Dissemination of OXA-48- and NDM-1-Producing Enterobacterales Isolates in an Algerian Hospital
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Characterization and Antibiotic Susceptibility
2.2. Molecular Detection of Resistant Determinants
2.3. Virulence Profiles of the Carbapanemase-Producing Enterobacterales
2.4. Clonal Relationships among Species
2.5. Plasmid Typing of the Carbapanemase-Producing Enterobacterales
3. Discussion
4. Materials and Methods
4.1. Bacterial Collection
4.2. Antibiotic Susceptibility Testing
4.3. Phenotypic Characterization of Carbapenemase Production
4.4. Molecular Characterization of Antibiotic Resistance Genes
4.5. Clonal Relationships
4.6. Phylogenetic Grouping and Screening for Virulence Determinants
4.7. Plasmid Typing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potter, R.F.; D’Souza, A.W.; Dantas, G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updates 2016, 29, 30–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, K.; Bradford, P.A. Epidemiology of β-lactamase-producing pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Vandel, L.; Sougakoff, W.; Livermore, D.M.; Nordmann, P. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A beta-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob. Agents Chemother. 1994, 38, 1262–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iovleva, A.; Doi, Y. Carbapenem-Resistant Enterobacteriaceae. Clin. Lab. Med. 2017, 37, 303–315. [Google Scholar] [CrossRef]
- Mairi, A.; Pantel, A.; Sotto, A.; Lavigne, J.P.; Touati, A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 587–604. [Google Scholar] [CrossRef]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM metallo-beta-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019, 32, e00115-18. [Google Scholar] [CrossRef] [Green Version]
- Touati, A.; Mairi, A. Carbapenemase-Producing Enterobacterales in Algeria: A Systematic Review. Microb. Drug Resist. 2020, 26, 475–482. [Google Scholar] [CrossRef]
- Cherak, Z.; Loucif, L.; Moussi, A.; Rolain, J.M. Carbapenemase-producing Gram-negative bacteria in aquatic environments: A review. J. Glob. Antimicrob. Resist. 2021, 25, 287–309. [Google Scholar] [CrossRef]
- Loucif, L.; Chelaghma, W.; Cherak, Z.; Bendjama, E.; Beroual, F.; Rolain, J.M. Detection of NDM-5 and MCR-1 antibiotic resistance encoding genes in Enterobacterales in long-distance migratory bird species Ciconia ciconia, Algeria. Sci. Total Environ. 2022, 814, 152861. [Google Scholar] [CrossRef]
- Brahmia, S.; Lalaoui, R.; Nedjai, S.; Djahmi, N.; Chettibi, S.; Rolain, J.M.; Bakour, S. First Clinical Cases of KPC-2-Producing Klebsiella pneumoniae ST258 in Algeria and Outbreak of Klebsiella pneumoniae ST101 Harboring blaOXA-48 Gene in the Urology Department of Annaba Hospital. Microb. Drug Resist. 2021, 27, 652–659. [Google Scholar] [CrossRef]
- Chaalal, N.; Touati, A.; Bakour, S.; Aissa, M.A.; Sotto, A.; Lavigne, J.P.; Pantel, A. Spread of OXA-48 and NDM-1-Producing Klebsiella pneumoniae ST48 and ST101 in Chicken Meat in Western Algeria. Microb. Drug Resist. 2021, 27, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abderrahim, A.; Djahmi, N.; Pujol, C.; Nedjai, S.; Bentakouk, M.C.; Kirane-Gacemi, D.; Dekhil, M.; Sotto, A.; Lavigne, J.P.; Pantel, A. First case of NDM-1-producing Klebsiella pneumoniae in Annaba University Hospital, Algeria. Microb. Drug Resist. 2017, 23, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Yanat, B.; Rodriguez-Martinez, J.M.; Touati, A. Plasmid-mediated quinolone resistance in Enterobacteriaceae: A systematic review with a focus on Mediterranean countries. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Agabou, A.; Pantel, A.; Ouchenane, Z.; Lezzar, N.; Khemissi, S.; Satta, D.; Sotto, A.; Lavigne, J.P. First description of OXA-48-producing Escherichia coli and the pandemic clone ST131 from patients hospitalised at a military hospital in Algeria. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Belbel, Z.; Lalaoui, R.; Bakour, S.; Nedjai, S.; Djahmi, N.; Rolain, J.M. First report of colistin resistance in an OXA-48- and a CTX-M-15 producing Klebsiella pneumoniae clinical isolate in Algeria due to PmrB protein modification and mgrB inactivation. J. Glob. Antimicrob. Resist. 2018, 14, 158–160. [Google Scholar] [CrossRef]
- Bouguenoun, W.; Bakour, S.; Bentorki, A.A.; Al Bayssari, C.; Merad, T.; Rolain, J.M. Molecular epidemiology of environmental and clinical carbapenemase-producing Gram-negative bacilli from hospitals in Guelma, Algeria: Multiple genetic lineages and first report of OXA-48 in Enterobacter cloacae. J. Glob. Antimicrob. Resist. 2016, 7, 135–140. [Google Scholar] [CrossRef]
- Bourafa, N.; Chaalal, W.; Bakour, S.; Lalaoui, R.; Boutefnouchet, N.; Diene, S.M.; Rolain, J.M. Molecular characterization of carbapenem-resistant Gram-negative bacilli clinical isolates in Algeria. Infect. Drug Resist. 2018, 11, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Cuzon, G.; Bentchouala, C.; Vogel, A.; Hery, M.; Lezzar, A.; Smati, F.; Dortet, L.; Naas, T. First outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Constantine, Algeria. Int. J. Antimicrob. Agents 2015, 46, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Djahmi, N.; Dunyach-Remy, C.; Pantel, A.; Dekhil, M.; Sotto, A.; Lavigne, J.P. Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean countries. BioMed Res. Int. 2014, 2014, 305784. [Google Scholar] [CrossRef] [Green Version]
- Bachiri, T.; Bakour, S.; Lalaoui, R.; Belkebla, N.; Allouache, M.; Rolain, J.M.; Touati, A. Occurrence of carbapenemase-producing Enterobacteriaceae isolates in the wildlife: First report of OXA-48 in wild boars in Algeria. Microb. Drug Resist. 2018, 24, 337–345. [Google Scholar] [CrossRef]
- Bouaziz, A.; Loucif, L.; Ayachi, A.; Guehaz, K.; Bendjama, E.; Rolain, J.M. Migratory white stork (Ciconia ciconia): A potential vector of the OXA-48-producing Escherichia coli ST38 clone in Algeria. Microb. Drug Resist. 2018, 24, 461–468. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dortet, L.; Poirel, L.; Nordmann, P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. BioMed Res. Int. 2014, 2014, 249856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loucif, L.; Kassah-Laouar, A.; Saidi, M.; Messala, A.; Chelaghma, W.; Rolain, J.M. Outbreak of OXA-48-producing Klebsiella pneumoniae involving a Sequence Type 101 clone in Batna University Hospital, Algeria. Antimicrob. Agents Chemother. 2016, 60, 7494–7497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajamer, V.R.; Bhattacharjee, A.; Paul, D.; Deshamukhya, C.; Singh, A.K.; Pradhan, N.; Tiwari, H.K. Escherichia coli encoding bla(NDM-5) associated with community-acquired urinary tract infections with unusual MIC creep-like phenomenon against imipenem. J. Glob. Antimicrob. Resist. 2018, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Abd El Ghany, M.; Sharaf, H.; Al-Agamy, M.H.; Shibl, A.; Hill-Cawthorne, G.A.; Hong, P.Y. Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh, Saudi Arabia. PLoS ONE 2018, 13, e0201613. [Google Scholar] [CrossRef]
- Paskova, V.; Medvecky, M.; Skalova, A.; Chudejova, K.; Bitar, I.; Jakubu, V.; Bergerova, T.; Zemlickova, H.; Papagiannitsis, C.C.; Hrabak, J. Characterization of NDM-encoding plasmids from Enterobacteriaceae recovered from Czech hospitals. Front. Microbiol. 2018, 9, 1549. [Google Scholar] [CrossRef]
- Cubero, M.; Cuervo, G.; Dominguez, M.A.; Tubau, F.; Marti, S.; Sevillano, E.; Gallego, L.; Ayats, J.; Pena, C.; Pujol, M.; et al. Carbapenem-resistant and carbapenem-susceptible isogenic isolates of Klebsiella pneumoniae ST101 causing infection in a tertiary hospital. BMC Microbiol. 2015, 15, 177. [Google Scholar] [CrossRef] [Green Version]
- Pitart, C.; Solé, M.; Roca, I.; Fàbrega, A.; Vila, J.; Marco, F. First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 beta-lactamase in Klebsiella pneumoniae in Spain. Antimicrob. Agents Chemother. 2011, 55, 4398–4401. [Google Scholar] [CrossRef] [Green Version]
- Argente, M.; Miro, E.; Marti, C.; Vilamala, A.; Alonso-Tarres, C.; Ballester, F.; Calderon, A.; Galles, C.; Gasos, A.; Mirelis, B.; et al. Molecular characterization of OXA-48 carbapenemase-producing Klebsiella pneumoniae strains after a carbapenem resistance increase in Catalonia. Enferm. Infecc. Microbiol. Clin. 2019, 37, 82–88. [Google Scholar] [CrossRef]
- Gharout-Sait, A.; Alsharapy, S.A.; Brasme, L.; Touati, A.; Kermas, R.; Bakour, S.; Guillard, T.; de Champs, C. Enterobacteriaceae isolates carrying the New Delhi metallo-beta-lactamase gene in Yemen. J. Med. Microbiol. 2014, 63, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papagiannitsis, C.C.; Miriagou, V.; Giakkoupi, P.; Tzouvelekis, L.S.; Vatopoulos, A.C. Characterization of pKP1780, a novel IncR plasmid from the emerging Klebsiella pneumoniae ST147, encoding the VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 2013, 68, 2259–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaase, M.; Schimanski, S.; Schiller, R.; Beyreiss, B.; Thürmer, A.; Steinmann, J.; Kempf, V.A.; Hess, C.; Sobottka, I.; Fenner, I.; et al. Multicentre investigation of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in German hospitals. Int. J. Med. Microbiol. 2016, 306, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lahlaoui, H.; Poirel, L.; Barguellil, F.; Moussa, M.B.; Nordmann, P. Carbapenem-hydrolyzing class D beta-lactamase OXA-48 in Klebsiella pneumoniae isolates from Tunisia. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Zenati, K.; Sahli, F.; Garcia, V.; Bakour, S.; Belhadi, D.; Rolain, J.M.; Touati, A. Occurrence and clonal diversity of multidrug-resistant Klebsiella pneumoniae recovered from inanimate surfaces in Algerian hospital environment: First report of armA, qnrB and aac(6’)-Ib-cr genes. J. Glob. Antimicrob. Resist. 2017, 10, 148–153. [Google Scholar] [CrossRef]
- Yaici, L.; Haenni, M.; Métayer, V.; Saras, E.; Mesbah, Z.F.; Ayad, M.; Touati, A.; Madec, J.Y. Spread of ESBL/AmpC-producing Escherichia coli and Klebsiella pneumoniae in the community through ready-to-eat sandwiches in Algeria. Int. J. Food Microbiol. 2017, 245, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Fasciana, T.; Gentile, B.; Aquilina, M.; Ciammaruconi, A.; Mascarella, C.; Anselmo, A.; Fortunato, A.; Fillo, S.; Petralito, G.; Lista, F.; et al. Co-existence of virulence factors and antibiotic resistance in new Klebsiella pneumoniae clones emerging in south of Italy. BMC Infect. Dis. 2019, 19, 928. [Google Scholar] [CrossRef] [Green Version]
- Obeng-Nkrumah, N.; Labi, A.K.; Blankson, H.; Awuah-Mensah, G.; Oduro-Mensah, D.; Anum, J.; Teye, J.; Kwashie, S.D.; Bako, E.; Ayeh-Kumi, P.F.; et al. Household cockroaches carry CTX-M-15-, OXA-48- and NDM-1-producing enterobacteria, and share beta-lactam resistance determinants with humans. BMC Microbiol. 2019, 19, 272. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; Barba, P.; Mena-López, S.; Espinel, N.; Zurita, J. Escherichia coli hyperepidemic clone ST410-A harboring bla(CTX-M-15) isolated from fresh vegetables in a municipal market in Quito-Ecuador. Int. J. Food Microbiol. 2018, 280, 41–45. [Google Scholar] [CrossRef]
- Báez, J.; Hernández-García, M.; Guamparito, C.; Díaz, S.; Olave, A.; Guerrero, K.; Cantón, R.; Baquero, F.; Gahona, J.; Valenzuela, N.; et al. Molecular characterization and genetic diversity of ESBL-producing Escherichia coli colonizing the migratory Franklin’s gulls (Leucophaeus pipixcan) in Antofagasta, North of Chile. Microb. Drug Resist. 2015, 21, 111–116. [Google Scholar] [CrossRef]
- Pitout, J.D.; Hanson, N.D.; Church, D.L.; Laupland, K.B. Population-based laboratory surveillance for Escherichia coli-producing extended-spectrum beta-lactamases: Importance of community isolates with blaCTX-M genes. Clin. Infect. Dis. 2004, 38, 1736–1741. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, L.; Hopkins, K.L.; Gutierrez, B.; Ovejero, C.M.; Shukla, S.; Douthwaite, S.; Prasad, K.N.; Woodford, N.; Gonzalez-Zorn, B. Association of the novel aminoglycoside resistance determinant RmtF with NDM carbapenemase in Enterobacteriaceae isolated in India and the UK. J. Antimicrob. Chemother. 2013, 68, 1543–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ye, M.; Jia, X.; Yu, F.; Wang, M. Coexistence of armA and genes encoding aminoglycoside-modifying enzymes in Acinetobacter baumannii. Afr. J. Microbiol. Res. 2012, 6, 5325–5330. [Google Scholar]
- Jakobsen, L.; Sandvang, D.; Hansen, L.H.; Bagger-Skjot, L.; Westh, H.; Jorgensen, C.; Hansen, D.S.; Pedersen, B.M.; Monnet, D.L.; Frimodt-Moller, N.; et al. Characterisation, dissemination and persistence of gentamicin resistant Escherichia coli from a Danish university hospital to the waste water environment. Environ. Int. 2008, 34, 108–115. [Google Scholar] [CrossRef]
- Noppe-Leclercq, I.; Wallet, F.; Haentjens, S.; Courcol, R.; Simonet, M. PCR detection of aminoglycoside resistance genes: A rapid molecular typing method for Acinetobacter baumannii. Res. Microbiol. 1999, 150, 317–322. [Google Scholar] [CrossRef]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.B.; Wang, M.; Park, C.H.; Kim, E.C.; Jacoby, G.A.; Hooper, D.C. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 3582–3584. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef] [Green Version]
- Yamane, K.; Wachino, J.; Suzuki, S.; Arakawa, Y. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob. Agents Chemother. 2008, 52, 1564–1566. [Google Scholar] [CrossRef] [Green Version]
- Compain, F.; Babosan, A.; Brisse, S.; Genel, N.; Audo, J.; Ailloud, F.; Kassis-Chikhani, N.; Arlet, G.; Decre, D. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J. Clin. Microbiol. 2014, 52, 4377–4380. [Google Scholar] [CrossRef] [Green Version]
- Turton, J.F.; Perry, C.; Elgohari, S.; Hampton, C.V. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J. Med. Microbiol. 2010, 59, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compain, F.; Poisson, A.; Le Hello, S.; Branger, C.; Weill, F.X.; Arlet, G.; Decre, D. Targeting relaxase genes for classification of the predominant plasmids in Enterobacteriaceae. Int. J. Med. Microbiol. 2014, 304, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Fernandez, A.; Fortini, D.; Veldman, K.; Mevius, D.; Carattoli, A. Characterization of plasmids harbouring qnrS1, qnrB2 and qnrB19 genes in Salmonella. J. Antimicrob. Chemother. 2009, 63, 274–281. [Google Scholar] [CrossRef]
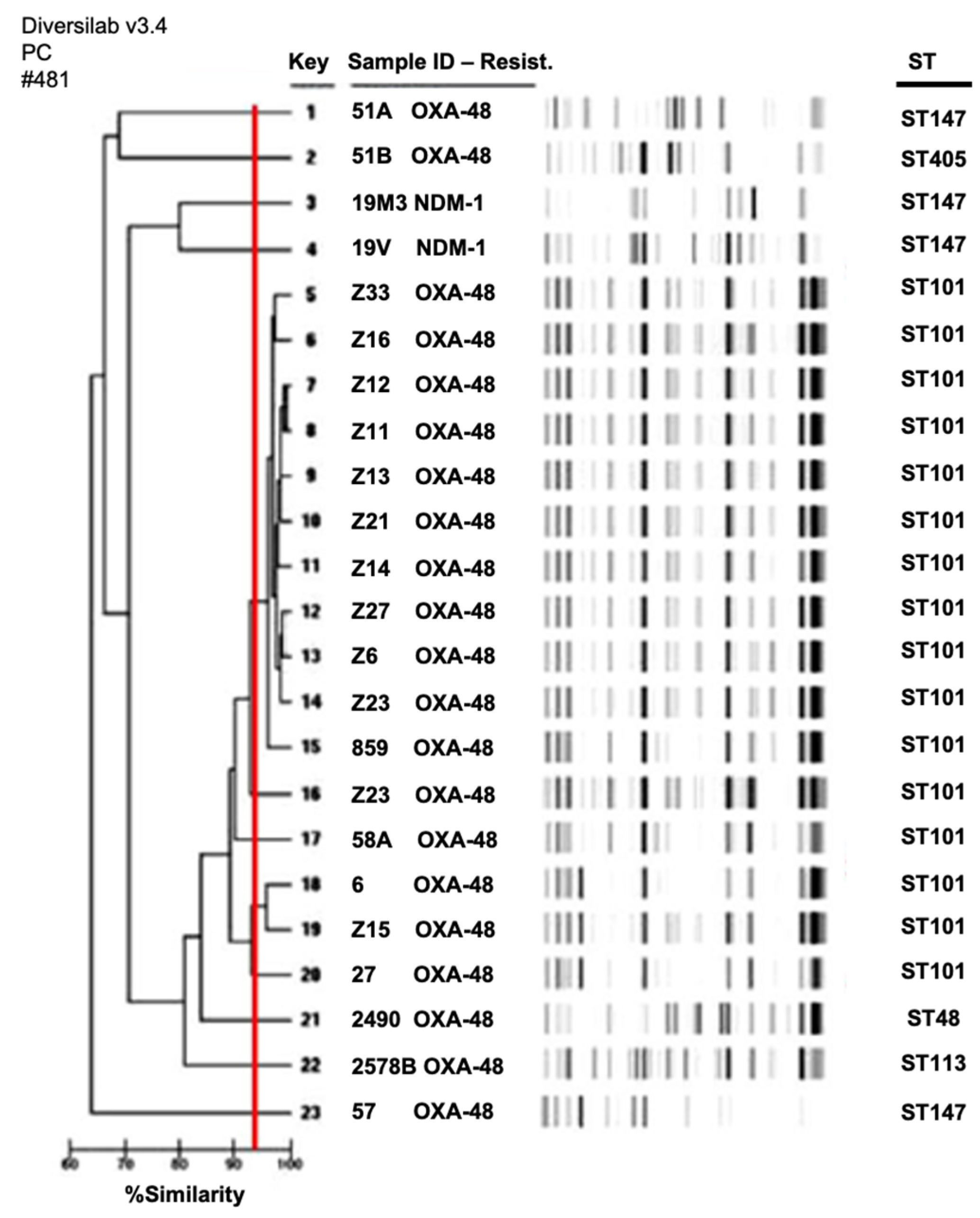
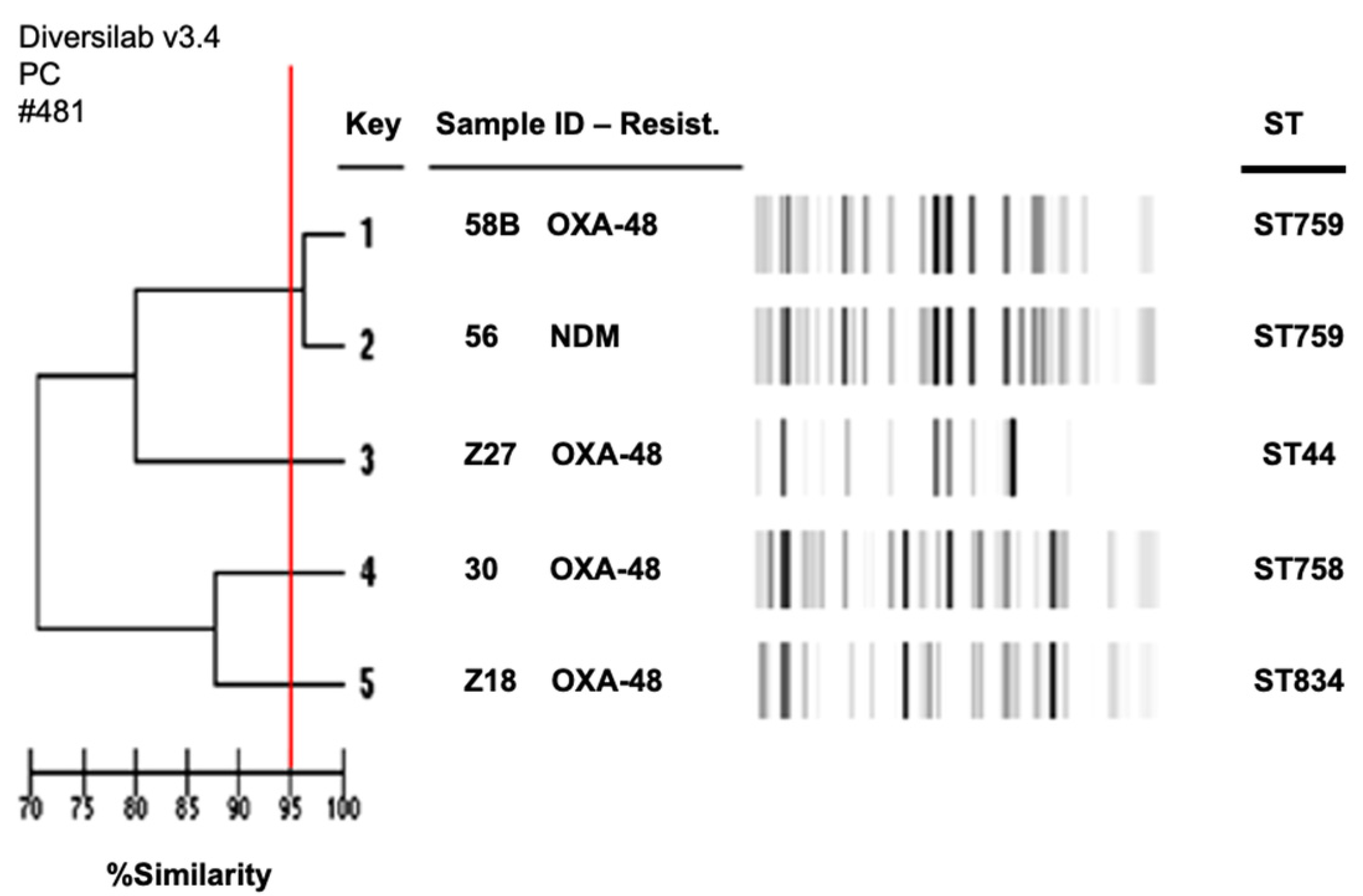
| Isolates | Species | Carbapenemase Genes | Additional Antibiotic Resistance Genes | Sequence Type | Virulence Genes | Phylogenetic Groups (E. coli) | Plasmid Type |
|---|---|---|---|---|---|---|---|
| 2490 | K. pneumoniae | blaOXA-48 | qnrB, qnrS, oqxAB | ST48 | ybtS, mrkD, entB, ugeF, wabG, ureA, fimH | NA * | IncM, IncR, MobC11 |
| 859 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrB, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, ugeF, wabG, ureA, fimH, kfu | NA | IncM, MobQu |
| 2578B | K. pneumoniae | blaOXA-48 | qnrB, oqxAB | ST113 | mrkD, entB, ugeF, wabG, ureA, fimH | NA | IncM |
| 19M3 | K. pneumoniae | blaNDM-1 | qnrB, aac(6′)-Ib-cr, oqxAB, aac(3)-II | ST147 | ybtS, mrkD, entB, ugeF, wabG, ureA, fimH | NA | IncR |
| 19V | K. pneumoniae | blaNDM-1 | blaCTX-M-15, qnrB, oqxABaac(6′)-Ib-cr, aac(3)-II | ST147 | ybtS, mrkD, entB, ugeF, wabG, ureA, fimH | NA | IncR |
| 27 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, colE NT2 |
| 57 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST147 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, IncR FIIK |
| 51A | K. pneumoniae | blaOXA-48 | blaCTX-M-15, oqxAB, aac(6′)-Ib-cr, rmtB | ST147 | ybtS, mrkD, entB, iutA, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, FV, IncAC, X3 |
| 51B | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrB, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST405 | ybtS, mrkD, entB, ugeF, ureA, fimH | NA | IncM, FIIK |
| 58A | K. pneumoniae | blaOXA-48 | blaCTX-M-15, oqxAB, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, colE NT1, colE NT2 |
| 6 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, ureA, fimH | NA | IncM, IncR, colE NT2 |
| Z6 | K. pneumoniae | blaOXA-48 | qnrS, oqxAB | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z7 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr, aac(3)-II, ant(2″) | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z9 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z11 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z12 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z13 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z14 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z15 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z16 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrB, qnrS, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, colE NT2 |
| Z21 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, qnrA, qnrS, oqxAB, aac(6′)-Ib-cr | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, MobQu |
| Z23 | K. pneumoniae | blaOXA-48 | blaCTX-M-15, oqxAB, aac(6′)-Ib-cr, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, MobQu |
| Z33 | K. pneumoniae | blaOXA-48 | qnrA, qnrS, oqxAB, aac(3)-II | ST101 | ybtS, mrkD, entB, kfu, ugeF, wabG, ureA, fimH | NA | IncM, FIIK, MobQu |
| 2578A | E. cloacae | blaOXA-48 | blaCTX-M-9, qnrB | ST68 | NA | NA | IncM, colE NT2 |
| 30 | E. coli | blaOXA-48 | aac(6′)-Ib-cr | ST758 | traT, fimH | B1 | IncM, AC, FV, colE NT1 |
| 58B | E. coli | blaOXA-48 | blaCTX-M-15, aac(6′)-Ib-cr | ST759 | traT, fimH, iutA | E | IncM, colE NT1, colE NT2, IncAC, FV, X3 |
| 56 | E. coli | blaNDM-1 | blaCTX-M-15, aac(6′)-Ib-cr, rmtB | ST759 | traT, fimH, hlyA | E | colE NT2, FV, X3 |
| Z18 | E. coli | blaOXA-48 | aac(3)-II | ST834 | traT, fimH, hlyA | A | IncM, FV, X3 |
| Z27 | E. coli | blaOXA-48 | blaCTX-M-15, aac(6′)-Ib-cr, aac(3)-II | ST44 | traT, fimH, hlyA, irp2, malX, papAH, papC, iutA, papG2, kpsMT2 | E | IncM, FV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abderrahim, A.; Djahmi, N.; Loucif, L.; Nedjai, S.; Chelaghma, W.; Gameci-Kirane, D.; Dekhil, M.; Lavigne, J.-P.; Pantel, A. Dissemination of OXA-48- and NDM-1-Producing Enterobacterales Isolates in an Algerian Hospital. Antibiotics 2022, 11, 750. https://doi.org/10.3390/antibiotics11060750
Abderrahim A, Djahmi N, Loucif L, Nedjai S, Chelaghma W, Gameci-Kirane D, Dekhil M, Lavigne J-P, Pantel A. Dissemination of OXA-48- and NDM-1-Producing Enterobacterales Isolates in an Algerian Hospital. Antibiotics. 2022; 11(6):750. https://doi.org/10.3390/antibiotics11060750
Chicago/Turabian StyleAbderrahim, Amel, Nassima Djahmi, Lotfi Loucif, Sabrina Nedjai, Widad Chelaghma, Djamila Gameci-Kirane, Mazouz Dekhil, Jean-Philippe Lavigne, and Alix Pantel. 2022. "Dissemination of OXA-48- and NDM-1-Producing Enterobacterales Isolates in an Algerian Hospital" Antibiotics 11, no. 6: 750. https://doi.org/10.3390/antibiotics11060750






