang, H.; Yao, G.; Lambert, H. Antimicrobial Use in COVID-19 Patients in the First Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2021, 10, 745. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Jin, L.S.; Fisher, D. MDRO transmission in acute hospitals during the COVID-19 pandemic. Curr. Opin. Infect. Dis. 2021, 34, 365–371. [Google Scholar]
- Wong, S.C.; Lam, G.M.; Chen, J.K.; Li, X.; Ip, F.F.; Yuen, L.H.; Chan, V.M.; AuYeung, C.Y.; So, S.C.; Ho, P.L.; et al. Air dispersal of multidrug-resistant Acinetobacter baumannii: Implications for nosocomial transmission during the COVID-19 pandemic. J. Hosp. Infect. 2021, 116, 78–86. [Google Scholar] [CrossRef]
- Silva, A.R.O.; Salgado, D.R.; Lopes, L.P.N.; Castanheira, D.; Emmerick, I.C.M.; Lima, E.C. Increased Use of Antibiotics in the Intensive Care Unit During Coronavirus Disease (COVID-19) Pandemic in a Brazilian Hospital. Front. Pharmacol. 2021, 12, 778386. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Kerr, F.; Hughes, S.; Urch, J.; Lanzman, M.; Yau, T.; Cockburn, A.; Patel, R.; Sheikh, A.; Gormley, C.; et al. Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom. Antibiotics 2021, 10, 110. [Google Scholar] [CrossRef]
- Walker, M.J.; Brouwer, S.; Forde, B.M.; Worthing, K.A.; McIntyre, L.; Sundac, L.; Maloney, S.; Roberts, L.W.; Barnett, T.; Richter, J.; et al. Detection of Epidemic Scarlet Fever Group A Streptococcus in Australia. Clin. Infect. Dis. 2019, 69, 1232–1234. [Google Scholar] [CrossRef]
- Brouwer, S.; Barnett, T.C.; Ly, D.; Kasper, K.J.; De Oliveira, D.M.; Rivera-Hernandez, T.; Cork, A.J.; McIntyre, L.; Jespersen, M.G.; Richter, J.; et al. Prophage exotoxins enhance colonization fitness in epidemic scarlet fever-causing Streptococcus pyogenes. Nat. Commun. 2020, 11, 5018. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-C.; Chen, J.-K.; Yuen, L.-H.; Chan, V.-M.; AuYeung, C.-Y.; Leung, S.-M.; So, S.-C.; Chan, B.-K.; Li, X.; Leung, J.-Y.; et al. Air dispersal of meticillin-resistant Staphylococcus aureus in residential care homes for the elderly: Implications for transmission during the COVID-19 pandemic. J. Hosp. Infect. 2022, 123, 52–60. [Google Scholar] [CrossRef]
- Wong, S.C.; Chen, J.H.; So, S.Y.; Ho, P.L.; Yuen, K.Y.; Cheng, V.C. Gastrointestinal colonization of meticillin-resistant Staphylococcus aureus: An unrecognized burden upon hospital infection control. J. Hosp. Infect. 2022, 121, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kok, K.H.; Wong, S.C.; Chan, W.M.; Wen, L.; Chu, A.W.H.; Ip, J.D.; Lee, L.K.; Wong, I.T.F.; Lo, H.W.H.; Cheng, V.C.C.; et al. Co-circulation of two SARS-CoV-2 variant strains within imported pet hamsters in Hong Kong. Emerg. Microbes. Infect. 2022, 11, 689–698. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Ip, J.D.; Chu, A.W.H.; Tam, A.R.; Chan, W.M.; Abdullah, S.M.U.; Chan, B.P.C.; Wong, S.C.; Kwan, M.Y.W.; Chua, G.T.; et al. Rapid spread of SARS-CoV-2 Omicron subvariant BA.2 in a single-source community outbreak. Clin. Infect. Dis. 2022, ciac203. [Google Scholar] [CrossRef] [PubMed]




| From 2012 to 2019 (Period 1) | From 2020 to 2021 (Period 2) | p Value | |
|---|---|---|---|
| Streptococcus pyogenes | |||
| Total number of blood cultures | 587 | 51 | |
| Mean (range) blood cultures per year | 73 (41–117) | 26 (21–30) | <0.001 |
| Per 100,000 patient admissions | 4.31 | 1.50 | <0.001 |
| Per 10,000 blood culture requests | 2.89 | 0.95 | <0.001 |
| Streptococcus pneumoniae | |||
| Total number of blood cultures | 1226 | 52 | |
| Mean (range) blood cultures per year | 153 (130–187) | 26 (28–24) | <0.001 |
| Per 100,000 patient admissions | 8.99 | 1.53 | <0.001 |
| Per 10,000 blood culture requests | 6.04 | 0.96 | <0.001 |
| Haemophilus influenzae | |||
| Total number of blood cultures | 211 | 11 | |
| Mean (range) blood culture per year | 26 (20–32) | 6 (1–10) | 0.007 |
| Per 100,000 patient admissions | 1.55 | 0.32 | 0.012 |
| Per 10,000 blood culture requests | 1.04 | 0.20 | 0.006 |
| Neisseria meningitidis | |||
| Total number of blood cultures | 33 | 1 | |
| Mean (range) blood cultures per year | 4 (1–7) | 1 (0–1) | 0.004 |
| Per 100,000 patient admissions | 0.24 | 0.03 | 0.002 |
| Per 10,000 blood culture requests | 0.16 | 0.02 | 0.002 |
| Methicillin-sensitive Staphylococcus aureus | |||
| Total number of blood cultures | 5451 | 1508 | |
| Mean (range) blood cultures per year | 681 (578–744) | 754 (719–789) | 0.020 |
| Per 100,000 patient admissions | 39.98 | 44.29 | 0.197 |
| Per 10,000 blood culture requests | 26.84 | 27.98 | 0.432 |
| Methicillin-resistant Staphylococcus aureus | |||
| Total number of blood cultures | 3784 | 1067 | |
| Mean (range) blood cultures per year | 473 (396–552) | 543 (515–552) | 0.009 |
| Per 100,000 patient admissions | 27.76 | 31.34 | <0.001 |
| Per 10,000 blood culture requests | 18.63 | 19.80 | 0.024 |
| Escherichia coli | |||
| Total number of blood cultures | 45,645 | 12,296 | |
| Mean (range) blood cultures per year | 5706 (4681–6428) | 6148 (6076–6220) | 0.070 |
| Per 100,000 patient admissions | 334.80 | 361.10 | 0.176 |
| Per 10,000 blood culture requests | 224.70 | 228.10 | 0.516 |
| Notifications of | |||
| Scarlet fever | |||
| Total number of notifications | 12,567 | 351 | |
| Mean (range) of notifications per year | 1571 | 176 | <0.001 |
| Per 100,000 inhabitants | 21.47 | 2.36 | <0.001 |
| Tuberculosis | |||
| Total number of notifications | 35,512 | 7397 | |
| Mean (range) of notifications per year | 4439 (4003–4858) | 3699 (3656–3741) | <0.001 |
| Per 100,000 inhabitants | 60.66 | 49.66 | <0.001 |
| Chickenpox | |||
| Total number of notifications | 68,776 | 3577 | |
| Mean (range) of notifications per year | 8597 (6898–10,926) | 1789 (1590–1987) | <0.001 |
| Per 100,000 inhabitants | 117.49 | 24.02 | <0.001 |
| From 2012 to 2019 (Period 1) | From 2020 to 2021 (Period 2) | p Value | |
|---|---|---|---|
| Patient aged ≤ 12 years | |||
| Streptococcus pyogenes | |||
| Total number of blood cultures | 42 | 0 | |
| Mean (range) blood cultures per year | 5 (1–10) | 0 | NA c |
| Streptococcus pneumoniae | |||
| Total number of blood cultures | 101 | 1 | |
| Mean (range) blood cultures per year | 13 (7–16) | 0.5 (0–1) | <0.001 |
| Haemophilus influenzae | |||
| Total number of blood cultures | 10 | 0 | |
| Mean (range) blood cultures per year | 1 (1–3) | 0 | NA c |
| Methicillin-sensitive Staphylococcus aureus | |||
| Total number of blood cultures | 115 | 24 | |
| Mean (range) blood cultures per year | 14 (11–18) | 12 (9–15) | 0.337 |
| Methicillin-resistant Staphylococcus aureus | |||
| Total number of blood cultures | 15 | 3 | |
| Mean (range) blood cultures per year | 2 (1–3) | 2 (0–3) | 0.755 |
| Escherichia coli | |||
| Total number of blood cultures | 350 | 71 | |
| Mean (range) blood cultures per year | 44 (34–54) | 36 (30–41) | 0.079 |
| Patient aged > 12 years | |||
| Streptococcus pyogenes | |||
| Total number of blood cultures | 544 | 51 | |
| Mean (range) number of blood cultures per year | 68 (37–108) | 26 (21–30) | <0.001 |
| Streptococcus pneumoniae | |||
| Total number of blood cultures | 1125 | 51 | |
| Mean (range) blood cultures per year | 141 (119–175) | 26 (23–28) | <0.001 |
| Haemophilus influenzae | |||
| Total number of blood cultures | 201 | 11 | |
| Mean (range) blood cultures per year | 25 (20–31) | 6 (1–10) | 0.009 |
| Methicillin-sensitive Staphylococcus aureus | |||
| Total number of blood cultures | 5333 | 1483 | |
| Mean (range) blood cultures per year | 667 (564–733) | 742 (704–799) | 0.021 |
| Methicillin-resistant Staphylococcus aureus | |||
| Total number of blood cultures | 3768 | 1064 | |
| Mean (range) blood cultures per year | 471 (394–551) | 532 (515–549) | 0.007 |
| Escherichia coli | |||
| Total number of blood cultures | 45,295 | 12,225 | |
| Mean (range) blood cultures per year | 5662 (4627–6390) | 6113 (6046–6179) | 0.065 |
| From 2012 to 2019 (Period 1) | From 2020 to 2021 (Period 2) | p Value | |
|---|---|---|---|
| Streptococcus pyogenes | |||
| Total number of blood cultures | 19 | 1 | |
| Mean (range) blood cultures per year | 2 (0–6) | 1 (0–1) | 0.043 |
| Per 100,000 patient admissions | 0.14 | 0.03 | 0.053 |
| Per 10,000 blood culture requests | 0.09 | 0.02 | 0.036 |
| Streptococcus pneumoniae | |||
| Total number of blood cultures | 50 | 3 | |
| Mean (range) blood cultures per year | 6 (3–11) | 2 (1–2) | <0.001 |
| Per 100,000 patient admissions | 0.37 | 0.09 | <0.001 |
| Per 10,000 blood culture requests | 0.25 | 0.06 | <0.001 |
| Haemophilus influenzae | |||
| Total number of blood cultures | 29 | 1 | |
| Mean (range) blood cultures per year | 4 (1–7) | 1 (0–1) | 0.006 |
| Per 100,000 patient admissions | 0.21 | 0.03 | 0.010 |
| Per 10,000 blood culture requests | 0.14 | 0.02 | 0.006 |
| Methicillin-sensitive Staphylococcus aureus | |||
| Total number of blood cultures | 2963 | 833 | |
| Mean (range) blood cultures per year | 370 (308–464) | 417 (397–436) | 0.081 |
| Per 100,000 patient admissions | 21.73 | 24.46 | 0.001 |
| Per 10,000 blood culture requests | 14.59 | 15.45 | 0.042 |
| Methicillin-resistant Staphylococcus aureus | |||
| Total number of blood cultures | 3498 | 1012 | |
| Mean (range) blood cultures per year | 437 (353–538) | 506 (455–557) | 0.094 |
| Per 100,000 patient admissions | 25.66 | 29.72 | <0.001 |
| Per 10,000 blood culture requests | 17.22 | 18.78 | 0.179 |
| Escherichia coli | |||
| Total number of blood cultures | 8034 | 1075 | |
| Mean (range) blood culture per year | 1004 (816–1125) | 988 (963–1012) | 0.695 |
| Per 100,000 patient admissions | 58.93 | 58.00 | 0.807 |
| Per 10,000 blood culture requests | 39.55 | 36.64 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, V.C.-C.; Wong, S.-C.; So, S.Y.-C.; Chen, J.H.-K.; Chau, P.-H.; Au, A.K.-W.; Chiu, K.H.-Y.; Li, X.; Ip, P.; Chuang, V.W.-M.; et al. Decreased Antibiotic Consumption Coincided with Reduction in Bacteremia Caused by Bacterial Species with Respiratory Transmission Potential during the COVID-19 Pandemic. Antibiotics 2022, 11, 746. https://doi.org/10.3390/antibiotics11060746
Cheng VC-C, Wong S-C, So SY-C, Chen JH-K, Chau P-H, Au AK-W, Chiu KH-Y, Li X, Ip P, Chuang VW-M, et al. Decreased Antibiotic Consumption Coincided with Reduction in Bacteremia Caused by Bacterial Species with Respiratory Transmission Potential during the COVID-19 Pandemic. Antibiotics. 2022; 11(6):746. https://doi.org/10.3390/antibiotics11060746
Chicago/Turabian StyleCheng, Vincent Chi-Chung, Shuk-Ching Wong, Simon Yung-Chun So, Jonathan Hon-Kwan Chen, Pui-Hing Chau, Albert Ka-Wing Au, Kelvin Hei-Yeung Chiu, Xin Li, Patrick Ip, Vivien Wai-Man Chuang, and et al. 2022. "Decreased Antibiotic Consumption Coincided with Reduction in Bacteremia Caused by Bacterial Species with Respiratory Transmission Potential during the COVID-19 Pandemic" Antibiotics 11, no. 6: 746. https://doi.org/10.3390/antibiotics11060746
APA StyleCheng, V. C.-C., Wong, S.-C., So, S. Y.-C., Chen, J. H.-K., Chau, P.-H., Au, A. K.-W., Chiu, K. H.-Y., Li, X., Ip, P., Chuang, V. W.-M., Lung, D. C., Tse, C. W.-S., Lee, R. A., Fung, K. S.-C., To, W.-K., Lai, R. W.-M., Que, T.-L., Lo, J. Y.-C., & Yuen, K.-Y. (2022). Decreased Antibiotic Consumption Coincided with Reduction in Bacteremia Caused by Bacterial Species with Respiratory Transmission Potential during the COVID-19 Pandemic. Antibiotics, 11(6), 746. https://doi.org/10.3390/antibiotics11060746









