The Role of Gut Microbiota in the Skeletal Muscle Development and Fat Deposition in Pigs
Abstract
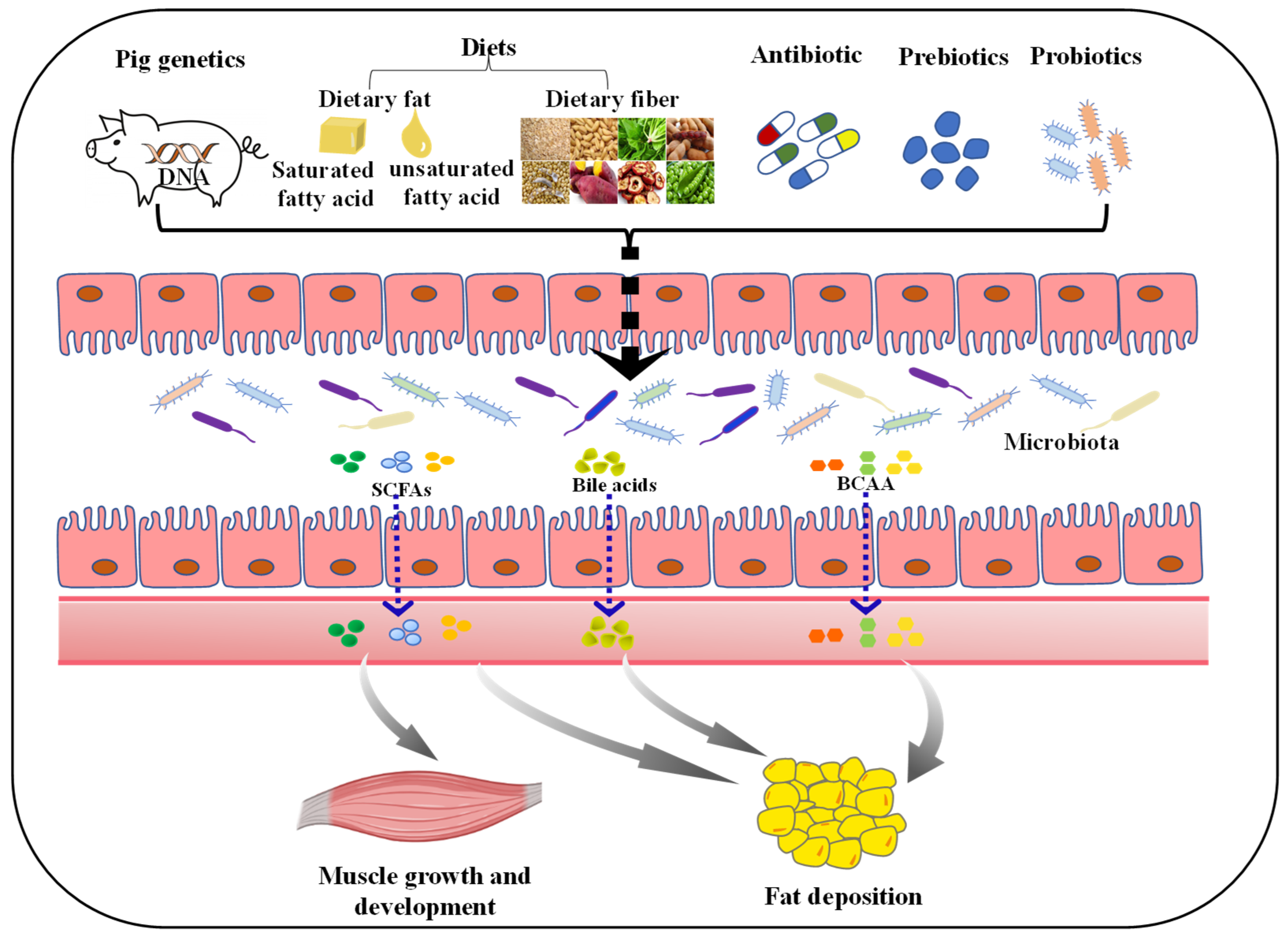
:1. Introduction
2. Gut Microbiota Linked to Muscle Growth and Development
2.1. Muscle Mass and Composition
2.2. Effects of SCFAs on Skeletal Muscle Metabolism
3. The Role of Gut Microbiota in Fat Deposition
3.1. Gut Microbiota Metabolites
3.2. Microbiota–Gut–Brain Axis in Fat Deposition
3.3. Fecal Microbiota Transplantation (FMT)
4. Factors Affecting Gut Microbial Composition and Function
4.1. Host Genetics
4.2. Diets
4.3. Antibiotic
4.4. Prebiotics and Probiotics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Ren, C.; Bai, Y.; Ijaz, M.; Hou, C.; Chen, L. Effects of protein posttranslational modifications on meat quality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 289–331. [Google Scholar] [CrossRef]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- Yang, H.; Xiang, Y.; Robinson, K.; Wang, J.; Zhang, G.; Zhao, J.; Xiao, Y. Gut Microbiota Is a Major Contributor to Adiposity in Pigs. Front. Microbiol. 2018, 9, 3045. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Yan, W.; Sun, C.; Ji, C.; Zhou, Q.; Zhang, D.; Zheng, J.; Yang, N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019, 13, 1422–1436. [Google Scholar] [CrossRef]
- Ma, J.; Duan, Y.; Li, R.; Liang, X.; Li, T.; Huang, X.; Yin, Y.; Yin, J. Gut microbial profiles and the role in lipid metabolism in Shaziling pigs. Anim. Nutr. 2022, 10, 12. [Google Scholar] [CrossRef]
- Hu, J.; Ma, L.; Nie, Y.; Chen, J.; Zheng, W.; Wang, X.; Xie, C.; Zheng, Z.; Wang, Z.; Yang, T.; et al. A Microbiota-Derived Bacteriocin Targets the Host to Confer Diarrhea Resistance in Early-Weaned Piglets. Cell Host Microbe 2018, 24, 817–832.e818. [Google Scholar] [CrossRef] [Green Version]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Yu, J.; Li, Y.; Yang, F.; Yu, H.; Xue, M.; Zhang, F.; Jiang, X.; Ji, X.; Bao, Z. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Annu. Med. 2021, 53, 508–522. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, 5662. [Google Scholar] [CrossRef] [Green Version]
- Agranyoni, O.; Meninger-Mordechay, S.; Uzan, A.; Ziv, O.; Salmon-Divon, M.; Rodin, D.; Raz, O.; Koman, I.; Koren, O.; Pinhasov, A.; et al. Gut microbiota determines the social behavior of mice and induces metabolic and inflammatory changes in their adipose tissue. NPJ Biofilms Microb. 2021, 7, 28. [Google Scholar] [CrossRef]
- Chen, C.Y.; Fang, S.M.; Wei, H.; He, M.Z.; Fu, H.; Xiong, X.W.; Zhou, Y.Y.; Wu, J.Y.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 110. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Jin, J.; Xu, Z.; Zuo, B. Functions and Regulatory Mechanisms of lncRNAs in Skeletal Myogenesis, Muscle Disease and Meat Production. Cells 2019, 8, 1107. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Liu, J.; Du, T.; Liu, Y.; Zhang, X.; Guo, Y.; Wang, J.; Zhou, X.; Li, X.; Yang, G.; et al. Circular RNA screening identifies circMYLK4 as a regulator of fast/slow myofibers in porcine skeletal muscles. Mol. Genet. Genom. 2021, 21, 835. [Google Scholar] [CrossRef]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef]
- Qi, R.; Sun, J.; Qiu, X.; Zhang, Y.; Wang, J.; Wang, Q.; Huang, J.; Ge, L.; Liu, Z. The intestinal microbiota contributes to the growth and physiological state of muscle tissue in piglets. Sci. Rep. 2021, 11, 11237. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Kallabis, S.; Abraham, L.; Müller, S.; Dzialas, V.; Türk, C.; Wiederstein, J.L.; Bock, T.; Nolte, H.; Nogara, L.; Blaauw, B.; et al. High-throughput proteomics fiber typing (ProFiT) for comprehensive characterization of single skeletal muscle fibers. Skelet. Muscle 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef]
- Yan, H.; Diao, H.; Xiao, Y.; Li, W.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci. Rep. 2016, 6, 31786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Xiong, X.; Su, Y.; Huang, L.; Chen, C. 16S rRNA gene-based association study identified microbial taxa associated with pork intramuscular fat content in feces and cecum lumen. BMC Microbiol. 2017, 17, 162. [Google Scholar] [CrossRef]
- Tang, S.; Xin, Y.; Ma, Y.; Xu, X.; Zhao, S.; Cao, J. Screening of Microbes Associated with Swine Growth and Fat Deposition Traits Across the Intestinal Tract. Front. Microbiol. 2020, 11, 586776. [Google Scholar] [CrossRef]
- Wu, C.; Lyu, W.; Hong, Q.; Zhang, X.; Yang, H.; Xiao, Y. Gut Microbiota Influence Lipid Metabolism of Skeletal Muscle in Pigs. Front. Nutr. 2021, 8, 675445. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-Activated Protein Kinase and Stimulation of Energy Metabolism by Acetic Acid in L6 Myotube Cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Kim, I.S.; Jung, S.H.; Lee, S.G.; Son, H.Y.; Myung, C.S. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41. PLoS ONE 2014, 9, e95268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fushimi, T.; Sato, Y. Effect of acetic acid feeding on the circadian changes in glycogen and metabolites of glucose and lipid in liver and skeletal muscle of rats. Br. J. Nutr. 2005, 94, 714–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakakibara, S.; Yamauchi, T.; Oshima, Y.; Tsukamoto, Y.; Kadowaki, T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem. Biophys. Res. Commun. 2006, 344, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, T.; Tayama, K.; Fukaya, M.; Kitakoshi, K.; Nakai, N.; Tsukamoto, Y.; Sato, Y. Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. J. Nutr. 2001, 131, 1973–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Henagan, T.M.; Stefanska, B.; Fang, Z.; Navard, A.M.; Ye, J.; Lenard, N.R.; Devarshi, P.P. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br. J. Pharmacol. 2015, 172, 2782–2798. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Kim, J.H.; Kim, H.M.; Lee, E.S.; Shin, D.H.; Kim, S.; Shin, M.; Kim, S.H.; Lee, J.H.; Kim, Y.J. Acetic acid enhances endurance capacity of exercise-trained mice by increasing skeletal muscle oxidative properties. Biosci. Biotechnol. Biochem. 2015, 79, 1535–1541. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Yu, J.; Zheng, P.; Huang, Z.; Luo, Y.; Luo, J.; Mao, X.; Yan, H.; He, J.; et al. Butyrate promotes slow-twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC-1α expression1. J. Anim. Sci. 2019, 97, 3180–3192. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, G.; Apovian, C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Lyu, W.; Liu, X.; Lu, L.; Dai, B.; Wang, W.; Yang, H.; Xiao, Y. Cecal Microbiota Modulates Fat Deposition in Muscovy Ducks. Front. Vet. Sci. 2021, 8, 609348. [Google Scholar] [CrossRef]
- He, M.; Fang, S.; Huang, X.; Zhao, Y.; Ke, S.; Yang, H.; Li, Z.; Gao, J.; Chen, C.; Huang, L. Evaluating the Contribution of Gut Microbiota to the Variation of Porcine Fatness with the Cecum and Fecal Samples. Front. Microbiol. 2016, 7, 2108. [Google Scholar] [CrossRef] [Green Version]
- Stokes, C.R. The development and role of microbial-host interactions in gut mucosal immune development. J. Anim. Sci. Biotechnol. 2017, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Huttenhower, C. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. Gut microbiota-derived metabolites in obesity: A systematic review. Biosci. Microb. Food Health 2020, 39, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Jiao, A.R.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Mao, X.B.; et al. Oral administration of short chain fatty acids could attenuate fat deposition of pigs. PLoS ONE 2018, 13, 6867. [Google Scholar] [CrossRef] [Green Version]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Mao, X.; Chen, D. Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 2020, 11, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.R.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.H.; Luo, J.Q.; Wang, Q.Y.; Wang, H.F.; et al. Infusion of short chain fatty acids in the ileum improves the carcass traits, meat quality and lipid metabolism of growing pigs. Anim. Nutr. 2021, 7, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Duan, Y.; Duan, Y.; Li, F.; Li, Y.; Guo, Q.; Ji, Y.; Tan, B.; Li, T.; Yin, Y. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids 2016, 48, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.H.; Li, F.N.; Wen, C.Y.; Wang, W.L.; Guo, Q.P.; Li, Y.H.; Yin, Y.L. Branched-chain amino acid ratios in low-protein diets regulate the free amino acid profile and the expression of hepatic fatty acid metabolism-related genes in growing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e43–e51. [Google Scholar] [CrossRef]
- Li, Y.; Wei, H.; Li, F.; Duan, Y.; Guo, Q.; Yin, Y. Effects of Low-Protein Diets Supplemented with Branched-Chain Amino Acid on Lipid Metabolism in White Adipose Tissue of Piglets. J. Agric. Food Chem. 2017, 65, 2839–2848. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Yang, Y.; Yin, Y.; Gong, S.; Han, M.; Yin, Y. Different Proportions of Branched-Chain Amino Acids Modulate Lipid Metabolism in a Finishing Pig Model. J. Agric. Food Chem. 2021, 69, 7037–7048. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Wada, T.; Febbraio, M.; He, J.; Matsubara, T.; Lee, M.J.; Gonzalez, F.J.; Xie, W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology 2010, 139, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- Ruan, Z.; Yang, Y.; Wen, Y.; Zhou, Y.; Fu, X.; Ding, S.; Liu, G.; Yao, K.; Wu, X.; Deng, Z.; et al. Metabolomic analysis of amino acid and fat metabolism in rats with L-tryptophan supplementation. Amino Acids 2014, 46, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Min, Z.Z.; Yuan, J.M. Apparent ileal digestible tryptophan requirements of 22-to 42-day-old broiler chicks. J. Appl. Poult. Res. 2016, 25, 54–61. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Foley, M.H.; O’Flaherty, S.; Barrangou, R.; Theriot, C.M. Bile salt hydrolases: Gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019, 15, e1007581. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRS and FXR: The yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Parséus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagug. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef] [Green Version]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Z.; Yu, Y.J.; Adeli, K. Role of Gut Microbiota in Neuroendocrine Regulation of Carbohydrate and Lipid Metabolism via the Microbiota-Gut-Brain-Liver Axis. Microorganisms 2020, 8, 527. [Google Scholar] [CrossRef] [Green Version]
- Alamshah, A.; McGavigan, A.K.; Spreckley, E.; Kinsey-Jones, J.S.; Amin, A.; Tough, I.R.; O’Hara, H.C.; Moolla, A.; Banks, K.; France, R.; et al. L-arginine promotes gut hormone release and reduces food intake in rodents. Diabetes Obes. Metab. 2016, 18, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Goswami, C.; Iwasaki, Y.; Yada, T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 2018, 57, 130–135. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [Green Version]
- de La Serre, C.B.; de Lartigue, G.; Raybould, H.E. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol. Behav. 2015, 139, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Colpitts, S.L.; Kasper, L.H. Influence of the Gut Microbiome on Autoimmunity in the Central Nervous System. J. Immunol. 2017, 198, 596–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic inflammation and obesity: A mechanistic review. Arch. Pharm. Res. 2019, 42, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Yacyshyn, B.R.; Yacyshyn, M.B. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes. Metab. 2019, 21, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellekilde, M.; Selfjord, E.; Larsen, C.S.; Jakesevic, M.; Rune, I.; Tranberg, B.; Vogensen, F.K.; Nielsen, D.S.; Bahl, M.I.; Licht, T.R.; et al. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci. Rep. 2014, 4, 5922. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Kong, F.; Xiang, Y.; Zhou, W.; Wang, J.; Yang, H.; Zhang, G.; Zhao, J. Comparative biogeography of the gut microbiome between Jinhua and Landrace pigs. Sci. Rep. 2018, 8, 5985. [Google Scholar] [CrossRef]
- Danne, C.; Rolhion, N.; Sokol, H. Recipient factors in faecal microbiota transplantation: One stool does not fit all. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 503–513. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurilshikov, A.; Wijmenga, C.; Fu, J.; Zhernakova, A. Host Genetics and Gut Microbiome: Challenges and Perspectives. Trends Immunol. 2017, 38, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Espin-Garcia, O.; Xu, W.; Silverberg, M.S.; Kevans, D.; Smith, M.I.; Guttman, D.S.; Griffiths, A.; Panaccione, R.; Otley, A.; et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat. Genet. 2016, 48, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrich, J.K.; Davenport, E.R.; Clark, A.G.; Ley, R.E. The Relationship Between the Human Genome and Microbiome Comes into View. Annu. Rev. Genet. 2017, 51, 413–433. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Huang, X.; Fang, S.; Yang, H.; He, M.; Zhao, Y.; Huang, L. Contribution of Host Genetics to the Variation of Microbial Composition of Cecum Lumen and Feces in Pigs. Front. Microbiol. 2018, 9, 2626. [Google Scholar] [CrossRef]
- Pajarillo, E.A.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Seo, K.S.; Kang, D.K. Pyrosequencing-based analysis of fecal microbial communities in three purebred pig lines. J. Microbiol. 2014, 52, 646–651. [Google Scholar] [CrossRef]
- Crespo-Piazuelo, D.; Migura-Garcia, L.; Estellé, J.; Criado-Mesas, L.; Revilla, M.; Castelló, A.; Muñoz, M.; García-Casco, J.M.; Fernández, A.I.; Ballester, M.; et al. Association between the pig genome and its gut microbiota composition. Sci. Rep. 2019, 9, 8791. [Google Scholar] [CrossRef]
- Reverter, A.; Ballester, M.; Alexandre, P.A.; Mármol-Sánchez, E.; Dalmau, A.; Quintanilla, R.; Ramayo-Caldas, Y. A gene co-association network regulating gut microbial communities in a Duroc pig population. Microbiome 2021, 9, 52. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Pu, G.; Li, P.; Du, T.; Niu, Q.; Fan, L.; Wang, H.; Liu, H.; Li, K.; Niu, P.; Wu, C.; et al. Adding Appropriate Fiber in Diet Increases Diversity and Metabolic Capacity of Distal Gut Microbiota Without Altering Fiber Digestibility and Growth Rate of Finishing Pig. Front. Microbiol. 2020, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, P.; Wu, Y.; Guo, P.; Liu, L.; Ma, N.; Levesque, C.; Chen, Y.; Zhao, J.; Zhang, J.; et al. Dietary Fiber Increases Butyrate-Producing Bacteria and Improves the Growth Performance of Weaned Piglets. J. Agric. Food Chem. 2018, 66, 7995–8004. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E.; Upadhyay, V.; Turnbaugh, J.A.; Ly, K.; Turnbaugh, P.J. Meta-Analysis Reveals Reproducible Gut Microbiome Alterations in Response to a High-Fat Diet. Cell Host Microbe 2019, 26, 265–272.e264. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Qiu, Y.Q.; Wang, L.; Gao, K.G.; Jiang, Z.Y. A high-fat diet increases body fat mass and up-regulates expression of genes related to adipogenesis and inflammation in a genetically lean pig. J. Zhejiang Univ. Sci. B 2018, 19, 884–894. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leone, V.A.; Devkota, S.; Wang, Y.; Brady, M.J.; Chang, E.B. Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. JPEN J. Parenter. Enteral Nutr. 2013, 37, 746–754. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Tian, M.; Chen, J.; Chen, F.; Guan, W. Different Sources of High Fat Diet Induces Marked Changes in Gut Microbiota of Nursery Pigs. Front. Microbiol. 2020, 11, 859. [Google Scholar] [CrossRef]
- Gunness, P.; Williams, B.A.; Gerrits, W.J.; Bird, A.R.; Kravchuk, O.; Gidley, M.J. Circulating triglycerides and bile acids are reduced by a soluble wheat arabinoxylan via modulation of bile concentration and lipid digestion rates in a pig model. Mol. Nutr. Food Res. 2016, 60, 642–651. [Google Scholar] [CrossRef]
- Yang, J.T.; Chen, Y.J.; Huang, C.W.; Wang, Y.C.; Mersmann, H.J.; Wang, P.H.; Ding, S.T. Docosahexaenoic Acid Suppresses Expression of Adipogenic Tetranectin through Sterol Regulatory Element-Binding Protein and Forkhead Box O Protein in Pigs. Nutrients 2021, 13, 2315. [Google Scholar] [CrossRef]
- López-García, A.; Benítez, R.; Núñez, Y.; Gómez-Izquierdo, E.; de Mercado, E.; García-Casco, J.M.; González-Recio, Ó.; López-Bote, C.; Estellé, J.; Óvilo, C. Influence of genetic background and dietary oleic acid on gut microbiota composition in Duroc and Iberian pigs. PLoS ONE 2021, 16, e0251804. [Google Scholar] [CrossRef]
- Che, L.; Zhou, Q.; Liu, Y.; Hu, L.; Peng, X.; Wu, C.; Zhang, R.; Tang, J.; Wu, F.; Fang, Z.; et al. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. 2019, 10, 8149–8160. [Google Scholar] [CrossRef] [PubMed]
- Sappok, M.A.; Peréz Gutiérrez, O.; Smidt, H.; Pellikaan, W.F.; Verstegen, M.W.; Bosch, G.; Hendriks, W.H. Adaptation of faecal microbiota in sows after diet changes and consequences for in vitro fermentation capacity. Animal 2015, 9, 1453–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Lu, N.; Xue, Y.; Liu, S.; Lei, H.; Tu, W.; Lu, Y.; Xia, D. Crude fiber modulates the fecal microbiome and steroid hormones in pregnant Meishan sows. Gen. Comp. Endocrinol. 2019, 277, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiong, Y.; Zhong, M.; Li, Y.; Wan, H.; Wu, D.; Liu, Q. Effects of purified fibre-mixture supplementation of gestation diet on gut microbiota, immunity and reproductive performance of sows. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1144–1154. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, C.; Zhang, X.; Peng, J. Inclusion of Soluble Fiber in the Gestation Diet Changes the Gut Microbiota, Affects Plasma Propionate and Odd-Chain Fatty Acids Levels, and Improves Insulin Sensitivity in Sows. Int. J. Mol. Sci. 2020, 21, 635. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, J.E. Fiber effects in nutrition and gut health in pigs. J Anim. Sci. Biotechnol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Han, P.; Li, P.; Zhou, W.; Fan, L.; Wang, B.; Liu, H.; Gao, C.; Du, T.; Pu, G.; Wu, C.; et al. Effects of various levels of dietary fiber on carcass traits, meat quality and myosin heavy chain I, IIa, IIx and IIb expression in muscles in Erhualian and Large White pigs. Meat Sci. 2020, 169, 108160. [Google Scholar] [CrossRef]
- Wu, X.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; He, J. Effect of different dietary non-starch fiber fractions on growth performance, nutrient digestibility, and intestinal development in weaned pigs. Nutrition 2018, 51, 20–28. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, L.; He, X.; Smidt, H.; Zhu, W. Dietary fibres modulate the composition and activity of butyrate-producing bacteria in the large intestine of suckling piglets. Antonie Van Leeuwenhoek 2017, 110, 687–696. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of antibiotic use in global pig production: A systematic review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yu, B.; Degroote, J.; Spranghers, T.; Van Noten, N.; Majdeddin, M.; Van Poucke, M.; Peelman, L.; De Vrieze, J.; Boon, N.; et al. Antibiotic affects the gut microbiota composition and expression of genes related to lipid metabolism and myofiber types in skeletal muscle of piglets. BMC Vet. Res. 2020, 16, 392. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Fouhse, J.M.; Zijlstra, R.T.; Willing, B.P. The role of gut microbiota in the health and disease of pigs. Anim. Front. 2016, 6, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Schokker, D.; Zhang, J.; Zhang, L.L.; Vastenhouw, S.A.; Heilig, H.G.; Smidt, H.; Rebel, J.M.; Smits, M.A. Early-life environmental variation affects intestinal microbiota and immune development in new-born piglets. PLoS ONE 2014, 9, e100040. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Su, Y.; Zoetendal, E.G.; Zhu, W. Differences in Microbiota Membership along the Gastrointestinal Tract of Piglets and Their Differential Alterations Following an Early-Life Antibiotic Intervention. Front. Microbiol. 2017, 8, 797. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Yu, K.; Yu, M.; Zhang, C.; Su, Y.; Zhu, W. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids 2017, 49, 771–781. [Google Scholar] [CrossRef]
- Kim, H.B.; Borewicz, K.; White, B.A.; Singer, R.S.; Sreevatsan, S.; Tu, Z.J.; Isaacson, R.E. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc. Natl. Acad. Sci. USA 2012, 109, 15485–15490. [Google Scholar] [CrossRef] [Green Version]
- Looft, T.; Allen, H.K.; Cantarel, B.L.; Levine, U.Y.; Bayles, D.O.; Alt, D.P.; Henrissat, B.; Stanton, T.B. Bacteria, phages and pigs: The effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 2014, 8, 1566–1576. [Google Scholar] [CrossRef] [Green Version]
- Allen, H.K.; Looft, T.; Bayles, D.O.; Humphrey, S.; Levine, U.Y.; Alt, D.; Stanton, T.B. Antibiotics in feed induce prophages in swine fecal microbiomes. mBio 2011, 2, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monger, X.C.; Gilbert, A.A.; Saucier, L.; Vincent, A.T. Antibiotic Resistance: From Pig to Meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- Sprockett, D.; Fukami, T.; Relman, D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, C.; Yang, Y.; Mu, C.; Su, Y.; Yu, K.; Zhu, W. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. J. Anim. Sci. Biotechnol. 2017, 8, 60. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.; Smidt, H.; Rebel, J.M.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef] [Green Version]
- Stark, C.M.; Susi, A.; Emerick, J.; Nylund, C.M. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut 2019, 68, 62–69. [Google Scholar] [CrossRef]
- Schulfer, A.F.; Schluter, J.; Zhang, Y.; Brown, Q.; Pathmasiri, W.; McRitchie, S.; Sumner, S.; Li, H.; Xavier, J.B.; Blaser, M.J. The impact of early-life sub-therapeutic antibiotic treatment (STAT) on excessive weight is robust despite transfer of intestinal microbes. ISME J. 2019, 13, 1280–1292. [Google Scholar] [CrossRef] [Green Version]
- Mu, C.; Zhu, W. Antibiotic effects on gut microbiota, metabolism, and beyond. Appl. Microbiol. Biotechnol. 2019, 103, 9277–9285. [Google Scholar] [CrossRef]
- Li, J.; Yang, K.; Ju, T.; Ho, T.; McKay, C.A.; Gao, Y.; Forget, S.K.; Gartner, S.R.; Field, C.J.; Chan, C.B.; et al. Early life antibiotic exposure affects pancreatic islet development and metabolic regulation. Sci. Rep. 2017, 7, 41778. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830s–837s. [Google Scholar] [CrossRef] [Green Version]
- Grela, E.R.; Świątkiewicz, M.; Florek, M.; Bąkowski, M.; Skiba, G. Effect of Inulin Source and a Probiotic Supplement in Pig Diets on Carcass Traits, Meat Quality and Fatty Acid Composition in Finishing Pigs. Animals 2021, 11, 2438. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv. Nutr. 2016, 7, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Wu, W.; Zhang, L.; Wen, X.; Xie, J.; Zhang, H. Gut microbiota mediates the effects of inulin on enhancing sulfomucin production and mucosal barrier function in a pig model. Food Funct. 2021, 12, 10967–10982. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Luo, Y.; Yu, J.; Luo, J.; Yan, H.; et al. Effects of dietary inulin supplementation on growth performance, intestinal barrier integrity and microbial populations in weaned pigs. Br. J. Nutr. 2020, 124, 296–305. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Xie, H.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Luo, Y.; Yu, J.; Luo, J.; et al. Synergetic responses of intestinal microbiota and epithelium to dietary inulin supplementation in pigs. Eur. J. Nutr. 2021, 60, 715–727. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Wang, J.; Zhu, W. Differential Effects of Early-Life and Postweaning Galacto-oligosaccharide Intervention on Colonic Bacterial Composition and Function in Weaning Piglets. Appl. Environ. Microbiol. 2022, 88, e0131821. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Changes in Ileal Microbial Composition and Microbial Metabolism by an Early-Life Galacto-Oligosaccharides Intervention in a Neonatal Porcine Model. Nutrients 2019, 11, 1753. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Zhao, X.; Azad, M.A.K.; Ma, C.; Gao, Q.; He, J.; Kong, X. Dietary supplementation with Bacillus subtilis and xylo-oligosaccharides improves growth performance and intestinal morphology and alters intestinal microbiota and metabolites in weaned piglets. Food Funct. 2021, 12, 5837–5849. [Google Scholar] [CrossRef]
- Tian, L.; Bruggeman, G.; van den Berg, M.; Borewicz, K.; Scheurink, A.J.; Bruininx, E.; de Vos, P.; Smidt, H.; Schols, H.A.; Gruppen, H. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol. Nutr. Food Res. 2017, 61, 186. [Google Scholar] [CrossRef]
- de Vries, H.; Geervliet, M.; Jansen, C.A.; Rutten, V.; van Hees, H.; Groothuis, N.; Wells, J.M.; Savelkoul, H.F.J.; Tijhaar, E.; Smidt, H. Impact of Yeast-Derived β-Glucans on the Porcine Gut Microbiota and Immune System in Early Life. Microorganisms 2020, 8, 1573. [Google Scholar] [CrossRef]
- Trachsel, J.; Briggs, C.; Gabler, N.K.; Allen, H.K.; Loving, C.L. Dietary Resistant Potato Starch Alters Intestinal Microbial Communities and Their Metabolites, and Markers of Immune Regulation and Barrier Function in Swine. Front. Immunol. 2019, 10, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Liao, R.; Tu, W.; Lu, Y.; Cai, X. Pyrodextrin enhances intestinal function through changing the intestinal microbiota composition and metabolism in early weaned piglets. Appl. Microbiol. Biotechnol. 2020, 104, 4141–4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [Green Version]
- Lopreiato, V.; Mezzetti, M.; Cattaneo, L.; Ferronato, G.; Minuti, A.; Trevisi, E. Role of nutraceuticals during the transition period of dairy cows: A review. J. Anim. Sci. Biotechnol. 2020, 11, 96. [Google Scholar] [CrossRef]
- Wang, W.; Gänzle, M. Toward rational selection criteria for selection of probiotics in pigs. Adv. Appl. Microbiol. 2019, 107, 83–112. [Google Scholar] [CrossRef]
- Guerra-Ordaz, A.A.; González-Ortiz, G.; La Ragione, R.M.; Woodward, M.J.; Collins, J.W.; Pérez, J.F.; Martín-Orúe, S.M. Lactulose and Lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl. Environ. Microbiol. 2014, 80, 4879–4886. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.Y.; Zhu, Y.H.; Zhang, W.; Zhou, D.; Zhai, C.C.; Wang, J.F. Influence of orally fed a select mixture of Bacillus probiotics on intestinal T-cell migration in weaned MUC4 resistant pigs following Escherichia coli challenge. Vet. Res. 2016, 47, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisi, P.; Latorre, R.; Priori, D.; Luise, D.; Archetti, I.; Mazzoni, M.; D’Inca, R.; Bosi, P. Effect of feed supplementation with live yeast on the intestinal transcriptome profile of weaning pigs orally challenged with Escherichia coli F4. Animal 2017, 11, 33–44. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Shanmugam, S.K.; Kang, D.K.; Kim, I.H. Preliminary assessment on potentials of probiotic B. subtilis RX7 and B. methylotrophicus C14 strains as an immune modulator in Salmonella-challenged weaned pigs. Trop. Anim. Health Prod. 2017, 49, 1065–1070. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, Y.H.; Zhang, W.; Wang, M.L.; Fan, W.Y.; Song, D.; Yang, G.Y.; Jensen, B.B.; Wang, J.F. Oral administration of a select mixture of Bacillus probiotics generates Tr1 cells in weaned F4ab/acR- pigs challenged with an F4+ ETEC/VTEC/EPEC strain. Vet. Res. 2015, 46, 95. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Q.; Zhu, Y.H.; Zhang, H.F.; Yue, Y.; Cai, Z.X.; Lu, Q.P.; Zhang, L.; Weng, X.G.; Zhang, F.J.; Zhou, D.; et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: Intestinal microbiota and immune imbalances. PLoS ONE 2012, 7, e40666. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79. [Google Scholar] [CrossRef]
- Samolińska, W.; Kowalczuk-Vasilev, E.; Grela, E.R. Comparative effect of different dietary inulin sources and probiotics on growth performance and blood characteristics in growing-finishing pigs. Arch. Anim. Nutr. 2018, 72, 379–395. [Google Scholar] [CrossRef] [PubMed]

| Dietary Sources of Prebiotics | Study Model | Effect on Gut Microbiota | Effect on Host Health | Effect on Growth Performance | Reference |
|---|---|---|---|---|---|
| A diet supplemented with 5% microcrystalline cellulose or inulin for 72 days | Pig | Alter the composition of ileal and colonic mucosal microbiota ↑Akkermansia and ↑production of butyrate | Enhance sulfomucin production and mucosal barrier function | Not mentioned | [144] |
| A basal diet containing 2.5, 5.0, and 10.0 g/kg inulin | Thirty-two male weaned pigs | ↑Lactobacillus population ↓Escherichia coli population in the caecum ↑Production of acetic and butyric acid | Elevate serum insulin-like growth factor-1 concentration but reduced diamine oxidase concentration; | 2.5 g/kg inulin increase the average daily feed intake (ADFI) and average daily body weight gain (ADG) of pigs | [145] |
| A basal diet containing 0.5% inulin for 21 days | Twenty growing-pigs | ↑Lactobacillus spp. in the ileum and ↑Bacteroides spp. in the cecum ↑Production of acetate and butyrate in cecum |
Increase villus height and the abundance of zonula occludens-1; Decrease IL-6 and TNFαexpression, and reduce gut epithelial cell apoptosis in ileum and cecum | Not mentioned | [146] |
| The combined of the early-life galacto-oligosaccharides (GOS) and postweaning GOS intervention | Weaning Piglets | ↑ The abundances of (SCFA) producers ↑Total SCFA concentration | Reduce the expression of MyD88-NF-κB signaling and the proinflammatory cytokines | Not mentioned | [147] |
| Piglets in the GOS group were given 10 mL of GOS solution daily | Neonatal Porcine Model | ↑Lactobacillus and unclassified Lactobacillaceae, ↓Clostridium sensu stricto on day 8 and day 21 after GOS intervention. ↓Escherichia on day 21 following the early-life GOS intervention | Increase microbial metabolites (such as SCFAs, and Lactate), endocrine peptides, and the mRNA expression of anti-inflammatory cytokines and antimicrobial peptides | Not mentioned | [148] |
| xylo-oligosaccharides group (basal diet + 250 g t-1 XOS) | weaned piglets | ↑The concentrations of butyrate in the ileum and tryptamine and spermidine in the colon ↓The concentration of indole in the colon | Improve the growth performance | Increase the ADFI and ADG | [149] |
| Piglets were fed a low-methyl esterified pectin enriched diet, a high-methyl esterified pectin enriched diet, a hydrothermal treated soybean meal enriched diet or a control diet | weaning pigs | ↓Abundance of the genus Lactobacillus ↑Abundance of Prevotella |
Affect the digestion processes; Shape the colonic microbiota from a Lactobacillus-dominating flora to a Prevotella-dominating community, with potential health-promoting effects | Not mentioned | [150] |
| piglets were fed with yeast-derived β-glucans | piglets | ↓Abundance of Methanobrevibacter ↑Abundance of genera Fusobacterium and Ruminococcaceae_UCG-002 |
Did not affect the vaccination response; Affect modestly fecal microbiota composition and immune parameters | Not mentioned | [151] |
| Pigs received a diet amended with 5% resistant potato starch | piglets | ↑anaerobic Clostridia and ↑Production of butyrate |
Increase the abundance of regulatory T cells in the cecum; Modulate the microbiota and host immune status, altering markers of cecal barrier function and immunological tolerance | Not mentioned | [152] |
| Piglets fed a diet with 0.5% PD | weaned piglets | ↓Abundance of pathogenic organisms, such as Defluviicoccus and Gardnerella ↑Psychrobacter and Prevotella ↑SCFA-producing bacteria | Increase the concentration of SCFAs in the feces | At 42 days of age, dietary PD supplementation increase the body weight (P = 0.06); Increase the feed efficiency | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Huang, X.; Yan, F.; Yin, J.; Xiao, Y. The Role of Gut Microbiota in the Skeletal Muscle Development and Fat Deposition in Pigs. Antibiotics 2022, 11, 793. https://doi.org/10.3390/antibiotics11060793
Han Q, Huang X, Yan F, Yin J, Xiao Y. The Role of Gut Microbiota in the Skeletal Muscle Development and Fat Deposition in Pigs. Antibiotics. 2022; 11(6):793. https://doi.org/10.3390/antibiotics11060793
Chicago/Turabian StyleHan, Qi, Xingguo Huang, Fuyong Yan, Jie Yin, and Yingping Xiao. 2022. "The Role of Gut Microbiota in the Skeletal Muscle Development and Fat Deposition in Pigs" Antibiotics 11, no. 6: 793. https://doi.org/10.3390/antibiotics11060793
APA StyleHan, Q., Huang, X., Yan, F., Yin, J., & Xiao, Y. (2022). The Role of Gut Microbiota in the Skeletal Muscle Development and Fat Deposition in Pigs. Antibiotics, 11(6), 793. https://doi.org/10.3390/antibiotics11060793





