Abstract
The guidelines on ventilator-associated pneumonia (VAP) recommend an empiric therapy against methicillin-resistant Staphylococcus aureus (MRSA) according to its prevalence rate. Considering the MRSA and MSSA VAP prevalence over the last 9 years in our tertiary care hospital, we assessed the clinical value of the MRSA nasal-swab screening in either predicting or ruling out MRSA VAP. We extracted the data of 1461 patients with positive bronchoalveolar lavage (BAL). Regarding the MRSA nasal-swab screening, 170 patients were positive for MRSA or MSSA. Overall, MRSA had a high prevalence in our ICU. Despite the COVID-19 pandemic, there was a significant downward trend in MRSA prevalence, while MSSA remained steady over time. Having VAP due to MRSA did not have any impact on LOS and mortality. Finally, the MRSA nasal-swab testing demonstrated a very high negative predictive value for MRSA VAP. Our results suggested the potential value of a patient-centered approach to improve antibiotic stewardship.
1. Introduction
Ventilator-associated pneumonia (VAP) is a frequent infection in the Intensive Care Unit (ICU) setting [1], with a high mortality rate [2].
While a microbiological confirmation is clearly encouraged, on most occasions clinicians have to start an empiric therapy, since delaying treatment is associated with higher mortality [3]. Regarding the initial selection of the antimicrobial regimen, an empiric therapy, based on the awareness of local resistance patterns, should be one of the cornerstones [4,5].
Particularly, while the guidelines recommend an empiric therapy against methicillin-resistant Staphylococcus aureus (MRSA) rather than methicillin-susceptible Staphylococcus aureus (MSSA) if more than 20% of the Staphylococcus aureus isolates in the local ICU are MRSA [6], there is a paucity of real-life data supporting it. Thus, we may require an individual-based approach for VAP rather than a primarily prevalence-based approach [7]. To go along with this alternative mindset, it is mandatory to identify more targeted risk factors for MRSA infection, such as MRSA colonization [8].
With this in mind, the primary aim of this study was to evaluate MRSA and MSSA VAP prevalence over the last nine years in our hospital, Fondazione IRCCS Policlinico San Matteo of Pavia (IRCCS-FPSMP). The secondary aim was to assess the clinical value of the MRSA nasal-swab screening, in either predicting or conversely ruling out MRSA VAP.
2. Results
Overall, data about 1461 positive BAL samples were extracted. Among these, 170 (11.6%) were positive for MRSA or MSSA.
Characteristics of patients with MSSA and MRSA VAP are shown in Table 1. Significantly, there were no differences in ICU between patients with MSSA and MRSA VAP.

Table 1.
Characteristics of patients with MSSA and MRSA VAP.
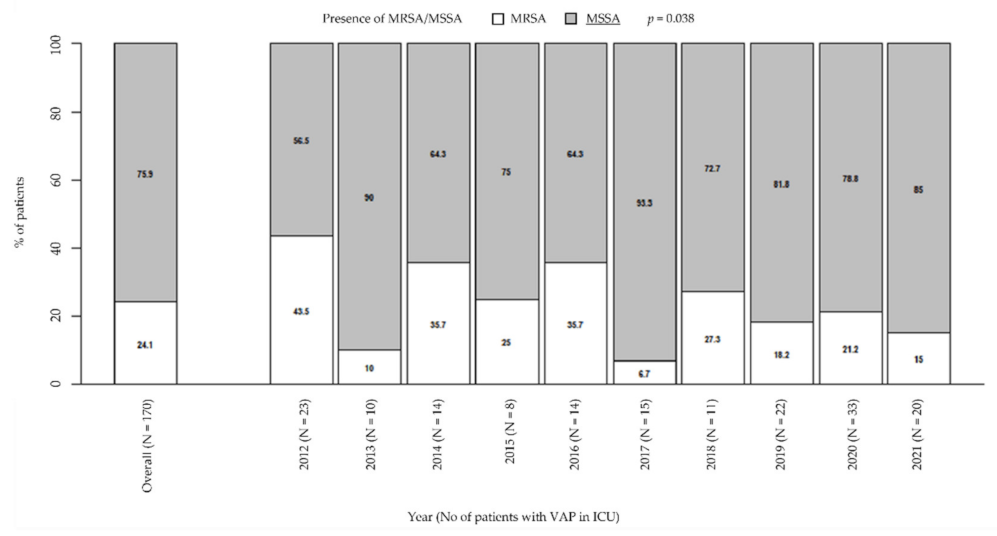
Among VAP due to Staphylococcus spp., the prevalence of MSSA significantly increased over the years from 56.5% in 2012 to 85% in 2021; by contrast, the prevalence of MRSA significantly decreased from 43.5% in 2012 to 15% in 2021 (p = 0.038; Figure 1). However, MRSA had a very high prevalence overall (24.1%). During the considered period, the prevalence of MRSA was higher than 10–20%, that is, the guidelines threshold to define a low-risk ICU for VAP due to this bacterium, or slightly lower, as it happened in 2019 (18.2%) and in the current year (15%). The exceptions are in 2013 and 2017, when prevalence was significantly lower than in the other years (10% and 6.7%, respectively).
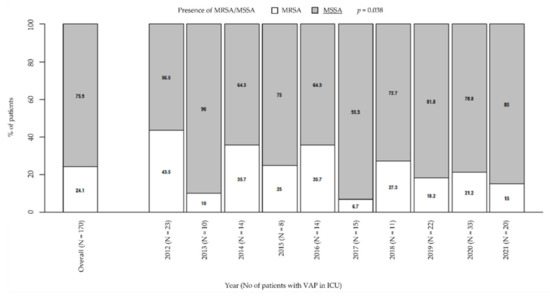
Figure 1.
Prevalence of MRSA/MSSA in patients with VAP in ICU. p-value by Chi-squared test for trend in proportions. ICU: intensive care unit. MRSA/MSSA: methicillin-resistant Staphylococcus aureus/methicillin-susceptible Staphylococcus aureus. VAP: ventilator-associated pneumonia.
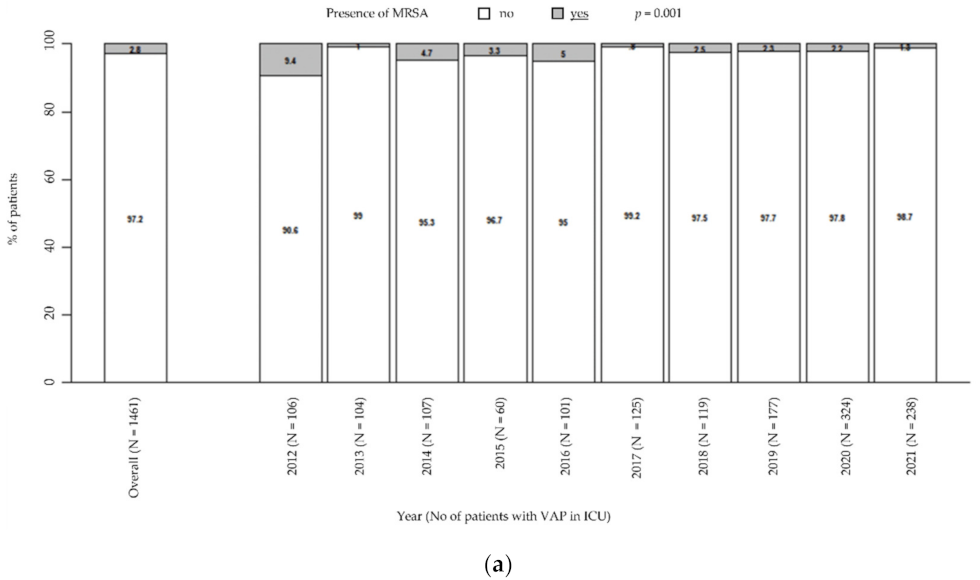
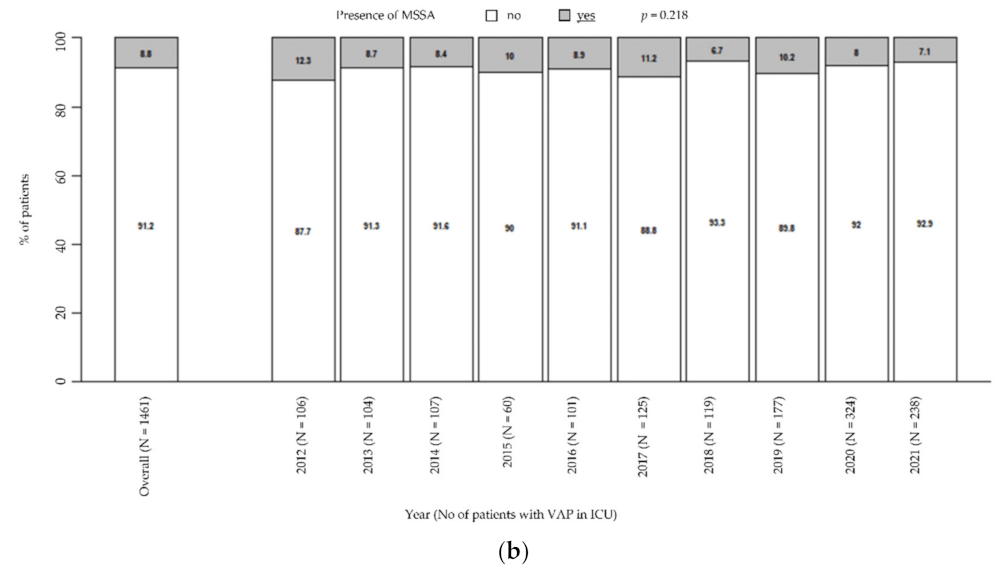
Moreover, there was a general downward trend in MRSA prevalence (from 9.4% in 2012 to 1.3% in 2021, p = 0.001; Figure 2a), while MSSA remained steady over time (from 12.3% in 2021 to 7.1% in 2021, p = 0.218; Figure 2b). Particularly, these trends were unaffected by the COVID pandemic.
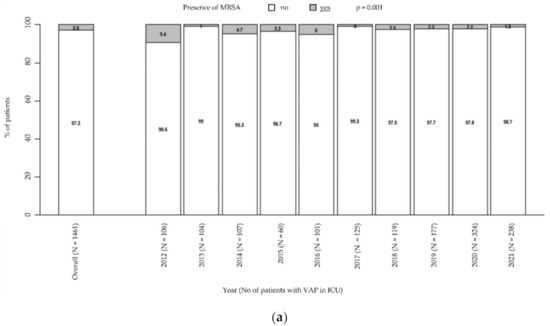
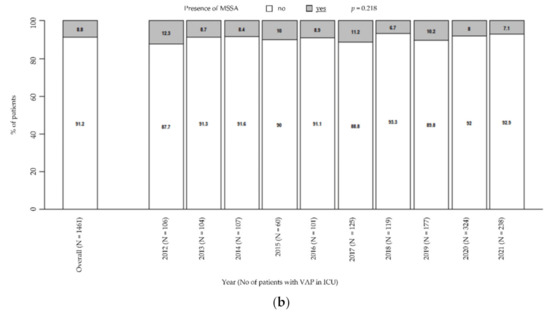
Figure 2.
Prevalence of MRSA in patients with VAP in ICU (a). Prevalence of MSSA in patients with VAP in ICU (b). p-value by Chi-squared test for trend in proportions. ICU: intensive care unit. MRSA: methicillin-resistant Staphylococcus aureus. MSSA: methicillin-susceptible Staphylococcus aureus. VAP: ventilator-associated pneumonia.
With regard to the secondary outcome, among the 1461 patients with VAP, 976 (66.8%) were tested for MRSA nasal colonization before BAL. Accordingly with the fact that the nasal screening for MRSA was not routinely performed before 2016, the majority of the nasal tests, namely, 896, were collected from 2016 onwards. Among the specimens collected from 2016 onwards, only 22 resulted positive for MRSA (2.46%) (Table 2).

Table 2.
MRSA nasal-swab screening.
The MRSA nasal-swab testing demonstrated a 42.1% sensitivity and 98.4% specificity, with a PPV of 36.4% and a NPV of 98.7% (Table 3).

Table 3.
Statistical analysis.
3. Discussion
Our results showed that, despite a downward trend in prevalence of VAP due to MRSA over the last nine years, it has overall remained above 20% in the ICU of IRCCS-FPSMP.
These findings are in line with the ECDC reports, which include countries with both low and high percentages of MRSA and outline a decreasing trend of MRSA infections during the period 2015–2018 [9]. In our case, this trend was notably unaffected by the COVID pandemic. This result is not entirely concordant with the current state of the literature, since the hard COVID-19 knock on our health systems widely increased hospital-acquired infection rates, including MRSA-related infections [10]. However, there are also some well-known and positive consequences of the measures of control and prevention implemented during the pandemic, especially on a microorganism which resides in the nose and on the skin, always covered by masks and gowns [11].
Regarding our choice of using data from positive BAL samples, it has been made according to the clinical practice guidelines on the management of VAP in adults, which recommend both invasive and noninvasive sampling with semiquantitative cultures as the preferred diagnostic methodology [12]. We believe that, although some cases of VAP might have been missed, obtaining microbiological data from BAL samples was crucial for the purposes of our study.
In our study population, MRSA is responsible for only a few cases of VAP, frequently resulting in many unnecessary empiric treatments. MSSA remains more involved in VAP, which warrants greater consideration [13].
In light of the very low absolute prevalence of MRSA as a cause of VAP in our reality and the disproportionate number of patients who would need to be treated unnecessarily empirically, we would really rethink the appropriateness of using the threshold proposed by the guidelines (10–20% relative prevalence of MRSA) [14].
Instead, it might be more meaningful to consider each patient’s individual risk factors for MRSA infection such as nasal colonization, previous antibiotic treatment and prolonged hospital stay, as prompted by recent findings [7]. In our opinion, this individual-based approach might be valuable also in a context where novel multiplex real-time PCR assays on respiratory materials will have a growing role in early diagnosis of VAP [15].
We want to highlight that MRSA nasal colonization, which is a recognized risk factor for MRSA VAP, has a significantly high NPV in our analysis. This finding has been already widely demonstrated in the current literature, also for extrapulmonary MRSA infections [16]. However, it should be mentioned that our result may be driven by the very low prevalence of MRSA VAP in our cohort of patients. This consideration prevents us from surely asserting that MRSA nares screening is a valuable tool to rule out MRSA VAP; though, it validates our initial hypothesis that moving forward the prevalence-based approach is crucial.
The limits of this study are the monocentric and retrospective nature, the lack of data on antibiotic treatments performed, and the clinical comorbidities, which are unfortunately not fully available from the ICD-9CM codes.
4. Materials and Methods
4.1. Study Design and Participants
This is a single-center, retrospective, observational cohort study.
The SkyNet database is a Relational Database Management System which includes microbiological and clinical data from all the patients admitted to IRCSS-FPSMP.
Data of positive bronchoalveolar lavage (BAL) samples of patients admitted to the ICU from 1 January 2012 to 13 December 2021 were retrospectively extracted from the SkyNet database.
According to the new National Healthcare Safety Network (NHSN) definition [17], only the BAL from patients with signs, symptoms, and imaging test results consistent with pneumonia were included. Moreover, BAL samples without leucocytes and/or inflammatory cells were excluded.
The samples of the MRSA nasal-swab testing of ICU patients with VAP from 1 January 2012 to 13 December 2021 were retrospectively extracted from the SkyNet database. Any patient with at least one positive nasal swab for MRSA at any time before VAP onset was considered colonized by MRSA. MRSA nasal-swab screening was extensively performed on all new ICU admissions from 2016, so only data from that date onwards were considered in our statistical analysis. Since the same year, a nasal decolonization with mupirocin of patients found colonized with MRSA has been routinely performed in our ICU.
4.2. Outcomes
The primary outcome was to evaluate MRSA and MSSA VAP prevalence over the last nine years in our tertiary care hospital. The secondary outcome was to evaluate the performance of the MRSA nasal swab in predicting or ruling out MRSA VAP.
4.3. Statistical Methods
The trend of positive BAL for MRSA or MSSA over the years and the differences in prevalence were evaluated by Chi-square tests for trend. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the MRSA nasal swab were calculated.
5. Conclusions
In conclusion, our results showed that the trend of MRSA VAP in our ICU has declined over the years, despite the COVID pandemic. Furthermore, we suggested the potential value of a patient-centered approach in order to improve antibiotic stewardship.
Author Contributions
M.C. (Marta Colaneri) and L.N.M. wrote the manuscript. D.D.C. and A.A. developed the theory and performed the computations. M.C. (Marta Corbella), G.P., P.C. and A.M. encouraged M.C. (Marta Colaneri) and D.D.C. to investigate the microbiology. C.B., A.D.M., P.S., F.M. and R.B. supervised the findings of this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was written in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The SkyNet Database was approved by the local Ethical Committee (Comitato Etico Pavia, No. prot. 20210068914).
Informed Consent Statement
All participants have signed an informed consent for data collection.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.; Klarin, B.; Koeman, M.; A Krueger, W.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Shorr, A.F.; Jiang, X.; Day, A.; Heyland, D.K. The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: An important determinant of outcome. J. Crit. Care 2012, 27, 322.e7–322.e14. [Google Scholar] [CrossRef] [PubMed]
- de Bus, L.; Depuydt, P.; Steen, J.; Dhaese, S.; de Smet, K.; Tabah, A.; Akova, M.; Cotta, M.O.; de Pascale, G.; Dimopoulos, G.; et al. Antimicrobial de-escalation in the critically ill patient and assessment of clinical cure: The DIANA study. Intensive Care Med. 2020, 46, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.R.; Williamson, J.C.; Johnson, J.W.; Ohl, C.A.; Karchmer, T.B.; Bowton, D.L. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest 2006, 130, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef] [PubMed]
- Vandana Kalwaje, E.; Rello, J. Management of ventilator-associated pneumonia: Need for a personalized approach. Expert Rev. Anti. Infect. Ther. 2018, 16, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Laehn, S.; Huang, A.; Carver, T.; Peppard, W. 701: Risk factors for MRSA pneumonia in critically ill trauma/surgical ICU patients. Crit. Care Med. 2016, 44, 251. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe 2018. Stockholm: ECDC. 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf (accessed on 14 December 2021).
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; E Poland, R.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of Coronavirus Disease 2019 (COVID-19) on Healthcare-Associated Infections. Clin. Infect. Dis. 2021, 74, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.I.; Conceicao, E.P.; Tan, J.Y.; Magesparan, K.D.; Amin, I.B.M.; Ismail, B.B.S.; Toh, H.X.; Jin, P.; Zhang, J.; Wee, E.G.L.; et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am. J. Infect. Control 2020, 49, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Peng, P.; Liu, Z.; Xue, Z.; Yao, C. Risk Factors and Clinical Outcomes of Hospital-Acquired MRSA Infections in Chongqing, China. Infect. Drug Resist. 2019, 12, 3709–3717. [Google Scholar] [PubMed] [Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [PubMed] [Green Version]
- Kim, H.J.; Kang, E.-R.; Park, M.Y.; Kim, B.K.; Kim, M.J.; Jung, S.; Roh, K.H.; Sung, N.; Yang, J.-H.; Lee, M.-W.; et al. Development of a multiplex real-time PCR assay for the simultaneous detection of four bacterial pathogens causing pneumonia. PLoS ONE 2021, 16, e0253402. [Google Scholar]
- Butler-Laporte, G.; De L’Étoile-Morel, S.; Cheng, M.P.; McDonald, E.G.; Lee, T.C. MRSA colonization status as a predictor of clinical infection: A systematic review and meta-analysis. J. Infect. 2018, 77, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ncezid, C.D.C.; DHQP. National Healthcare Safety Network (NHSN) Patient Safety Component Manual. 2021. Available online: https://www.cdc.gov/nhsn/pdfs/validation/2021/pcsmanual_2021-508.pdf (accessed on 14 December 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).