1. Introduction
Staphylococcus aureus, a Gram-positive bacteria, is a pathobiont of humans and animals including pets, livestock and wildlife, with animal infections and reservoirs being a potential source for human infections and vice versa [
1]. Currently, the epidemiology of
S. aureus including methicillin resistant strains is classified into three hospital or healthcare-associated
S. aureus (HA-SA and HA-MRSA, respectively), community-associated
S. aureus (CA-SA) and livestock-associated
S. aureus (LA-SA) [
2].
Spa typing and multi-locus sequence typing (MLST) are widely used to derive
spa types (t) and sequence types (STs) or clonal complexes (CCs), which have been determined globally. Some
spa and ST types are commonly associated with LA-SA. Typically, LA-SA from pigs in Europe have been associated with CC398, whereas CC9 is the predominant type in Asia [
3].
Spa types t011, t034, t108, t567, t571, t899, t1254, t1451, t2011 and t2510 are associated with CC398, and are among those more closely linked to LA-SA [
4].
While a wide range of livestock are implicated with LA-SA, pigs are considered a major reservoir of these
S. aureus strains [
5]. Notably, the LA-SA have been detected in persons with occupational contact with pigs including farm and slaughterhouse workers and veterinarians [
6,
7]. Additionally, LA-SA has been isolated in persons without occupational contact to the animals [
8]. Therefore, LA-SA are a source of concern as they can be passed on from animals to humans and from humans to humans. While LA-SA were mostly associated with colonisation and minor infections, blood stream infections (invasive infections) with livestock-associated methicillin-resistant
S. aureus (LA-MRSA) have been reported in Germany and Denmark [
9,
10]. This is worrisome as it shows that these strains are not only circulating in the community but are entering hospitals thereby blurring the distinctions between the epidemiological groups of
S. aureus. Such transmission is of major concern, because while the use of antibiotics in farm animals such as pigs may select for antimicrobial resistance, LA-SA have generally been more susceptible to antibiotics compared to the HA-SA due to excessive use of antibiotics and or poor antibiotic stewardship in hospitals. Therefore, the entry of LA-SA into health care institutions may lead to these strains acquiring resistance which could be passed back into their communities of origin as adapted strains.
While emphasis is mainly placed on MRSA, methicillin susceptible
S. aureus (MSSA) strains are equally important in the evolution of
S. aureus. Using whole genome sequencing, it has been shown that LA-CC398-MRSA evolved from an ancestor which was a human-adapted HA-MSSA CC398 [
11]. The CC398-MSSA ancestor could have acquired resistance to methicillin and tetracycline while losing the prophage that carries the immune evasion cluster genes (IEC). The IEC genes protect
S. aureus against the immune system in humans [
12]. The presence or absence of the IEC genes can indicate whether
S. aureus strains are human or livestock-associated, respectively. Several studies from European countries show that the LA-CC398 MSSA have emerged as a subpopulation of causative agents of invasive infections in hospitals [
13,
14,
15,
16,
17]. Of interest is a subset of the CC398 MSSA which is independent of livestock but is human adapted [
15]. However, there is sparse information on this clade. Therefore, more studies are warranted to further understand such lineages. Furthermore, given the role of human and animal interactions in the emergence of such lineages, it is critical to conduct such studies in a comprehensive manner using a “One Health” approach. Despite heightened interest in the epidemiology of LA-SA across the globe, there is still a paucity of data on the prevalence and characteristics of pig related LA-SA on the African continent. A recent systematic review revealed that only 19 studies specifically reported on the prevalence or incidence, antimicrobial susceptibility profiles and genetic characteristics of pig-associated
S. aureus in Africa between 2000 and 2019 [
18].
Pig farming is an important economic activity in Zambia, with most pig farmers being smallholder farmers in the rural areas of the country. However, commercial pig farming has over the years become more common in the more urban parts of the country especially in Lusaka Province. This shift could entail an increase in antibiotic use in pig rearing establishments, which has been shown to be a risk factor for the emergence of MRSA. Zambia, similarly to many other countries, has reported the presence of
S. aureus infections in the clinical settings including multidrug resistant strains of MRSA [
18,
19], in pets [
20] and wildlife [
21]. However, there is scarce information on LA-SA. Therefore, this study aimed to determine the prevalence, phenotypic and genotypic characteristics of
S. aureus in pigs and workers from farms and abattoirs handling pigs in Lusaka Province of Zambia.
3. Discussion
This study aimed at determining the prevalence, phenotypic and molecular characteristics of
S. aureus from pigs and workers from pig farms and abattoirs in the Lusaka Province of Zambia. This is the first report on the presence of
S. aureus in pigs and workers from farms and abattoirs in Zambia. The overall prevalence rate (33.1%) of
S. aureus in the present study was relatively high and is in congruence with similar studies on the African continent that have reported prevalences ranging from 0% to 55% [
18]. However, specific comparisons of prevalences is difficult due to the variations in the conduct of these studies, for example, most studies have only studied isolates either from farms or abattoirs and not from both sites [
1]. In addition, some studies may sample from more than one body part of the pigs [
1]. A comparatively higher prevalence of
S. aureus was detected in the pigs (37.8%) than in workers (11.3%), similar to the findings from a recent study in Nigeria [
22]. However, the studies from Nigeria and South Africa detected more
S. aureus from pigs than in our study [
22,
23]. The current study further showed that hand and nasal prevalence of
S. aureus was the same among workers.
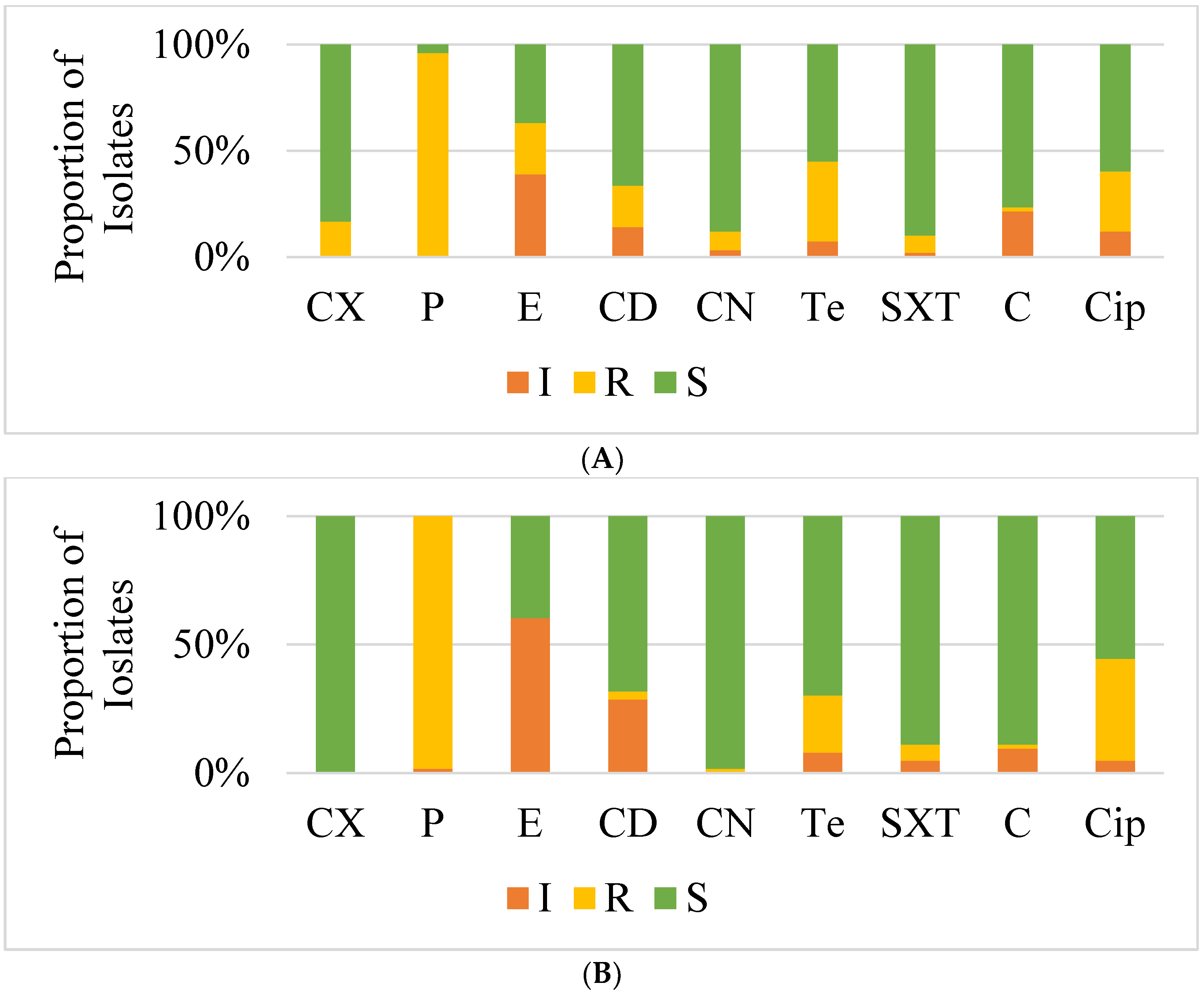
The antimicrobial susceptibility profiles of the isolates revealed that most isolates from farms and abattoirs were resistant to several antibiotics, with the highest resistance being to penicillin (98%). This finding is significantly higher than that from the study in Nigeria which reported a lower resistance to penicillin of 55% [
22]. The high resistance to penicillin reflects possible overuse of the antibiotic, as penicillin is generally among most frequently used antibiotics in many farms in many countries [
22,
24,
25]. Resistance to tetracycline, erythromycin and ciprofloxacin was also recorded in 25% to 35% of isolates in our present study. Notably, tetracycline is also commonly used to treat infections in both humans and animals and its resistance can be used as an indicative marker of LA-SA [
13,
26]. Only 18% of farm isolates were resistant to cefoxitin implying methicillin resistance. However, all these isolates were susceptible to vancomycin with the MICs ranging between 1.5 μg/mL to 3 μg/mL. Vancomycin is the drug of choice for MDR
S. aureus infections in human health and is rarely used to treat animal infections [
27]. This finding which is similar to that of a previous study that studied vancomycin susceptibility of clinical
S. aureus show that vancomycin is still a viable treatment option of
S. aureus infections in Zambia [
28].
The isolates in the present study were more susceptible to co-trimoxazole, gentamicin and chloramphenicol ranging from 79% to 92%. Inducible resistance to macrolides, lincosamides, and group B streptogramins (MLSBi) phenotype was only detected in one isolate. MLSBi phenotype positive isolates appear to be erythromycin-resistant and clindamycin sensitive in vitro, but when given in vivo, they have constitutive
erm mutations that render clindamycin ineffective [
29]. A recent study at the largest referral hospital in Zambia found that none of the isolates had the MLSBi phenotype [
28]. However, an earlier study at the same hospital reported a high rate of the MLSBi phenotype of 68.3% [
19]. Many studies on
S. aureus in animals do not report on the MLSBi phenotype probably because clindamycin is not used to treat infections in animals, however, a study from South Africa reported the MLSBi phenotype among the studied isolates from pigs [
23]. Although multi-drug resistance was observed to two or more antibiotics in more than 40% of the isolates, generally our findings suggest that there are seemingly still several antibiotics that would be viable to treat infections caused by these isolates from the pig and pork production sector in Zambia.
Unexpectedly, despite the phenotypic resistance to methicillin based on resistance to oxacillin using cefoxitin disc that was detected in some of the isolates, neither the
mecA nor
mecC genes that encode for methicillin resistance were detected in any of the isolates. A possible explanation to the phenotypic resistance could be that the isolates are hyperproducers of penicillinases that confer some resistance to cefoxitin [
30]. While the
mecA is the mainstay gene responsible for methicillin resistance in clinical isolates, the
mecC gene is linked to livestock associated staphylococcus especially LA-MRSA [
31]. A recent study from South Africa reported the presence of the
mecC in pig-associated
S. aureus for the first time in Africa [
32]. Relatively few countries have reported typical LA-MRSA pig-related
S. aureus in Africa [
18]. Studies from other parts of the world such as Europe and America state that intensive pig farming methods and heavy use of antibiotics are risk factors for the emergence and spread of methicillin resistance as well as resistance to other antibiotics in
S. aureus among pigs and attending workers at farms and slaughterhouses [
9,
33,
34]. However, none of the facilities included in the current study practises such intensive pig rearing. Markedly, MSSA cannot be overlooked as they form the reservoir from which MRSA arise [
11,
35]. The presence of antimicrobial resistance genes in the present study including
tetM,
tetK and
tetL genes encoding for tetracycline resistance and
ermB and
ermC genes encoding resistance to erythromycin in some of the isolates indicate the need to closely monitor these strains as they may become a source of antimicrobial resistance given that some of these genes are harboured on plasmids which can be easily transferred between microorganisms [
36].
Genes encoding the PVL and SEs were not detected in any of the isolates in the present study. While this was the first study to look for the presence of these genes in isolates from pigs in Zambia, the PVL has been reported in a previous study of clinical isolates howbeit only three out of 33 isolates were positive [
37]. A study in Senegal on pigs and workers at commercial farm reported a high prevalence of the PVL gene [
38]. The PVL is associated with skin and soft tissue infections and has a provenance for humans, but our study indicates that it is dispensible for pig colonisation. The role of SEs in Staphylococcal foodborne disease has been documented in several studies [
39,
40,
41]. Therefore, the non-detection of SEs could indicate the relative safety of the pork and pork products on the Zambian market for consumers. Interestingly, several isolates harboured the IEC genes with the
sak being the most prevalent. The staphylokinase and chemotaxis inhibitory proteins form the IEC and contribute to immune evasion in humans [
12]. While IEC genes are less prevalent in livestock-adapted
S. aureus lineages, they are considered good genetic markers for identification of human-associated
S. aureus clones [
42]. Therefore, the finding of IEC genes among
S. aureus isolates from pigs in the present study potentiate the notion of possible anthropogenic nature of some of the
S. aureus in Africa but could also indicate the presence of LA-SA that are well adapted to human hosts [
15,
18].
Our study found six
spa types among which t1430 was the most prevalent followed by t304 mostly among
S. aureus isolates from pigs both at the farms and abattoirs. Of significance is that t1430 and t034 are associated with CC9 and CC398 which are Livestock-associated lineages of
S. aureus in Asia and Europe, respectively [
3,
4]. Therefore, our findings suggest that typical LA-SA lineages are present in pig and pork production facilities in Zambia. Generally, the
spa types detected in pigs were different from those detected in humans in the present study, only t1430 was found in pigs and workers isolates. This would suggest distinct
S. aureus lineages in the two populations. However, given that we could not identify the
spa types of many isolates, we recommend further investigations into the clonal lineages using other molecular methods such as multilocus sequence typing (MLST) and whole genome sequencing (WGS) which could not be performed in the present study. Previous studies on pig related
S. aureus isolates on the African continent are relatively few but show that the isolates have diverse
spa types [
18]. Furthermore, studies looking into the presence of LA-SA as a cause of disease among hospitalised patients in Africa are needed as this has not been reported yet but have a large impact on epidemiology of
S. aureus infections.