Identifying Targets for Antibiotic Use for the Management of Carbapenem-Resistant Acinetobacter baumannii (CRAb) in Hospitals—A Multi-Centre Nonlinear Time-Series Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Methods
4.1. Study Design and Population
4.2. Microbiology and Pharmacy Data
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABHR | Alcohol-Based Hand Rub |
| AMR | Antimicrobial Resistance |
| AMS | Antimicrobial Stewardship |
| ARMA | Autoregressive Moving Average |
| ATC | Anatomical Therapeutic Classification |
| CLSI | Clinical and Laboratory Standards Institute |
| CRAb | Carbapenem-Resistant Acinetobacter Baumannii |
| CRE | Carbapenem-resistant Enterobacteriaceae |
| CSF | Cerebrospinal fluid |
| CVP | Central venous pressure |
| DDD | Defined Daily Dose |
| ET | Endotracheal tube |
| GAM | Generalized Additive Models |
| HAIs | Health-care-associated infections |
| J01 | Antibacterials for systemic use |
| L | Liter |
| MARS | Multivariate Adaptive Regression Splines |
| MDRAb | Multidrug-resistant Acinetobacter baumannii |
| MDRO | Multidrug-resistant organism |
| MOH | Ministry of Health |
| OBD | Occupied Bed-Days |
| OMASS | Oman Antimicrobial Surveillance System |
| SCA | Scientific Computing Associates |
| VP | Ventriculoperitoneal |
| WHO | World Health Organization |
References
- Aldeyab, M.; López-Lozano, J.M.; Gould, I.M. Global antibiotics use and resistance. In Global Pharmaceutical Policy; Babar, Z.U.D., Ed.; Palgrave Macmillan: Singapore, 2020; pp. 331–344. ISBN 978-981-15-2723-4. [Google Scholar]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance. 2014. Available online: https://amr-review.org/Publications.html (accessed on 13 May 2022).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Hecker, M.T.; Aron, D.C.; Patel, N.P.; Lehmann, M.K.; Donskey, C.J. Unnecessary use of antimicrobials in hospitalized patients: Current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch. Intern. Med. 2003, 163, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Jirjees, F.J.; Al-Obaidi, H.J.; Sartaj, M.; Conlon-Bingham, G.; Farren, D.; Scott, M.G.; Gould, I.M.; López-Lozano, J.M.; Aldeyab, M.A. Antibiotic use and resistance in hospitals: Time-series analysis strategy for determining and prioritising interventions. Hosp Pharm Eur. 2020, 95, 13–19. Available online: https://hospitalpharmacyeurope.com/news/reviews-research/antibiotic-use-and-resistance-in-hospitals-time-series-analysis-strategy-for-determining-and-prioritising-interventions/ (accessed on 12 May 2022).
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Davey, P.; Brown, E.; Charani, E.; Fenelon, L.; Gould, I.M.; Holmes, A.; Ramsay, C.R.; Wiffen, P.J.; Wilcox, M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.B. Balancing the drug-resistance equation. Trends Microbiol. 1994, 2, 341–342. [Google Scholar] [CrossRef]
- López-Lozano, J.M.; Lawes, T.; Nebot, C.; Beyaert, A.; Bertrand, X.; Hocquet, D.; Aldeyab, M.; Scott, M.; Conlon-Bingham, G.; Farren, D.; et al. A nonlinear time-series analysis approach to identify thresholds in associations between population antibiotic use and rates of resistance. Nat. Microbiol. 2019, 4, 1160–1172. [Google Scholar] [CrossRef]
- Hayajneh, W.A.; Al-Azzam, S.; Yusef, D.; Lattyak, W.J.; Lattyak, E.A.; Gould, I.; López-Lozano, J.M.; Conway, B.R.; Conlon-Bingham, G.; Aldeyab, M.A. Identification of thresholds in relationships between specific antibiotic use and carbapenem-resistant Acinetobacter baumannii (CRAb) incidence rates in hospitalized patients in Jordan. J. Antimicrob. Chemother. 2021, 76, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Yusef, D.; Hayajneh, W.A.; Bani Issa, A.; Haddad, R.; Al-Azzam, S.; Lattyak, E.A.; Lattyak, W.J.; Gould, I.; Conway, B.R.; Bond, S.; et al. Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J. Antimicrob. Chemother. 2021, 76, 516–523. [Google Scholar] [CrossRef]
- Oman: Antimicrobial Resistance (AMR) National Action Plan. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/oman_national-action-plan-on-amr.pdf?sfvrsn=7f35d99_1&download=true (accessed on 12 May 2022).
- Oman Antimicrobial Resistance Surveillance System (OMASS). The First National Annual Antimicrobial Resistance Report (2017); Directorate General for Disease Surveillance and Control, Ministry of Health: Muscat, Oman, 2018. [Google Scholar]
- Oman Antimicrobial Resistance Surveillance System (OMASS). The Annual Antimicrobial Resistance Report (2018); Directorate General for Disease Surveillance and Control: Ministry of Health: Muscat, Oman, 2019. [Google Scholar]
- Balkhair, A.; Al-Muharrmi, Z.; Al’Adawi, B.; Al Busaidi, I.; Taher, H.B.; Al-Siyabi, T.; Al Amin, M.; Hassan, K.S. Prevalence and 30-day all-cause mortality of carbapenem-and colistin-resistant bacteraemia caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae: Description of a decade-long trend. Int. J. Infect. Dis. 2019, 85, 10–15. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO guidelines on hand hygiene in health care. In First Global Patient Safety Challenge—Clean Ecare Is Safer Care; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Kampf, G.; Löffler, H.; Gastmeier, P. Hand hygiene for the prevention of nosocomial infections. Dtsch. Arztebl. Int. 2009, 106, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Lotfinejad, N.; Peters, A.; Tartari, E.; Fankhauser-Rodriguez, C.; Pires, D.; Pittet, D. Hand hygiene in health care: 20 years of ongoing advances and perspectives. Lancet Infect. Dis. 2021, 21, e209–e221. [Google Scholar] [CrossRef]
- Lawes, T.; López-Lozano, J.-M.; Nebot, C.A.; Macartney, G.; Subbarao-Sharma, R.; Dare, C.; Wares, K.D.; Gould, I.M. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: A non-linear time-series study. Lancet Infect. Dis. 2015, 15, 1438–1449. [Google Scholar] [CrossRef]
- Lawes, T.; López-Lozano, J.-M.; Nebot, C.; Macartney, G.; Subbarao-Sharma, R.; Dare, C.R.J.; Edwards, G.F.S.; Gould, I.M. Turning the tide or riding the waves? Impacts of antibiotic stewardship and infection control on MRSA strain dynamics in a Scottish region over 16 years: Non-linear time series analysis. BMJ Open 2015, 5, e006596. [Google Scholar] [CrossRef]
- Lawes, T.; López-Lozano, J.-M.; Nebot, C.A.; Macartney, G.; Subbarao-Sharma, R.; Wares, K.D.; Sinclair, C.; Gould, I.M. Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: A non-linear time-series analysis. Lancet Infect. Dis. 2017, 17, 194–206. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2018, 63, e01110-18. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Lozano, J.M.; Monnet, D.L.; Yagüe, A.; Burgos, A.; Gonzalo, N.; Campillos, P.; Saez, M. Modelling and forecasting antimicrobial resistance and its dynamic relationship to antimicrobial use: A time series analysis. Int. J. Antimicrob. Agents 2000, 14, 21–31. [Google Scholar] [CrossRef]
- Shardell, M.; Harris, A.D.; El-Kamary, S.S.; Furuno, J.P.; Miller, R.R.; Perencevich, E.N. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin. Infect. Dis. 2007, 45, 901–907. [Google Scholar]
- Austin, D.J.; Kristinsson, K.G.; Anderson, R.M. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc. Natl. Acad. Sci. USA 1999, 96, 1152–1156. [Google Scholar] [CrossRef] [Green Version]
- National Surgical Antimicrobial Prophylaxis Guidelines. Available online: https://moh.gov.om/documents/236878/0/national+surgical+antimicrobial+prophylaxis/dd57462f-2f8b-47c6-b78b-f2821ad17fc9 (accessed on 12 May 2022).
- National Antimicrobial Guidelines. Available online: https://www.moh.gov.om/documents/236878/0/national+antimicrobial+guidelines/c82511c5-63e9-4205-8f49-0f60df4d7aa4 (accessed on 12 May 2022).
- MoH Code of Practice for Infection Prevention and Control. Available online: https://www.moh.gov.om/documents/236878/0/MOH+Code+Practice/4e55e63c-a1dc-4a2d-b813-37ec6915dc60 (accessed on 12 May 2022).
- Allegranzi, B.; Pittet, D. Role of hand hygiene in healthcare-associated infection prevention. J. Hosp. Infect. 2009, 73, 305–315. [Google Scholar] [CrossRef]
- Barrera, L.; Zingg, W.; Mendez, F.; Pittet, D. Effectiveness of a hand hygiene promotion strategy using alcohol-based handrub in 6 intensive care units in Colombia. Am. J. Infect. Control 2011, 39, 633–639. [Google Scholar] [CrossRef]
- Kingston, L.; O’Connell, N.H.; Dunne, C.P. Hand hygiene-related clinical trials reported since 2010: A systematic review. J. Hosp. Infect. 2016, 92, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Faidah, H.S.; Algethamy, M.; Alghamdi, S.; Alhazmi, G.A.; Alshomrani, A.O.; Alqethami, B.R.; Alotibi, H.S.; Almutiri, M.Z.; Almuqati, K.S.; et al. Antimicrobial Usage and Resistance in Makkah Region Hospitals: A Regional Point Prevalence Survey of Public Hospitals. Int. J. Environ. Res. Public Health 2021, 19, 254. [Google Scholar] [CrossRef] [PubMed]
- Al-Maliky, G.R.; Al-Ward, M.M.; Taqi, A.; Balkhair, A.; Al-Zakwani, I. Evaluation of antibiotic prescribing for adult inpatients at Sultan Qaboos University Hospital, Sultanate of Oman. Eur. J. Hosp. Pharm. 2018, 25, 195–199. [Google Scholar] [CrossRef]
- Al-Yamani, A.; Khamis, F.; Al-Zakwani, I.; Al-Noomani, H.; Al-Noomani, J.; Al-Abri, S. Patterns of Antimicrobial Prescribing in a Tertiary Care Hospital in Oman. Oman Med. J. 2016, 31, 35–39. [Google Scholar] [CrossRef]
- Mahmood, R.K.; Gillani, S.W.; Saeed, M.W.; Hafeez, M.U.; Gulam, S.M. Systematic Review: Study of the Prescribing Pattern of Antibiotics in Outpatients and Emergency Departments in the Gulf Region. Front. Pharmacol. 2020, 11, 585051. [Google Scholar] [CrossRef]
- Elhajji, F.D.; Al-Taani, G.M.; Anani, L.; Al-Masri, S.; Abdalaziz, H.; Qabba’H, S.H.; Al Bawab, A.Q.; Scott, M.; Farren, D.; Gilmore, F.; et al. Comparative point prevalence survey of antimicrobial consumption between a hospital in Northern Ireland and a hospital in Jordan. BMC Health Serv. Res. 2018, 18, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Matar, M.; Enani, M.; Binsaleh, G.; Roushdy, H.; Alokaili, D.; Al Bannai, A.; Khidir, Y.; Al-Abdely, H. Point prevalence survey of antibiotic use in 26 Saudi hospitals in 2016. J. Infect. Public Health 2019, 12, 77–82. [Google Scholar] [CrossRef]
- Alothman, A.; Al Thaqafi, A.; Al Ansary, A.; Zikri, A.; Fayed, A.; Khamis, F.; Al Salman, J.; Al Dabal, L.; Khalife, N.; AlMusawi, T.; et al. Prevalence of infections and antimicrobial use in the acute-care hospital setting in the Middle East: Results from the first point-prevalence survey in the region. Int. J. Infect. Dis. 2020, 101, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Hill, R.L.; Thomson, H.; Charlett, A.; Turton, J.; Pike, R.; Patel, B.C.; Manuel, R.; Gillespie, S.; Balakrishnan, I.; et al. Antimicrobial treatment and clinical outcome for infections with carbapenem- and multiply-resistant Acinetobacter baumannii around London. Int. J. Antimicrob. Agents 2010, 35, 19–24. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Amaya-Villar, R.; Ferrándiz-Millón, C.; Díaz-Martín, A.; López-Sánchez, J.M.; Gutiérrez-Pizarraya, A. Optimum treatment strategies for carbapenem-resistantAcinetobacter baumanniibacteremia. Expert Rev. Anti-Infect. Ther. 2015, 13, 769–777. [Google Scholar] [CrossRef]
- Hong, J.; Jang, O.J.; Bak, M.H.; Baek, E.H.; Park, K.-H.; Hong, S.I.; Cho, O.-H.; Bae, I.-G. Management of carbapenem-resistant Acinetobacter baumannii epidemic in an intensive care unit using multifaceted intervention strategy. Korean J. Intern. Med. 2018, 33, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Meschiari, M.; Lòpez-Lozano, J.-M.; Di Pilato, V.; Gimenez-Esparza, C.; Vecchi, E.; Bacca, E.; Orlando, G.; Franceschini, E.; Sarti, M.; Pecorari, M.; et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob. Resist. Infect. Control 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Aldeyab, M.A.; McELNAY, J.C.; Scott, M.G.; Elhajji, F.W.D.; Kearney, M.P. Hospital antibiotic use and its relationship to age-adjusted comorbidity and alcohol-based hand rub consumption. Epidemiol. Infect. 2014, 142, 404–408. [Google Scholar] [CrossRef]
- Aldeyab, M.A.; McElnay, J.C.; Scott, M.; Lattyak, W.J.; Elhajji, F.D.; Aldiab, M.A.; Magee, F.A.; Conlon, G.; Kearney, M.P. A modified method for measuring antibiotic use in healthcare settings: Implications for antibiotic stewardship and benchmarking. J. Antimicrob. Chemother. 2014, 69, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Sixth Edition: M100; CLSI: Wayne, PA, USA, 2016. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment, 2022. Oslo. 2021. Available online: https://www.whocc.no/filearchive/publications/2022guidelinesweb.pdf (accessed on 12 May 2022).
- Friedman, J. Multivariate adaptive regression splines. Ann. Statist. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized Additive Models; Chapman & Hall: London, UK, 1990. [Google Scholar]
- Liu, L.-M. Time Series Analysis and Forecasting, 2nd ed.; Scientific Computing Associates Corp.: River Forest, IL, USA, 2009. [Google Scholar]
- Neter, J.; Wasserman, W.; Kutner, M.H. Applied Linear Statistical Models, 3rd ed.; Irwin: New York, NY, USA, 1990. [Google Scholar]
- Muggeo, V.M.R. Interval estimation for the breakpoint in segmented regression: A smoothed score-based approach. Austral. N. Z. J. Stat. 2017, 59, 311–322. [Google Scholar] [CrossRef]
- Faraway, J. Extending the Linear Model with R; Chapman & Hall/CRC: New York, NY, USA, 2006. [Google Scholar]
- Box, G.E.P.; Jenkins, G.M. Time Series Analysis: Forecasting and Control; Holden Day: San Francisco, CA, USA, 1976. [Google Scholar]
- Box, G.E.P.; Jenkins, G.M.; Reinsel, G.C. Time Series Analysis: Forecasting and Control, 3rd ed.; Prentice Hall: Englewood Cliff, NJ, USA, 1994. [Google Scholar]
- Conlon-Bingham, G.M.; Aldeyab, M.; Scott, M.; Kearney, M.P.; Farren, D.; Gilmore, F.; McElnay, J. Effects of antibiotic cycling policy on incidence of healthcare-associated MRSA and Clostridioides difficile infection in secondary healthcare settings. Emerg. Infect. Dis. 2019, 25, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]

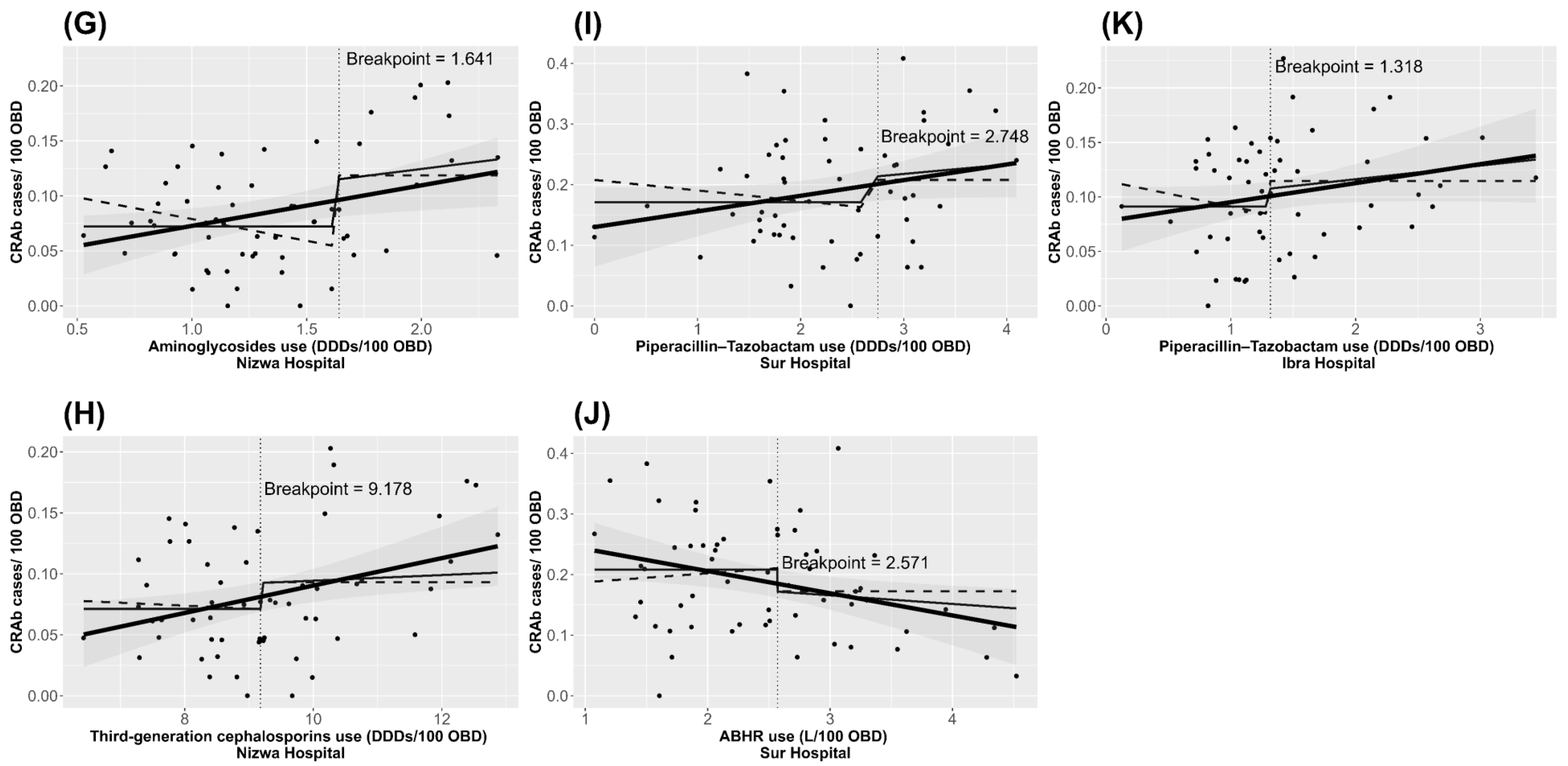
| Hospitals | Terms | Median Use (IQ Range) * | Lag (Months) | Threshold (95% Confidence Limit) | Relationship to Threshold | Regression Coefficient (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| a. Royal Hospital (R2 = 38.2) | Constant | N/A | N/A | N/A | N/A | 0.024 (0.020 to 0.029) | <0.0001 |
| Third-generation cephalosporins | 4.82 (4.24–5.85) | 1 | 5.87 (5.39 to 7.64) | Above | 0.017 (0.010 to 0.024) | <0.0001 | |
| Alcohol-based hand rub | 5.03 (4.20–5.50) | 3 | 5.09 (2.20 to 5.11) | Below | 0.008 (0.003 to 0.013) | 0.0025 | |
| b. Khawlah Hospital (R2 = 47.58) | Constant | N/A | N/A | N/A | N/A | 0.074 (0.057 to 0.091) | <0.0001 |
| Piperacillin-tazobactam | 3.40 (2.87–3.83) | 2 | 2.99 (2.35 to 3.86) | Above | 0.029 (0.010 to 0.047) | 0.0039 | |
| Aminoglycosides | 0.92 (0.66–1.22) | 1 | 0.84 (0.81 to 1.40) | Above | 0.053 (0.024 to 0.081) | 0.0005 | |
| Autoregressive | N/A | 1 | N/A | N/A | 0.328 (0.076 to 0.581) | 0.0154 | |
| c. As Sultan Qaboos Hospital (R2 = 30.04) | Constant | N/A | N/A | N/A | N/A | 0.109 (0.092 to 0.126) | <0.0001 |
| Aminoglycosides | 3.54 (3.21–4.13) | 4 | 3.50 (3.44 to 5.46) | Above | 0.046 (0.026 to 0.067) | <0.0001 | |
| Fluoroquinolones | 2.98 (2.57–3.41) | 4 | 2.52 (2.00 to 2.72) | Above | 0.026 (0.010 to 0.041) | 0.0022 | |
| d. Nizwa Hospital (R2 = 24.50) | Constant | N/A | N/A | N/A | N/A | 0.068 (0.055 to 0.082) | <0.0001 |
| Third-generation cephalosporins | 9.05 (8.08–10.02) | 4 | 9.18 (9.15 to 12.52) | Above | 0.014 (0.002 to 0.026) | 0.0246 | |
| Aminoglycosides | 1.27 (1.00–1.63) | 2 | 1.64 (1.32 to 1.70) | Above | 0.079 (0.009 to 0.148) | 0.0339 | |
| e. Sur Hospital (R2 = 42.9) | Constant | N/A | N/A | N/A | N/A | 0.204 (0.179 to 0.228) | <0.0001 |
| Piperacillin-tazobactam | 2.29 (1.76–2.99) | 3 | 2.75 (1.83 to 3.77) | Above | 0.070 (0.006 to 0.135) | 0.0387 | |
| Alcohol-based hand rub | 2.51 (1.87–3.08) | 2 | 2.57 (2.15 to 3.06) | Above | −0.079 (−0.118 to −0.041) | 0.0001 | |
| f. Ibra Hospital (R2 = 9.2) | Constant | N/A | N/A | N/A | N/A | 0.085 (0.066 to 0.104) | <0.0001 |
| Piperacillin-tazobactam | 1.30 (1.04–1.79) | 3 | 1.32 (1.12 to 2.98) | Above | 0.016 (0.003 to 0.028) | 0.0219 |
| Hospitals | Antibiotic | Patient Treatments per Month | |||
|---|---|---|---|---|---|
| Maximum Suggested by Threshold (Lower and Upper Bound) | Average Use in Last 12 Months of Study | Suggested Reduction in Use (%) | |||
| Standard | Conservative | ||||
| a. Royal Hospital | Third-generation cephalosporins | 139 (127–181) | 105 | Maintain below threshold | Maintain below threshold |
| b. Khawlah Hospital | Piperacillin-tazobactam | 44 (35–57) | 55 | 11 (20) | 20 (36) |
| Aminoglycoside | 12 (12–21) | 11 | Maintain below threshold | Maintain below threshold | |
| c. As Sultan Qaboos Hospital | Aminoglycoside | 52 (51–84) | 49 | Maintain below threshold | Maintain below threshold |
| Fluoroquinolones | 38 (30–41) | 44 | 6 (14) | 14 (32) | |
| d. Nizwa Hospital | Third-generation cephalosporins | 85 (84–115) | 74 | Maintain below threshold | Maintain below threshold |
| Aminoglycoside | 15 (12–16) | 9 | Maintain below threshold | Maintain below threshold | |
| e. Sur Hospital | Piperacillin-tazobactam | 13 (9–15) | 12 | Maintain below threshold | 3 (25) |
| f. Ibra Hospital | Piperacillin-tazobactam | 8 (7–19) | 11 | 3 (27) | 4 (36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hashimy, Z.S.; Conway, B.R.; Al-Yaqoobi, M.; Khamis, F.; Al Mawali, G.Z.; Al Maashani, A.M.; Al Hadhrami, Y.S.; Al Alawi, S.S.; Al Mamari, M.S.; Lattyak, W.J.; et al. Identifying Targets for Antibiotic Use for the Management of Carbapenem-Resistant Acinetobacter baumannii (CRAb) in Hospitals—A Multi-Centre Nonlinear Time-Series Study. Antibiotics 2022, 11, 775. https://doi.org/10.3390/antibiotics11060775
Al-Hashimy ZS, Conway BR, Al-Yaqoobi M, Khamis F, Al Mawali GZ, Al Maashani AM, Al Hadhrami YS, Al Alawi SS, Al Mamari MS, Lattyak WJ, et al. Identifying Targets for Antibiotic Use for the Management of Carbapenem-Resistant Acinetobacter baumannii (CRAb) in Hospitals—A Multi-Centre Nonlinear Time-Series Study. Antibiotics. 2022; 11(6):775. https://doi.org/10.3390/antibiotics11060775
Chicago/Turabian StyleAl-Hashimy, Zainab Said, Barbara R. Conway, Mubarak Al-Yaqoobi, Faryal Khamis, Ghalib Zahran Al Mawali, Aisha Mahad Al Maashani, Yaqoob Said Al Hadhrami, Said Salim Al Alawi, Mohammed Said Al Mamari, William J. Lattyak, and et al. 2022. "Identifying Targets for Antibiotic Use for the Management of Carbapenem-Resistant Acinetobacter baumannii (CRAb) in Hospitals—A Multi-Centre Nonlinear Time-Series Study" Antibiotics 11, no. 6: 775. https://doi.org/10.3390/antibiotics11060775







